Abstract
BACKGROUND:
von Willebrand factor is a blood glycoprotein that is required for normal hemostasis. Its level can be increased by endothelial cell damage.
HYPOTHESIS:
von Willebrand factor is a suitable marker of endothelial dysfunction.
METHODS:
von Willebrand factor activity was determined by ELISA in patients with acute coronary syndromes, acute stroke and chronic vascular diseases, and was compared with the values of healthy controls.
RESULTS:
von Willebrand factor activity of patients in each group was significantly higher (P<0.001) than that of the control group. The values of patients with acute coronary syndrome and acute stroke were significantly higher (P<0.05 and P<0.01, respectively) than those of patients with chronic vascular diseases. von Willebrand factor activity was significantly higher in patients with acute coronary syndrome and acute stroke (P<0.05 and P<0.01, respectively) on the sixth day than on admission.
CONCLUSIONS:
By measuring von Willebrand factor activity, a considerable, significant difference could be found between healthy people and chronic and acute vascular patients. The routine measurement of von Willebrand factor activity in vascular patients as an index of endothelial dysfunction may have clinical importance, because detection of this marker can be a noninvasive way of assisting diagnosis and indicating disease progression.
Keywords: Endothelial dysfunction, Marker, Vascular disorders, von Willebrand factor
Cardiovascular and cerebrovascular diseases are major causes of death in adult and elderly patients in developed and many developing countries. Atherosclerosis, a general, progressive process affecting the vascular system, is the underlying disease in these clinical conditions. The ‘response to injury’ hypothesis of atherogenesis proposes that ‘injury’ to the endothelium is the initiating event in this process (1). Endothelial cells form a continuous flow surface throughout the circulatory system and have numerous complex roles. The importance of endothelial dysfunction is the impaired ability of cells to participate adequately both in coagulation and fibrinolysis; these changes may predispose to thrombus and atherosclerosis formation (2). The most useful marker for vascular damage could be a specific, stable, circulating product of the endothelial cells.
von Willebrand factor (vWf) is a blood glycoprotein (GP) that is required for normal hemostasis. A deficiency of vWf results in von Willebrand disease, the most common inherited bleeding disorder. The role of vWf in the pathomechanism of different vascular diseases has been recognized only in the previous decade (3). vWf is produced by endothelial cells and megakaryocytes throughout the body (3,4). Levels of circulating vWf are increased following endothelial cell damage (2) and may also increase during acute phase responses, exercise and pregnancy (5). Although vWf release may merely be due to activated or stimulated endothelial cells (6), the highest levels of vWf are often associated with severe disease, and it is reasonable to assume that direct endothelial cell damage has occurred. This has facilitated its possible use as a marker of such injury in atherosclerosis (7).
vWf has four major functions. Factor VIII circulates in plasma noncovalently complexed with vWf and appears to be stabilized by this interaction. vWf may be viewed as forming a molecular bridge as a part of the adhesion process between platelets and the subendothelium of an injured vessel wall. In the subendothelial matrix, vWf binds to some types of collagen and heparin. vWf has a role in platelet aggregation with binding sites for specific platelet membrane GP Ib and GP IIb/IIIa (7). In the absence of injury, vWf does not appear to interact with circulating platelets. However, damage to the endothelium allows vWf to bind constituents of subendothelial connective tissue, and this enables vWf to bind platelets with sufficient affinity to snare them from the rapidly flowing blood and retain them at the site of injury. A remarkable feature of vWf-mediated platelet adhesion is its dependence on fluid shear stress. At low and medium shear rates of veins and normal, healthy great arteries, platelet adhesion is not stimulated by vWf. However, at a high shear rate, which occurs in small arterioles of 10 μm to 50 μm diameter and above atherosclerotic plaques in partially occluded arteries, platelet adhesion is strongly dependent on vWf (3).
The aim of the present study was to determine vWf activity as the marker of endothelium dysfunction in patients with acute coronary syndromes (ACS), acute stroke and chronic cardiovascular, cerebrovascular and peripheral vascular diseases compared with healthy controls.
PATIENTS AND METHODS
Patients
Control subjects were young, healthy people without any major cardiovascular risk factors or symptomatic vascular diseases. Patients in the chronic vascular disease groups had cardiovascular or cerebrovascular events in their medical history (greater than three months after onset), or peripheral vascular disease confirmed radiologically. They were recruited in outpatient departments. Patients with ACS were admitted to the authors’ coronary care unit with acute heart symptoms, and routine examinations (electrocardiogram, myocardial enzymes) confirmed the ACS in each case. Troponin was increased in 78% of the cases (troponin-positive) and in 22% troponin remained in the normal range (troponin-negative cases). Patients with acute stroke hospitalized within 24 h from onset were recruited for this study at the Department of Neurology. Diagnosis was based on history and clinical neurological and neuroradiological examinations, including computed tomography and magnetic resonance imaging of the brain. Acute focal ischemic stroke occurred in 50%, multiple ischemic stroke in 20% and hemorrhagic stroke in 30% of cases (Table 1).
TABLE 1.
Number, age and male:female ratio of controls and patients
| n | Age (years) | Male:female | |
|---|---|---|---|
| Controls | 23 | 36±12 | 14:9 |
| Chronic vascular patients | 56 | 67±10 | 38:18 |
| Patients with ACS | 29 | 67±13 | 18:11 |
| Patients with acute stroke | 15 | 67±12 | 10:5 |
Age is presented as mean ± SD. ACS Acute coronary syndromes
All patients were asked about their medical histories, including smoking habits, hypertension, diabetes mellitus and dyslipidemia, and their body weight and height were assessed. Patients were considered hypertensive if they were on antihypertensive agents, or if their blood pressure was greater than 140 mmHg systolic or 90 mmHg diastolic. Patients were considered to have diabetes mellitus if they were being treated for diabetes mellitus with oral antidiabetic agents or insulin or if their serum fasting blood sugar level exceeded 7.0 mmol/L. Patients were considered hyperlipidemic if they were taking lipid lowering drugs or if their serum cholesterol level exceeded 5.2 mmol/L or triglyceride level exceeded 2.0 mmol/L. Patients were considered to be obese if their body mass index exceeded 30 kg/m2. The incidence of vascular risk factors is shown in Table 2.
TABLE 2.
Incidence of risk factors in patients with vascular disease
| Chronic patients | ACS | Acute stroke | |
|---|---|---|---|
| Smoking (%) | 29 | 36 | 42 |
| Hypertension (%) | 88 | 75 | 92 |
| Diabetes mellitus (%) | 39 | 29 | 29 |
| Hypercholesterolemia (%) | 65 | 61 | 42 |
| Hypertriglyceridemia (%) | 29 | 36 | 17 |
| Obesity (%) | 61 | 62 | 31 |
ACS Acute coronary syndromes
Blood sampling
To detect vWf activity, venous blood samples were drawn from the antecubital vein of patients into vacutainer tubes containing trisodium citrate (3.2%) as an anticoagulant. Blood samples were taken in patients with ACS and acute stroke on admission, day 2 and day 6; except for acute measurements, all blood samples were drawn between 07:00 and 08:00, after 12 h of fasting. Low density lipoprotein cholesterol concentration was estimated by the Friedewald formula (8).
Measurement of vWf activity
A quantitative direct enzyme immunoassay (Shield Diagnostics Ltd, United Kingdom) was used for the detection of vWf activity (9). Plasma was separated by centrifugation for 10 min at 2500 × g, then 100 μL plasma was dispensed into the wells of microtitre strips coated with purified murine anti-vWf immunoglobulin G monoclonal antibody. During the first incubation vWf bound to the antibody layer, then the wells were washed to remove unbound plasma components. A horseradish-peroxidase-labelled mouse anti-human monoclonal anti-vWf conjugate bound to surface-associated antibody during second incubation. After a further washing step, specifically bound antibody was treated with substrate solution. The amount of bound conjugate was given in absorbance units measured with a Rosys Anthos 2010 absorption photometer (Anthos Labtec Instruments Ltd, Germany). The activity of vWf in a sample was estimated with interpolation using a dose-response curve according to the “4th International Standard for Factor VIII and von Willebrand factor in Plasma” (Code 97/586, National Institute for Biological Standards and Control, United Kingdom).
Statistical analyses
Results were expressed as mean ± SEM. Values distributed normally were evaluated by Student’s t test to calculate statistical significance (10).
RESULTS
None of the examined groups showed a significant difference in the vWf activity between men and women. There was no correlation between age and vWf activity in the control or patient groups.
vWf activity was significantly elevated in all patients diagnosed with vascular diseases compared with the control group (P<0.001) (Figure 1).
Figure 1).
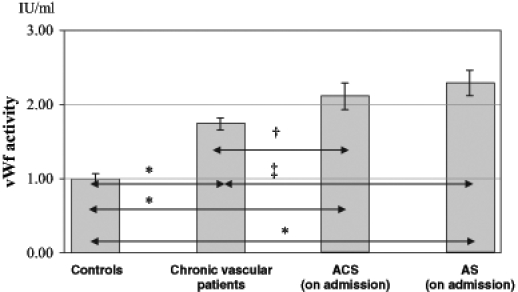
von Willebrand factor (vWf) activity of patients and control group (mean ± SEM). ACS Acute coronary syndromes; AS Acute stroke. †P<0.05; ‡P<0.01; *P<0.001
The vWf values of patients with acute coronary syndrome and acute stroke were significantly higher than in patients with chronic vascular disease (P<0.05 and P<0.01, respectively) (Figure 2). In the subgroups of patients with chronic vascular diseases, there was no significant difference among patients with cardiovascular, cerebrovascular and peripheral vascular disease. During the hospital phase, vWf activity in patients with acute acute coronary syndrome and acute stroke increased continuously and was significantly higher on day 6 than on admission (P<0.05 and P<0.01, respectively) (Figure 2).
Figure 2).
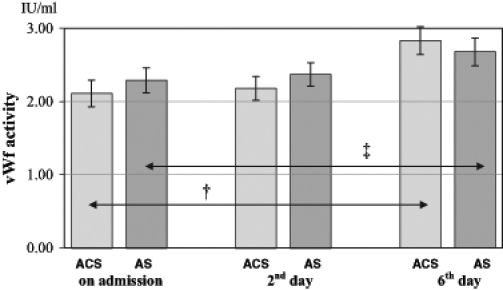
von Willebrand factor (vWf) activity of patients with acute coronary syndromes (ACS) and acute stroke (AS) on admission, day 2 and day 6 (mean ± SEM). †P<0.05; ‡P<0.01)
vWf activity was significantly (P<0.05) higher in troponin-positive patients (2.53±0.12 IU/mL) with ACS compared with the troponin-negative subjects (2.05±0.34 IU/mL). It showed an increasing tendency in patients with hemorrhagic stroke (2.70±0.18 IU/mL) and was significantly elevated (P<0.05) in patients with multiplex ischemic stroke (2.93±0.21 IU/mL) compared with focal ischemic stroke patients (2.44±0.13 IU/mL).
DISCUSSION
Vascular injury and thrombus formation are key events in the origin and progression of atherosclerosis (11). Identification of a molecule, that accurately reflects this injury, would be a useful adjunct in the study of these pathogenic processes. Plasma vWf is perhaps the most useful marker, because it is specific for endothelial cells, it is stable, it may be relevant to the disease process and it is simple to assay. Green first postulated that vWf may be of value as a marker of endothelial injury in atherosclerosis (12).
Because endothelial cell injury is important in the development of atherosclerosis, the elevation of vWf activity can be associated with well-known risk factors for this disease: hypertension, smoking, obesity, diabetes and dyslipidemia (7,13–16). In our study, the group of chronic vascular patients was made up of persons with chronic cardiovascular, cerebrovascular and peripheral vascular disease. Our results showed that vWf activity was significantly increased in these patients compared with control subjects. The majority of patients had some of the previously mentioned cardiovascular risk factors, which may account for raised vWf activity and can refer to the endothelial dysfunction and extensive atherosclerosis in these patients. There were no significant differences in vWf activity among the subgroups (cardiovascular, cerebrovascular and peripheral vascular diseases), suggesting that atherosclerosis is a general syndrome and the different types of vascular diseases are manifestations of the same disease localized at different sites of the circulatory system. According to our results, none of the examined groups had significant difference in the vWf activity of men and women. We did not find any correlation between age and vWf activity within the control or patients’ groups. The measurement of vWf activity may be of clinical value in two ways: as a noninvasive way of assisting diagnosis, or as an indicator of disease progression (16).
Because vWf is important in the aggregation of platelets and their adhesion to subendothelial cells, increased levels of circulating vWf per se may promote atherosclerosis and contribute to cardiovascular events (17). Critical steps during the development of ACS are the disruption of atherosclerotic plaque and the superimposed formation of platelet-rich thrombus leading to subtotal or total occlusion of the coronary circulation (18). Pathological stenosis can directly lead to shear-induced aggregation of platelets depending on the presence of plasma vWf and platelet receptors GP Ib/IX and GP IIb/IIIa. In patients with ACS, significant increases of high shear-induced platelet aggregation and plasma vWf levels were observed compared with patients with chronic coronary artery disease (19). According to our results, the values of patients with ACS were significantly higher than in the control group and in patients with chronic vascular disease. The significantly higher vWf activity in acute phase suggests on one hand, more severe endothelium dysfunction, and on the other hand, that it is related to the development of the acute event through increased platelet adhesion and aggregation. During the hospital phase, vWf activity in patients with ACS increased continuously and on day 6 was significantly higher than on admission. The increase of vWf after an acute event can be caused by the endothelial lesion, adrenergic stimulation or the acute phase reaction (20), and can lead to early reocclusion and reinfarction despite recanalization and antithrombotic therapy. The significantly higher vWf activity in troponin-positive patients with ACS compared with troponin-negative subjects can be associated with the distinct pathomechanisms or different severities of these two subtypes of ACS.
Abnormalities of coagulation and fibrinolysis may play an important role in the pathogenesis of ischemic stroke and vascular dementia. Recent evidence indicates that high shear-induced platelet aggregation is increased in patients with atherothrombotic stroke and transient ischemic attacks and is correlated with the increase of larger vWf multimers (21). This association between vWf and cerebral thrombosis may reflect either endothelial dysfunction associated with cerebral thrombosis (or its risk factors, such as hypertension) or ischemia-related release of vWf from infarcted tissue (16). Patients with thrombotic strokes have higher vWf concentrations than those with hemorrhagic strokes (22) but, according to other studies, there is no significant difference in vWf between the three pathological subtypes of stroke (ischemic/thrombotic, hemorrhagic and transient ischemic attack) (23). In our investigation, vWf values of patients with acute stroke were significantly higher than in patients with chronic vascular diseases. As with patients with ACS, vWF increased during the hospital phase and on day 6 it was significantly higher than on admission, which may be analogous to the ACS cases. vWf activity was significantly elevated in patients with multiplex ischemic stroke compared with focal ischemic stroke patients. These results can also refer to the distinct pathomechanisms or different severities of the subtypes of acute stroke. Improved understanding of the pathogenesis of stroke and the potential to predict patients at risk of stroke should herald the beginning of new approaches in stroke management.
There is sufficient evidence to suggest that vWf may be a useful tool for clinical evaluation. The physiological functions of the protein have led to suggestions that increased circulating concentrations of vWf may be relevant to coronary artery disease, but epidemiological studies have been inconclusive. Perhaps vWf is a marker of systemic inflammatory process, or a marker of factors that may produce local endothelial damage, or just a marker of the extent of subclinical atherosclerosis. Previous studies also suggest an association between vWf levels and risk of future coronary artery disease, but further studies have been proposed to determine whether this association is causal (24).
Study limitations
The control group was significantly younger than the patient group; nevertheless, there was no correlation between age and vWf activity in the control or patient groups. Our results require further confirmation in a larger population.
CONCLUSIONS
Our study adds further evidence that vWf activity may prove to be a suitable marker of endothelial dysfunction. By measuring vWf activity, a considerable, significant difference could be found between healthy people and chronic and acute vascular patients. We demonstrated a relationship between vWf activity and the severity of vascular diseases. The routine measurement of vWf activity in vascular patients as an index of endothelial dysfunction may have clinical importance because determination of this marker can be a noninvasive way of assisting diagnosis and indicative of disease progression (16).
Acknowledgments
This work was supported by grants from the Hungarian Scientific Research Foundation (OTKA F 030770).
Footnotes
Paper was presented at the Fourth International Symposium on Myocardial Cytoprotection, Pécs, Hungary, September 25 to 27, 2003
REFERENCES
- 1.Harker LA, Ross R, Glomset JA. The role of endothelial cell injury and platelet response in atherogenesis. Thromb Haemostas. 1978;39:312–21. [PubMed] [Google Scholar]
- 2.Boneu B, Abbai M, Plante J, Bierme R. Factor VIII complex and endothelial damage. Lancet. 1975;305:1430. doi: 10.1016/s0140-6736(75)92650-1. [DOI] [PubMed] [Google Scholar]
- 3.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 4.Ewenstein BE. Vascular biology of von Willebrand factor. In: Born GV, Schwartz CJ, editors. Vascular Endothelium. Stuttgart: Schattauer GmbH; 1997. pp. 107–22. [Google Scholar]
- 5.Pottinger BE, Read RC, Paleolog EM, Higgins PG, Pearson JD. von Willebrand factor is an acute phase reactant in man. Thrombosis Res. 1989;53:387–94. doi: 10.1016/0049-3848(89)90317-4. [DOI] [PubMed] [Google Scholar]
- 6.Pober JS. Cytokine-mediated activation of vascular endothelium. Am J Pathol. 1988;133:426–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Blann AD, McCollum CN. von Willebrand factor, endothelial cell damage and atherosclerosis. Eur J Vasc Surg. 1994;8:10–5. doi: 10.1016/s0950-821x(05)80112-4. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Fischer BE, Thomas KB, Dorner F. von Willebrand factor: measuring its antigen or function? Correlation between the level of antigen, activity, and multimer size using various detection systems. Thromb Res. 1998;91:39–40. doi: 10.1016/s0049-3848(98)00078-4. [DOI] [PubMed] [Google Scholar]
- 10.Electronic statistic textbook . Tulsa: StatSoft; 2004. Electronic Statistics Textbook. < http://www.statsoft.com/textbook/stathome.html>. (Version current at February 23, 2004). [Google Scholar]
- 11.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and acute coronary syndromes. N Engl J Med. 1992;326:242–50. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 12.Minick CR, Alonso DR, Rankin L. Role of immunologic arterial injury in atherogenesis. Thromb Haemostas. 1978;39:304–11. [PubMed] [Google Scholar]
- 13.Vita JA, Treasure CB, Yeung AC, et al. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation. 1992;85:1390–7. doi: 10.1161/01.cir.85.4.1390. [DOI] [PubMed] [Google Scholar]
- 14.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary flow modulation in humans: Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–62. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB. Lipids, diabetes and coronary heart disease. Insights from the Framingham study. Am Heart J. 1985;110:1100–7. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Blann AD. von Willebrand factor and its relevance to cardiovascular disorders. Br Heart J. 1995;74:580–3. doi: 10.1136/hrt.74.6.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kario K, Matsuo T, Hoshide S, et al. Lipid-lowering therapy corrects endothelial cell dysfunction in a short time but does not affect hypercoagulable state even after long-term use in hyperlipidemic patients. Blood Coagul Fibrinolysis. 1999;10:269–76. doi: 10.1097/00001721-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ.The role of plaque pathology in coronary thrombosis Clin Cardiol 1997201–7.8994729 [Google Scholar]
- 19.Isaka N, Tanigawa T, Nishikawa M, Nakano T. [High shear induced platelet aggregation (h-SIPA) and effects of antiplatelet therapy.] Nippon Rinsho. 1998;56:2624–9. Japanese. [PubMed] [Google Scholar]
- 20.Soskin P, Wiesel ML, Mossard JM, et al. von Willebrand factor in coronary disease. Arch Mal Coeur Vaiss. 1994;87:85–93. [PubMed] [Google Scholar]
- 21.Uchiyama S, Yamazaky M, Maruyama S, et al. Shear-induced platelet aggregation in cerebral ischemia. Stroke. 1994;25:1547–51. doi: 10.1161/01.str.25.8.1547. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Lin Z, Shen S. Changes of von Willebrand factor and antithrombin III levels in acute stroke: Differences between thrombotic and hemorrhagic stroke. Thromb Res. 1993;72:353–8. doi: 10.1016/0049-3848(93)90145-e. [DOI] [PubMed] [Google Scholar]
- 23.Lip GY, Blann AD, Faroqi IS, Zarifis J, Sagar G, Beevers DG. Abnormal hemorheology, endothelial dysfunction and thrombogenesis in relation to hypertension in acute (ictus <12 h) stroke patients: The West Birmingham Stroke Project. Blood Coagul Fibrinolysis. 2001;12:307–15. doi: 10.1097/00001721-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Whincup PH, Danesh J, Walker M, et al. Von Willebrand factor and coronary heart disease. Prospective study and meta-analysis. Eur Heart J. 2002;23:1764–70. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]


