Abstract
BACKGROUND:
Cardiovascular diseases are life-threatening conditions and, thus, have received a great deal of attention over the years. Several mechanisms, including hemorheology changes and inflammatory effects, are considered to be involved in the pathogenesis of these diseases. Because cardiovascular dysfunction is also known to worsen hemorheology changes and influence vital symptoms, it has become critical to formulate effective therapeutic strategies to combat the deleterious effects of cardiovascular diseases. Although a wide variety of drugs have been developed for the treatment of cardiovascular diseases, the effectiveness of any agent for therapy of a given disease cannot be indicated with certainty.
OBJECTIVES AND OBSERVATIONS:
Pentoxifylline (PTXF), a phosphodiesterase inhibitor, has been investigated for close to two decades because of its primary pharmacological actions on hemorheology and other anti-inflammatory effects. Several studies have been conducted to investigate the effects and mechanisms of PTXF in ischemic injury, peripheral vascular disease and heart failure. The present article is intended to emphasize the therapeutic potentials of PTXF in different types of cardiovascular diseases, focusing on the mechanisms of its pharmacological actions.
Keywords: Blood viscosity, Ischemia reperfusion injury, Pentoxifylline, Peripheral vascular disease, Platelet function
Pentoxifylline (PTXF), a synthetic methylxanthine, was approved in 1984 for the prevention of intermittent claudication in chronic occlusive arterial disease (1,2). Like other methylxanthine derivatives, PTXF is not only prescribed for peripheral vascular and cerebrovascular diseases, but is also indicated for the treatment of asthma (3). Additionally, PTXF is used to improve the effectiveness of microcirculation, increase red blood cell (RBC) deformability, decrease platelet aggregation and lower plasma viscosity (3–5). PTXF has also been shown to modify the immune system. For instance, this drug improves leukocyte deformability and chemotaxis, depresses neutrophil degranulation, decreases endothelial leukocyte adhesion and lowers the sensitivity of leukocytes to cytokines (6–13). Furthermore, it has been reported that PTXF can inhibit the production of inflammatory cytokines (14), and, thus, reduces neutrophil adhesiveness to endothelial cells, enhances chemotaxis and lowers the production of free radicals (15). In other studies, PTXF has been shown to augment the production of prostacyclins and a vasodilator, eicosanoid (16,17). PTXF is known to inhibit phosphodiesterase (PDE), an enzyme that breaks down cyclic AMP (cAMP), which elevates the level of intracellular cAMP and, thus, lowers platelet aggregation (18) and depresses the production of tumour necrosis factor-alpha (TNF-α) (19). Furthermore, PTXF has been reported to promote the oxygenation of ischemic areas and lower the amount of metabolic derangements associated with ischemia-reperfusion injury (20). Therefore, in view of the wide variety of effects and potent hemorheological properties of PTXF, the pharmacological actions of this agent and its analogues will be discussed for a clear understanding of its therapeutic potential for treatment of cardiovascular disease.
ANALOGUES AND METABOLITES OF PTXF
PTXF is a derivative of theobromine, a methylated xanthine. The most closely related methylated xanthines include caffeine, theophylline and aminophylline (Figure 1), which have been discovered in plants and have several similar pharmacological actions. All of these agents relax smooth muscle, particularly bronchial muscle, stimulate the central nervous system and act on the kidney to promote diuresis. Furthermore, caffeine and theophylline modify blood circulation activities in a similar manner. Although it is known that caffeine increases the capacity for muscular work in humans, three major actions of methylxanthines, namely translocation of intracellular calcium, accumulation of cyclic nucleotides and blockade of adenosine receptors, have received the most attention (1). Of the methylxanthines, the effects of caffeine on the cardiovascular system have been studied since 1966. Caffeine is a widely consumed compound because it is present in many common beverages such as tea, coffee and soft drinks. It is also a frequently used pharmacological substance because it has an antagonist affect on the adenosine A1 and A2α receptors (21). However, caffeine has toxic effects at high doses (22), and chronic caffeine intake is a risk factor for cardiovascular disease because it causes an increase in blood pressure, heart rate and aortic stiffness (23). Sardao et al (22) have reported that caffeine produced an increase in mitochondria state 4 respiration and a decrease in state 3 respiration, in addition to Ca2+ accumulation. Okafor et al (24) demonstrated that the administration of caffeine influenced the alteration of myofilament Ca2+ responsiveness and contractile activation.
Figure 1).
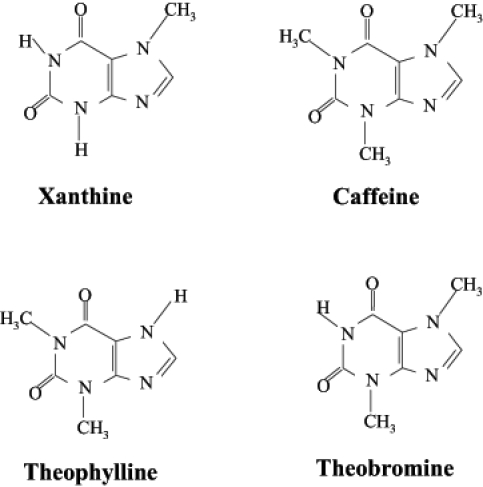
Chemical structure of xanthine and its derivatives
Theophylline, another antagonist of adenosine, has been reported to stabilize breathing in patients with brain damage (25). Other studies have demonstrated that theophylline has therapeutic effects with respect to arrhythmias and symptomatic bradycardia, which are secondary to atrioventricular nodal block and the sick sinus syndrome (26,27). Although some investigators (28) have indicated that theophylline lowers increased hemoglobin and hematocrit levels in the renal transplant recipient, Trivedi and Lal (29) reported that theophylline was ineffective in this condition. While the effects of theophylline on cardiovascular disease remain to be carefully examined, aminophylline has been shown to have beneficial effects on exercise-induced chest pain in humans due to vasodilation and inhibition of the myocardial steal phenomenon associated with transmural myocardial maldistribution of blood flow (30). In addition, Altun et al (31) have also indicated that aminophylline has a potential therapeutic effect on advanced atrioventricular block during acute inferior myocardial infarction. Because PTXF has fewer side effects and a larger therapeutic range than theobromine, most studies investigating treatments for cardiovascular diseases have focused on PTXF. In addition to PTXF, some PTXF analogues, including HWA-138 (albifylline), HWA-448 and A-802715, have been examined. HWA-138 has been considered a potential drug for treating cardiovascular disease because it has been shown to reduce cytokine production and inhibit coagulation disturbances (32). It has also been reported to protect the liver from shock-induced injury in rats by blocking leukocyte adhesion to the endothelium (32). Furthermore, HWA-138 was used to alleviate symptoms of endotoxin-induced acute lung injury in pigs (33). PTXF has been prescribed to increase blood flow to various organs such as the brain, skeletal muscle, kidney and lung. HWA-138 and HWA-448 have been demonstrated to impede the progression of renal damage associated with septic shock in rats (34) and were shown to induce prostocyclin synthesis in the endothelial cell (35). Another analogue, A-802715, has been reported to inhibit the production of TNF-α caused by lipopolysaccharides in serum (36). A-802715 was shown to be more potent than PTXF as an immunosupressant because it has been documented to suppress the cyclosporine-resistant signal-dependent pathway in T cell proliferation under both in vitro and in vivo conditions (37,38).
Thus, there are many analogues of PTXF which have been examined in both experimental and clinical settings. Some of these agents, such as A-802715, HWA-138 and HWA-448, have shown more potential therapeutic benefits than PTXF in treating cardiovascular disease.
Because PTXF has received much attention for its pharmacological effects on hemorheology and immune response, its metabolism has been examined extensively. This drug is clinically effective when administered either orally or intravenously. It is metabolized by erythrocytes and the liver, and is excreted by the kidney with a half-life of 3.4 h (3,39). Miller et al (40) have reported that the maximum plasma concentration of PTXF was achieved within 5 min following its injection. There are seven metabolites of PTXF (Figure 2); metabolite V is considered to be the major urinary metabolite of PTXF in humans (40). Honess et al (41) demonstrated no significant amount of metabolite I or PTXF detected in urine (3). Although the effects of these metabolites on cardiovascular disease have not been fully examined, some investigations of metabolites I and V indicate that these have hemorheological properties similar to those of PTXF (42). Moreover, metabolite I, known as lisofylline or BL149, was found to be the most active metabolite of PTXF for the treatment of intermittent claudication in humans (42). Additionally, lisofylline was found to depress TNF-α synthesis, decrease transforming growth factor-beta (TGF-β) activity and inhibit macrophage inflammatory protein 1-alpha production in mice (43). Hasegawa et al (44) reported that lisofylline treatment decreased pulmonary hypertension, hypoxemia and neutropenia due to sepsis. The mechanism of its effect on cardiovascular disease was suggested to involve antiphosphatidic acid signalling (42) due to inhibition of lysophosphatidic acid acyl transferase activity; the inhibition of this enzyme blocks the conversion of lysophosphatidic acid to phosphatidic acid (45). Although, lysofylline can be useful in the treatment of cardiovascular disease, PTXF is required clinically due to its diverse effects on hemorheology and inflammation with fewer side effects.
Figure 2).
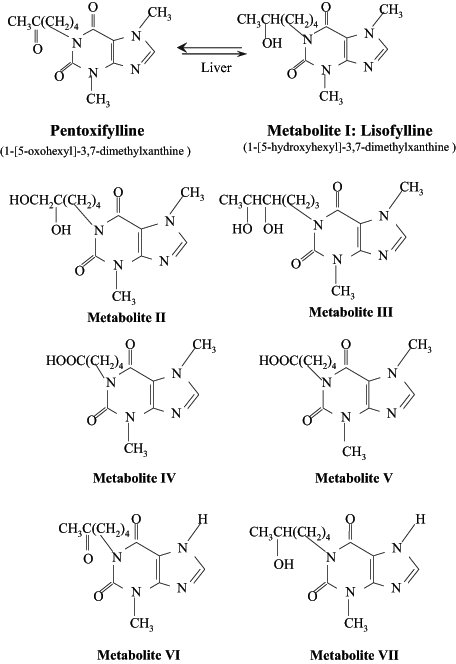
Pentoxifylline and its metabolites
EFFECT OF PTXF ON ISCHEMIC BRAIN
Stroke is a major global health concern because it is the third leading cause of death in North America (46–50) and is one of the primary factors that disable the elderly. Therefore, searching for an effective pharmacological intervention of ischemic cerebral disease has become important (46). Cerebral blood flow (CBF) is a major point of focus in treating stroke because it is necessary to maintain normal mental condition and consciousness. Reduced CBF is a significant symptom that leads to the death of brain cells as a consequence of cerebral ischemia. Other factors such as arteriosclerosis, thrombosis, and a number of vascular and hematolytic changes can also decrease CBF and, thus, may produce cerebral ischemia (47–50).
Deformation of RBCs is one example of a vascular event that results in marked abnormalities in patients with cerebral ischemia; this alteration in RBCs is both a consequence and a cause of this ischemia (50,51). There are three factors that determine the deformability of RBCs: the shape of the RBC, the viscoelastic properties of the membrane, and internal viscosity of the cell content (51). In addition, the flexibility of RBC membranes is determined by intracellular ATP and Ca2+ ion concentrations (50,52). An increase in plasma osmolarity and a lowered blood pH can also result in a rigid erythrocyte membrane (53–55). Because treatment of cell deformability with PTXF improves capillary perfusion and regional CBF (56), this reduces the damage that occurs due to cerebral ischemia. Thus, the increase in capillary perfusion and regional CBF are primarily due to the hemorheological properties of PTXF, which reduce blood viscosity, improve RBC flexibility and inhibit platelet aggregation (56,57).
Studies in rats also demonstrated that PTXF enhances the elasticity in RBCs by increasing the amount of ATP (3). Similar findings have been reported in humans (58). Bowton et al (59) found that this change in ATP level with oral administration of a single sustained release capsule of PTXF increased global and regional CBF in patients with cerebrovascular disease. Furthermore, studies have indicated that PTXF exerts beneficial effects in cerebrovascular disease by inhibiting brain edema, reducing disturbances of brain cell membrane permeability and removing mechanical obstacles in microcirculation (11,60,61). Thus, it can be seen that PTXF has a broad range of therapeutic effects in patients with cerebrovascular disorders. This drug has been used to treat transient ischemic attacks, cerebral thrombosis and hemorrhage, and chronic cerebrovascular insufficiency (3). As well, PTXF is useful in treating ischemic brain lesions, mainly by inhibiting membrane permeability of brain cells and preventing an increase in blood viscosity.
EFFECT OF PTXF ON ISCHEMIC HEART
Ischemic heart injury has become a major economic and health care concern. Almost 45% of all deaths in northern European countries in the past decade have been reported to result from this cardiovascular disease (62). A similar situation exists in Canada where more than 58% of cardiovascular deaths were attributed to ischemic cardiac lesions (63). There are a number of factors to consider before we can successfully treat ischemic injury in the heart. Cytokines, for example, are important mediators of cardiovascular diseases. A myocardial ischemic event prompts the release of cytokines and other inflammatory mediators that cause coronary vascular injury. The specific target of such mediators appears to be the endothelium and neutrophils. Inflammatory cytokines, including TNF-α and interleukin-1 (IL-1), act on neutrophils and adhere to the vascular endothelium. This induces the obstruction of capillary beds and causes the no-reflow phenomenon during reperfusion. Moreover, the accumulation of TNF-α and IL-1 within ischemic tissue directly injures the tissue and leads to the release of oxygen free radicals, which results in further damage to the endothelium (14). Other studies have demonstrated that TNF-α directly decreases contractile function in hamsters, dogs and humans (64,65). This acute negative inotropic effect of TNF-α is due to interference in Ca2+ homeostasis and, thus, TNF-α is considered to disrupt excitation-contraction coupling and desensitize the beta-adrenal receptors (66). The early contractile depression induced by TNF-α is mediated by sphingosine, an endogenous second messenger (67). In addition, TNF-α induces the production of nitric oxide and, thus, desensitizes myofilament sensitivity to Ca2+ leading to contractile dysfunction (68). Another mechanism of cardiac depression provoked by TNF-α is the induction of apoptosis in cardiomyocytes; this process appears to be mediated by sphingosine and nitric oxide (69–71). These studies indicate that anti-TNF-α therapy may be useful in ischemic injury.
Various pharmacodynamic investigations have demonstrated the beneficial effect of PTXF on ischemic myocardial and vascular disorders (15,72–77). In one study (3), 40 ischemic heart disease patients treated with PTXF 600 mg/day for 25 days to 30 days, showed a lowered level of glyceryl trinitrate consumption, greater ability to exercise and reduced tachycardia. Reduction in TNF-α production has been shown to be an important mechanism by which PTXF protects against ischemic injury; this has been shown to occur both in vitro and in vivo. PTXF decreases TNF-α synthesis via two mechanisms. First, one of its metabolites, lisofylline, inhibits the activity of lysophosphatidic acid acyl transferase that converts lysophosphatidic acid to phosphatidic acid (45). This induces a rise in Ca2+ concentration and a decrease in the synthesis of TNF-α (78). Second, PTXF acts as an inhibitor of PDE and induces prolonged cAMP levels resulting in the activation of protein kinase A, which blocks the nuclear factor kappa-B-induced TNF-α messenger RNA transcription (79). This indicates that PTXF, by inhibiting the PDE activity, blocks TNF-α gene transcription and protein synthesis (80) (Figure 3).
Figure 3).
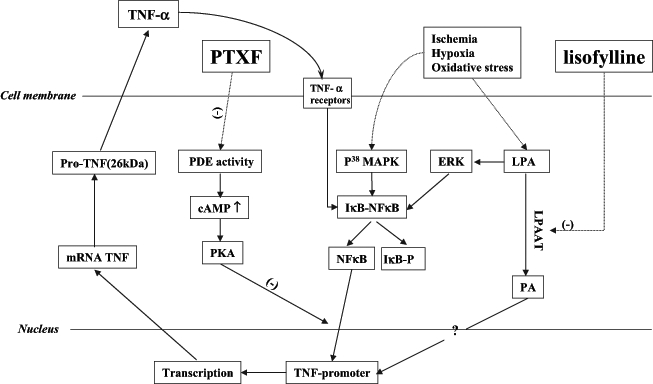
Tumour necrosis factor-alpha (TNF-α) synthesis induced by ischemia reperfusion and hypoxia in cardiomyocytes, and the mechanism of pentoxifylline’s (PTXF’s) anti-TNF-α effect. (−) Inhibit; ? Controversial; cAMP Cyclic AMP; ERK Extracellular signal-regulated kinases; IκB-NFκB Inhibit kappa B-nuclear factor kappa B; LPA Lysophosphatidic acid; LPAAT Lysophosphatidic acid acyl transferase; P38 MAPK P38 mitogen-activated protein kinase; PA Phosphatidic acid; PDE Phosphodiesterase; PKA Protein kinase A; TACE TNF-α converting enzyme
The primary pharmacodynamic effects of PTXF, such as increased RBC deformability and decreased blood viscosity. are also considered to be important mechanisms for protection of ischemic heart (3). Dauber et al (73) demonstrated that PTXF attenuates coronary microvascular protein leak and decreases endothelium-dependent relaxation in coronary epicardial arteries after ischemia and reperfusion. In addition, the increase in neutrophil cAMP induced by PTXF diminished superoxide anion production, adherence of neutrophils to vascular endothelium, and reduced the response of neutrophils to platelet-activating factor (PAF) and cytokines such as TNF-α and IL-1 (81–83). PTXF was also reported to decrease myeloperoxidase, an index of tissue leukocyte accumulation, and, thus, reduce leukocyte adhesion in ischemic myocardium (74,84). In addition, PTXF is an effective hydroxyl radical scavenger, preventing endothelial injury by reactive oxygen species (15). Studies from our laboratory have demonstrated that PTXF has a protective effect on both ischemic heart injury and Ca2+ paradox heart injury (85,86). Thus, PTXF, with its limited side effects and favorable hemorheological properties, may be considered to possess great potential for beneficial effects in ischemic heart disease.
EFFECT OF PTXF ON ISCHEMIC SKELETAL MUSCLE
Ischemia-reperfusion injury in skeletal muscle is a clinical disease that exhibits effects at the molecular and cellular levels (87). For instance, swelling of endothelial cells, leukocyte endothelial adhesion, modification of monocyte/neutrophil function, vascular thrombosis and even cell death have been identified in ischemic skeletal muscle (88–91). Finding a method to diminish the extent of endothelial injury and inhibit neutrophil adhesion in ischemic skeletal muscle are topics that have received considerable attention (20,88–90). It has been noted that neutrophils contain primary and secondary granules that consist of a variety of glycoproteins. Complement receptor-3 is one of the glycoproteins released by neutrophils that causes neutrophil adhesion when stimulated by a variety of cytokines (11). Because neutrophil adhesion plays a key role in ischemia-reperfusion injury, finding a way to block this adhesion is considered an important step in establishing a treatment for injuries to skeletal muscle (88,90).
Incidentally, it has been discovered that PTXF inhibits neutrophil adhesion by blocking the effects of complement receptor-3 up-modulation, preventing degranulation of myeloperoxidase and lysozyme, which are found in the granules of neutrophils, and modulating the cytoskeletal interactions at the adenosine A2 receptor (11,92). PTXF also prevents the adherence of neutrophils stimulated by TNF-α (10). Furthermore, it has been shown that PTXF interferes with the leukocyte-signalling pathway by activating phosphatidylinositol-3-kinase and phospholipase D via a number of different agonists, which eventually inhibits actin polymerization and superoxide anion production (93). Moreover, PAF in venous blood is subsequently decreased; this potent lipid mediator, which is produced by ischemic skeletal muscle during periods of reperfusion, would otherwise result in increased binding of neutrophils to endothelial cells (20,91,94). PTXF also blocks the response of granulocytes to PAF (20,95).
Administration of PTXF at a high dose was found to decrease the degree of skeletal muscle necrosis (91). Adams et al (20) reported that using PTXF (25 mg/kg) immediately before reperfusion extensively diminished the degree of muscle necrosis and PAF levels in the venous effluents of the isolated canine gracilis. As well, Hanazawa et al (96) reported that PTXF treatment prevented leukocyte adhesion after reperfusion in the rat cremaster muscle. The administration of PTXF has also been reported to decrease PAF levels and neutrophil adhesion in ischemic skeletal muscle (10,20,96). In summary, both in vitro and in vivo studies have revealed that the hemorheological and anti-inflammatory activities of PTXF were responsible for the therapeutic effects of PTXF. PTXF can immensely increase recovery in a variety of organs, including the brain, heart, intestines, testes and skeletal muscle, during ischemia-reperfusion injury (4,73,74,84,87,97–101). Thus, PTXF has great potential as a therapeutic intervention for helping patients recover from clinically common ischemia-reperfusion injuries.
EFFECT OF PTXF ON VASCULAR DISEASE
An effective treatment for peripheral arterial disease is necessary because this life-threatening condition affects eight to 10 million people in the United States (102). Furthermore, increased incidence of this disease is correlated with the development of arteriosclerosis and the hypercoagulable state (103–105). It is also well known that a decrease in peripheral blood flow and pathological hemorheological changes are involved in the development of peripheral arterial disease (56). The abnormal proliferation of vascular smooth muscle cells (VSMC) and the accumulation of extracellular matrix components, such as collagen and fibronectin, are major contributing factors for arteriosclerotic vascular disease (106). Moreover, TNF-α, which is an important cytokine that promotes leukocyte adhesion to a vessel wall due to an increase in cell adhesion receptors, is released by the endothelium (107,108). Considering all of these factors, treatment of peripheral arterial disease should focus on lowering blood coagulation that induces arteriosclerosis, affecting blood flow in the injured vessels (109).
As well, it has been uncovered that oral and/or intravenous administration of PTXF can decrease the level of fibrinogen by increasing fibrinolytic activity or reducing fibrinogen production in patients with peripheral vascular disease (3,110,111). It can also improve rest and exercise blood flow in patients with vascular diseases (3). Furthermore, the anti-TNF-α effects of PTXF may provide another way to treat the hypercoagulable state of circulatory failure that worsens arteriosclerosis (11,112).
It has been revealed by a number of studies that PTXF may impede the development of arteriosclerosis by inhibiting the production of the platelet-derived growth factor, which then prevents the proliferation of VSMC (103,113). This drug also reduces the amount of TGF-β produced, thereby, lowering the extent of collagen synthesis in VSMC (113). These antimitogenic and anticollagenic effects of PTXF are mainly associated with the cAMP-protein kinase A effector pathway, thus, decreasing the messenger RNA level of TGF-β1-stimulated collagen 24 h following PTXF administration (106). In addition, most in vitro and in vivo experiments show that PTXF induces vasodilation in both the skeletal muscle vascular bed and human forearm vascular bed (114–118). Consequently, perfusion in the microcirculatory vascular bed is improved (114–118). These PTXF-induced vasodilation effects may result from PDE activity inhibition, which causes an increase in cAMP levels. cAMP interacts with the adenosine receptor to induce the inhibition of adenosine uptake by blood cells and the endothelium (119–121). Although PTXF was proven to be therapeutically effective for peripheral arterial disease, it has been documented that PTXF does not have any greater beneficial effects over a placebo in treating the vasospastic peripheral vascular disorder known as Raynaud’s phenomenon (122). On the other hand, several investigations have suggested that the effectiveness of PTXF stems from its hemorheological property and other pharmacological actions, such as the reduction of blood viscosity, enhancement of fibrinolytic activity, depression of the production of TNF-α and vasodilation action (106,112,119). Thus, PTXF is considered to be of great potential for treating various circulatory disorders clinically (123).
EFFECT OF PTXF ON PLATELET FUNCTION
Platelets are fragments from giant bone marrow megakaryocytes, which are disk-shaped in structure. These fragments have a diameter close to one-third of an RBC’s width and they participate in various body functions including maintenance of vascular integrity, arterial thrombosis, activation of plasma coagulation and atherogenesis (52). A number of factors, such as the interactions between cAMP and Ca2+, as well as the formation of prostaglandin, are known to stimulate platelets (52). The cAMP content of platelets regulates the activation of cyclo-oxygenase, which converts arachidonic acid of platelet lipids to end peroxides, and prostaglandin G1 and H2; this subsequently leads to the production of thromboxane A2. As a result, the excessive thromboxane activity causes intravascular platelet aggregation. Prostacyclin, which is synthesized in vascular walls, activates adenylate cyclase to cause an increase in cAMP levels that inhibits prostaglandin-cyclo-oxygenase (52,124) (Figure 4). Thromboxane production is then reduced (5,125).
Figure 4).
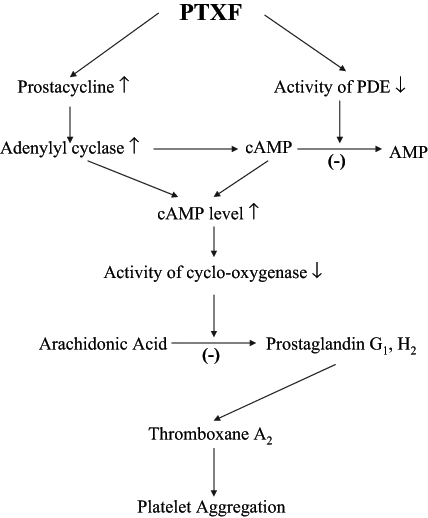
The mechanism of pentoxifylline (PTXF) on antiplatelet aggregation. ↓ Decrease; ↑ Increase; (−) Inhibit; cAMP Cyclic AMP; PDE Phosphodiesterase
Platelets release platelet factor 3 that can initiate the coagulation system and stimulate the activation of thrombin, via cleavage of prothrombin, to cause platelet aggregation and disintegration (52,124,125). Alteration of platelet functions impairs microcirculation and plays an important role in the development of cardiovascular diseases. Additionally, platelets are highly reactive due to their greater tendency to aggregate and release platelet factor 3 during a stage of cardiovascular dysfunction (125). There is an observed positive feedback phenomenon where platelet dysfunction induces cardiovascular diseases, which further worsens platelet function. Therefore, pharmacological improvement of platelet function is considered to be important for the treatment of all kinds of vascular diseases.
Various research groups have reported that PTXF produces a marked decrease in platelet adhesion and aggregation to the vessel wall in experimental animal models and patients with severe peripheral vascular disorder, cerebrovascular disorders or diabetes (3,105). It is believed that platelet aggregation is prevented through a variety of mechanisms. For instance, platelet membrane PDE activity that converts cAMP to AMP is inhibited by PTXF (11). PTXF also has the added benefit of decreasing platelet aggregation and thrombocytes by decreasing platelet pseudopodia formation, thus, depressing the release of platelet factor 3 (125,126). Following intravenous administration of PTXF, vasodilation is enhanced as serum prostacyclin levels become elevated (3,16,17,20). PTXF can also increase the level of cAMP via prostacyclin-activating adenylate cyclase (125) (Figure 4). Based on all of these findings, it was suggested that PTXF effectively prevents platelet aggregation and adhesion, which lends more credit to the notion that PTXF has a number of therapeutic effects for treating various vascular diseases.
EFFECT OF PTXF ON BLOOD VISCOSITY
Blood viscosity is another major contributing factor for the development of vascular disease. Greater blood viscosity induces vascular occlusion, which can occur in a wide variety of diseases such as heart disease, cerebrovascular disease, hypertension and diabetes (127–130). Furthermore, blood viscosity is a variable which changes along a vessel due to the combinatorial effects of many factors such as vascular geometry, flow separation and local blood composition (127). The flexibility of the RBC membrane, fibrinogen levels, shear stress and platelet aggregation are also close determinants of blood viscosity (105,131,132).
Rheology factors can differ greatly among various individuals and diseases. Such variations may influence oxygen supply in the blood (133). Therefore, counteracting an increase in blood viscosity may help treat many vascular diseases (129). A number of methods to decrease blood viscosity have been recognized. A prospective study (134), for instance, demonstrated that fibrinolytic therapy reduces fibrinogen in blood plasma and, in turn, reduces blood viscosity, which results in increased blood flow. Increasing RBC deformability is also another important way of improving blood flow (135,136). According to some studies, it has been found that PTXF decreases blood viscosity by increasing RBC flexibility, decreasing erythrocyte aggregation and stimulating fibrinolysis to reduce plasma fibrinogen concentration (46,137,138). Moreover, Schneider et al (139) reported that therapy with PTXF could decrease shear stress. Consequently, the flow properties of blood and microcirculation can be enhanced with PTXF treatment (128).
SUMMARY AND CONCLUDING REMARKS
Cardiovascular diseases are life-threatening conditions and, thus, have received a great deal of attention over the years. So, it is not surprising that various treatments to cure these diseases, by the use of drugs such as heparin and acetylsalicylic acid, have been approved (140–142). PTXF, however, is another drug now being used to treat various vascular diseases because this methylxanthine derivative has less severe side effects, as well as many potent hemorheological properties, which make it an effective drug for combating vascular disorders (3,57). PTXF may be used to treat ischemic heart disease because it can improve RBC deformability, decrease RBC aggregation (3), increase blood flow to the heart (59), and inhibit neutrophil adhesion and the production of some cytokines, such as TNF-α and IL-1 (14). This drug is also capable of decreasing PAF levels, reducing the effect of PAF during ischemia-reperfusion injury (20), depressing the proliferation of the VSMC (106), inhibiting platelet aggregation and improving blood flow (59). All these properties of PTXF are interrelated and originate from the inhibition of cAMP PDE (19,81,93). The mechanisms of PTXF action are shown in Figure 5. In addition, many studies have shown that PTXF can be therapeutically beneficial in treating liver fibrosis and cirrhosis due to its antifibrogenic action (143,144). Based on the cumulative action of all of these effects, PTXF has been recognized as an effective therapeutic strategy for treating various cardiovascular diseases. However, its mechanism for alleviating cardiovascular dysfunction and the optimum dosage for therapy have not been clearly identified. Hence, a great deal of research and appropriate clinical trials still need to be conducted.
Figure 5).
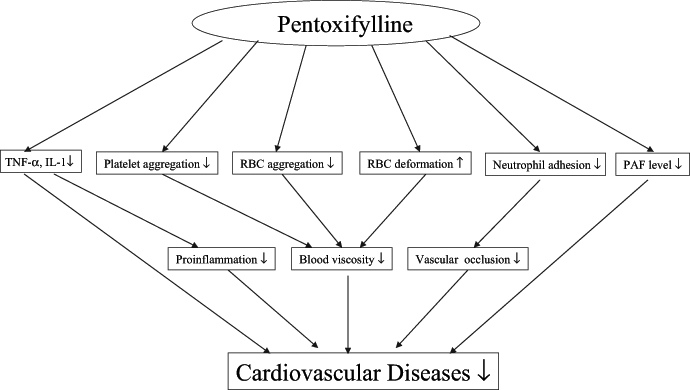
The role of pentoxifylline in cardiovascular disease. ↓ Decrease; ↑ Increase; IL-1 Interleukin-1; PAF Platelet-activating factor; RBC Red blood cell; TNF-α Tumour necrosis factor-alpha
Acknowledgments
This work has been supported by the Canadian Institute of Health Research (CIHR) Group in Experimental Cardiology. NSD holds the CIHR Pharmaceutical Research and Development Chair in Cardiovascular Research supported by Merck Frosst Canada. SM was a visiting scientist from CU Shah College of Pharmacy, Mumbai, India.
REFERENCES
- 1.Rall TW. Drug used in the treatment of asthma. In: Limbird LE, Milinoff PB, Ruddon RW, Gilman AG, Hardman JG, editors. The Pharmacological Basis of Therapeutics. New York: Pergramon Press; 1990. pp. 618–38. [Google Scholar]
- 2.Wesley GC, Brater DC, Jonson AR. Central nervous system stimulants. In: Kist K, editor. Goth’s Medical Pharmacology. St Louis: Mosby Year Book; 1992. pp. 300–7. [Google Scholar]
- 3.Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Eun BL, Liu XH, Barks JD. Pentoxifylline attenuates hypoxic-ischemic brain injury in immature rats. Pediatr Res. 2000;47:73–8. doi: 10.1203/00006450-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Muller R. Hemorheology and peripheral vascular diseases: A new therapeutic approach. J Med. 1981;12:209–35. [PubMed] [Google Scholar]
- 6.Betticher DC, Keller H, Maly FE, Reinhart WH. The effect of endotoxin and tumour necrosis factor on erythrocyte and leucocyte deformability in vitro. Br J Haematol. 1993;83:130–7. doi: 10.1111/j.1365-2141.1993.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 7.Fossat C, Fabre D, Alimi Y, et al. Leukocyte activation study during occlusive arterial disease of the lower limb: Effect of pentoxifylline infusion. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S96–100. doi: 10.1097/00005344-199500252-00021. [DOI] [PubMed] [Google Scholar]
- 8.Klinzing S, Lesser T, Schubert H, Bartel M, Klein U. May pentoxifylline improve lung function after one-lung flooding? Res Exp Med (Berl) 2001;200:69–76. doi: 10.1007/BF03220016. [DOI] [PubMed] [Google Scholar]
- 9.Salyer JL, Bohnsack JF, Knape WA, Shigeoka AO, Ashwood ER, Hill HR. Mechanisms of tumor necrosis factor-alpha alteration of PMN adhesion and migration. Am J Pathol. 1990;136:831–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Samlaska CP, James WD. Superficial thrombophlebitis. I. Primary hypercoagulable states. J Am Acad Dermatol. 1990;22:975–89. doi: 10.1016/0190-9622(90)70139-9. [DOI] [PubMed] [Google Scholar]
- 11.Samlaska CP, Winfield EA. Pentoxifylline. J Am Acad Dermatol. 1994;30:603–21. doi: 10.1016/s0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 12.Schmalzer EA, Chien S. Filterability of subpopulations of leukocytes: Effect of pentoxifylline. Blood. 1984;64:542–6. [PubMed] [Google Scholar]
- 13.Weiss DJ, Evanson OA. Evaluation of lipopolysaccharide-induced activation of equine neutrophils. Am J Vet Res. 2002;63:811–5. doi: 10.2460/ajvr.2002.63.811. [DOI] [PubMed] [Google Scholar]
- 14.Dhote-Burger P, Vuilleminot A, Lecompte T, et al. Neutrophil degranulation related to the reperfusion of ischemic human heart during cardiopulmonary bypass. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S124–9. doi: 10.1097/00005344-199500252-00026. [DOI] [PubMed] [Google Scholar]
- 15.Horton JW, White DJ. Free radical scavengers prevent intestinal ischemia-reperfusion-mediated intestinal dysfunction. J Surg Res. 1993;55:282–9. doi: 10.1006/jsre.1993.1141. [DOI] [PubMed] [Google Scholar]
- 16.Myers SI, Horton JW, Hernandez R, Walker PB, Vaughan WG. Pentoxifylline protects splanchnic prostacyclin synthesis during mesenteric ischemia/reperfusion. Prostaglandins. 1994;47:137–50. doi: 10.1016/0090-6980(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 17.Schermuly RT, Roehl A, Weissmann N, et al. Combination of nonspecific PDE inhibitors with inhaled prostacyclin in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1361–8. doi: 10.1152/ajplung.2001.281.6.L1361. [DOI] [PubMed] [Google Scholar]
- 18.Manrique RV, Manrique V. Platelet resistance to prostacyclin. Enhancement of the antiaggregatory effect of prostacyclin by pentoxifylline. Angiology. 1987;38:101–8. doi: 10.1177/000331978703800202. [DOI] [PubMed] [Google Scholar]
- 19.Strieter RM, Remick DG, Ward PA, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155:1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 20.Adams JG, Jr, Dhar A, Shukla SD, Silver D. Effect of pentoxifylline on tissue injury and platelet-activating factor production during ischemia-reperfusion injury. J Vasc Surg. 1995;21:742–8. doi: 10.1016/s0741-5214(05)80005-9. [DOI] [PubMed] [Google Scholar]
- 21.White PJ, Nguyen TT. Chronic caffeine treatment causes changes in cardiac adenosine receptor function in rats. Pharmacology. 2002;65:129–35. doi: 10.1159/000058038. [DOI] [PubMed] [Google Scholar]
- 22.Sardao VA, Oliveira PJ, Moreno AJ. Caffeine enhances the calcium-dependent cardiac mitochondrial permeability transition: Relevance for caffeine toxicity. Toxicol Appl Pharmacol. 2002;179:50–6. doi: 10.1006/taap.2001.9334. [DOI] [PubMed] [Google Scholar]
- 23.Vlachopoulos C, Hirata K, Stefanadis C, Toutouzas P, O’Rourke MF. Caffeine increases aortic stiffness in hypertensive patients. Am J Hypertens. 2003;16:63–6. doi: 10.1016/s0895-7061(02)03155-2. [DOI] [PubMed] [Google Scholar]
- 24.Okafor CC, Saunders L, Li X, et al. Myofibrillar responsiveness to cAMP, PKA, and caffeine in an animal model of heart failure. Biochem Biophys Res Commun. 2003;300:592–9. doi: 10.1016/s0006-291x(02)02885-1. [DOI] [PubMed] [Google Scholar]
- 25.Mitrouska I, Kondili E, Prinianakis G, Siafakas N, Georgopoulos D. Effects of theophylline on ventilatory poststimulus potentiation in patients with brain damage. Am J Respir Crit Care Med. 2003;167:1124–30. doi: 10.1164/rccm.200206-552OC. [DOI] [PubMed] [Google Scholar]
- 26.Cawley MJ, Al-Jazairi AS, Stone EA. Intravenous theophylline – an alternative to temporary pacing in the management of bradycardia secondary to AV nodal block. Ann Pharmacother. 2001;35:303–7. doi: 10.1345/aph.10106. [DOI] [PubMed] [Google Scholar]
- 27.Dixon WC, Bauch TD. Effects of theophylline on exercise indices in a patient with chronotropic incompetence. Clin Cardiol. 2000;23:787–9. doi: 10.1002/clc.4960231019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang S, Sherwood RA, Gamsu HR, Marsden JT, Peters TJ, Greenough A. Comparison of the effects of theophylline and caffeine on serum erythropoietin concentration in premature infants. Eur J Pediatr. 1998;157:406–9. doi: 10.1007/s004310050840. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi H, Lal SM. A prospective, randomized, open labeled crossover trial of fosinopril and theophylline in post renal transplant erythrocytosis. Ren Fail. 2003;25:77–86. doi: 10.1081/jdi-120017470. [DOI] [PubMed] [Google Scholar]
- 30.Yesildag O, Yazici M, Yilmaz O, Ucar R, Sagkan O. The effect of aminophylline infusion on the exercise capacity in patients with syndrome X. Acta Cardiol. 1999;54:335–7. [PubMed] [Google Scholar]
- 31.Altun A, Kirdar C, Ozbay G. Effect of aminophylline in patients with atropine-resistant late advanced atrioventricular block during acute inferior myocardial infarction. Clin Cardiol. 1998;21:759–62. doi: 10.1002/clc.4960211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzi I, Maier M, Herzog C, Bauer M. Influence of pentoxifylline and albifylline on liver microcirculation and leukocyte adhesion after hemorrhagic shock in the rat. J Trauma. 1996;40:90–6. doi: 10.1097/00005373-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann H, Weis M, Frank G, Birg A, Schonharting MM, Jochum M. Amelioration of endotoxin-induced acute lung injury in pigs by HWA 138 and A 80 2715: New analogs of pentoxifylline. Shock. 1995;4:166–70. doi: 10.1097/00024382-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Berens KL, Langston JD, Wasan KM, Luke DR. Influence of pentoxifylline and related analogues in endotoxemic renal failure. Circ Shock. 1991;34:344–8. [PubMed] [Google Scholar]
- 35.Schade UF. The role of prostacyclin in the protective effects of pentoxifylline and other xanthine derivatives in endotoxin action in mice. Eicosanoids. 1989;2:183–8. [PubMed] [Google Scholar]
- 36.Niehorster M, Schonharting M, Wendel A. A novel xanthine derivative counteracting in vivo tumor necrosis factor alpha toxicity in mice. Circ Shock. 1992;37:270–3. [PubMed] [Google Scholar]
- 37.Lin Y, Goebels J, Rutgeerts O, et al. Use of the methylxanthine derivative A802715 in transplantation immunology: I. Strong in vitro inhibitory effects on CD28-costimulated T cell activities. Transplantation. 1997;63:1813–8. doi: 10.1097/00007890-199706270-00019. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y, Segers C, Mikhalsky D, Tjandra-Maga TB, Schonharting M, Waer M. Use of the methylxanthine derivative A802715 in transplantation immunology: II. In vivo experiments. Transplantation. 1997;63:1734–8. doi: 10.1097/00007890-199706270-00005. [DOI] [PubMed] [Google Scholar]
- 39.Ambrus JL, Stadler S, Kulaylat M. Hemorrheologic effects of metabolites of pentoxifylline (Trental) J Med. 1995;26:65–75. [PubMed] [Google Scholar]
- 40.Miller K, Louie A, Baltch AL, Smith RP, Davis PJ, Gordon MA. Pharmacokinetics of pentoxifylline and its metabolites in healthy mice and in mice infected with Candida albicans. Antimicrob Agents Chemother. 1998;42:2405–9. doi: 10.1128/aac.42.9.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honess DJ, Dennis IF, Bleehen NM. Pentoxifylline: Its pharmacokinetics and ability to improve tumour perfusion and radiosensitivity in mice. Radiother Oncol. 1993;28:208–18. doi: 10.1016/0167-8140(93)90060-l. [DOI] [PubMed] [Google Scholar]
- 42.Lillibridge JA, Kalhorn TF, Slattery JT. Metabolism of lisofylline and pentoxifylline in human liver microsomes and cytosol. Drug Metab Dispos. 1996;24:1174–9. [PubMed] [Google Scholar]
- 43.Clarke E, Rice GC, Weeks RS, et al. Lisofylline inhibits transforming growth factor beta release and enhances trilineage hematopoietic recovery after 5-fluorouracil treatment in mice. Cancer Res. 1996;56:105–12. [PubMed] [Google Scholar]
- 44.Hasegawa N, Oka Y, Nakayama M, et al. The effects of post-treatment with lisofylline, a phosphatidic acid generation inhibitor, on sepsis-induced acute lung injury in pigs. Am J Respir Crit Care Med. 1997;155:928–36. doi: 10.1164/ajrccm.155.3.9117028. [DOI] [PubMed] [Google Scholar]
- 45.Bursten SL. Interaction of lipopolysaccharide with a mammalian lyso-phosphatidate acyltransferase (LPAAT) transfected into E. coli, and effect of lisofylline on LPAAT transfected into mammalian cells. Prog Clin Biol Res. 1998;397:345–56. [PubMed] [Google Scholar]
- 46.Rogers SJ, Sherman DG. Pathophysiology and treatment of acute ischemic stroke. Clin Pharm. 1993;12:359–76. [PubMed] [Google Scholar]
- 47.Kowal P. Hemorheology in cerebral ischemia. Neurol Neurochir Pol. 1996;30(Suppl 2):7–11. [PubMed] [Google Scholar]
- 48.Meyer JS. Regulation of cerebral hemodynamics in health and disease. Eur Neurol. 1983;22(Suppl 1):47–60. doi: 10.1159/000115611. [DOI] [PubMed] [Google Scholar]
- 49.Meyer JS, Okayasu H, Tachibana H, Okabe T. Stable xenon CT CBF measurements in prevalent cerebrovascular disorders (stroke) Stroke. 1984;15:80–90. doi: 10.1161/01.str.15.1.80. [DOI] [PubMed] [Google Scholar]
- 50.Sipos C, Popoviciu L, Motoc R, Marian R. Relationships between the degree and topography of the atherosclerotic lesions of the extracranial carotid axis with the clinical form of ischaemic attack, evaluated by duplex methodology. Rom J Neurol Psychiatry. 1990;28:157–62. [PubMed] [Google Scholar]
- 51.Dormandy JA. Red cell deformability. Eur Neurol. 1983;22(Suppl 1):23–9. doi: 10.1159/000115607. [DOI] [PubMed] [Google Scholar]
- 52.De Clerck F, David JL. Pharmacological control of platelet and red blood cell function in the microcirculation. J Cardiovasc Pharmacol. 1981;3:1388–412. doi: 10.1097/00005344-198111000-00026. [DOI] [PubMed] [Google Scholar]
- 53.Bolton LM, Thomas TH, Dunlop W. Erythrocyte ion and water balance and membrane potential in the puerperium of normal pregnancy. Br J Obstet Gynaecol. 1996;103:547–51. doi: 10.1111/j.1471-0528.1996.tb09804.x. [DOI] [PubMed] [Google Scholar]
- 54.Cabantchik ZI. Erythrocyte membrane transport. Novartis Found Symp. 1999;226:6–16. doi: 10.1002/9780470515730.ch2. [DOI] [PubMed] [Google Scholar]
- 55.Weng X, Cloutier G, Beaulieu R, Roederer GO. Influence of acute-phase proteins on erythrocyte aggregation. Am J Physiol. 1996;271:H2346–52. doi: 10.1152/ajpheart.1996.271.6.H2346. [DOI] [PubMed] [Google Scholar]
- 56.Marcel GA, George C. Pentoxifylline and cerebrovascular diseases. Eur Neurol. 1983;22(Suppl 1):89–97. doi: 10.1159/000115656. [DOI] [PubMed] [Google Scholar]
- 57.Frampton JE, Brogden RN. Pentoxifylline (oxpentifylline). A review of its therapeutic efficacy in the management of peripheral vascular and cerebrovascular disorders. Drugs Aging. 1995;7:480–503. doi: 10.2165/00002512-199507060-00007. [DOI] [PubMed] [Google Scholar]
- 58.Schubotz R, Muhlfellner O. The effect of pentoxifylline on erythrocyte deformability and on phosphatide fatty acid distribution in the erythrocyte membrane. Curr Med Res Opin. 1977;4:609–17. doi: 10.1185/03007997709115279. [DOI] [PubMed] [Google Scholar]
- 59.Bowton DL, Stump DA, Prough DS, Toole JF, Lefkowitz DS, Coker L. Pentoxifylline increases cerebral blood flow in patients with cerebrovascular disease. Stroke. 1989;20:1662–6. doi: 10.1161/01.str.20.12.1662. [DOI] [PubMed] [Google Scholar]
- 60.Ganser V, Boksay I. Effect of pentoxifylline on cerebral edema in cats. Neurology. 1974;24:487–93. doi: 10.1212/wnl.24.5.487. [DOI] [PubMed] [Google Scholar]
- 61.Muller R, Schroer R. Cerebrovascular circulatory disorders: New aspects of pathophysiology and therapy. J Med. 1979;10:347–64. [PubMed] [Google Scholar]
- 62.Ytrehus K. The ischemic heart – experimental models. Pharmacol Res. 2000;42:193–203. doi: 10.1006/phrs.2000.0669. [DOI] [PubMed] [Google Scholar]
- 63.Canadian Task Force for Cardiovascular Science A Joint Initiative of the Heart and Stroke Foundation of Canada and the Canadian Cardiovascular Society. Can J Cardiol. 1993;9:699–735. [PubMed] [Google Scholar]
- 64.Cain BS, Meldrum DR, Dinarello CA, Meng X, Banerjee A, Harken AH. Adenosine reduces cardiac TNF-alpha production and human myocardial injury following ischemia-reperfusion. J Surg Res. 1998;76:117–23. doi: 10.1006/jsre.1998.5304. [DOI] [PubMed] [Google Scholar]
- 65.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 66.Meldrum DR, Dinarello CA, Shames BD, et al. Ischemic preconditioning decreases postischemic myocardial tumor necrosis factor-alpha production. Potential ultimate effector mechanism of preconditioning. Circulation. 1998;98:II214–8. [PubMed] [Google Scholar]
- 67.Mathias S, Dressler KA, Kolesnick RN. Characterization of a ceramide-activated protein kinase: Stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1991;88:10009–13. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldhaber JI, Kim KH, Natterson PD, Lawrence T, Yang P, Weiss JN. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am J Physiol. 1996;271:H1449–55. doi: 10.1152/ajpheart.1996.271.4.H1449. [DOI] [PubMed] [Google Scholar]
- 69.Bergman MR, Holycross BJ. Pharmacological modulation of myocardial tumor necrosis factor alpha production by phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1996;279:247–54. [PubMed] [Google Scholar]
- 70.Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–65. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandenabeele P, Declercq W, Vanhaesebroeck B, Grooten J, Fiers W. Both TNF receptors are required for TNF-mediated induction of apoptosis in PC60 cells. J Immunol. 1995;154:2904–13. [PubMed] [Google Scholar]
- 72.Barnett JC, Touchon RC. Therapy of ischemic cardiomyopathy with pentoxifylline. Angiology. 1990;41:1048–52. doi: 10.1177/000331979004101204. [DOI] [PubMed] [Google Scholar]
- 73.Dauber IM, Lesnefsky EJ, Ashmore RC, et al. Coronary vascular injury due to ischemia-reperfusion is reduced by pentoxifylline. J Pharmacol Exp Ther. 1992;260:1250–6. [PubMed] [Google Scholar]
- 74.Gale SC, Hokama JY, Ritter LS, Gorman GD, Copeland JG, McDonagh PF. Pentoxifylline reduces coronary leukocyte accumulation early in reperfusion after cold ischemia. Ann Thorac Surg. 2001;71:1305–11. doi: 10.1016/s0003-4975(00)02655-2. [DOI] [PubMed] [Google Scholar]
- 75.Insel J, Halle AA, Mirvis DM. Efficacy of pentoxifylline in patients with stable angina pectoris. Angiology. 1988;39:514–9. doi: 10.1177/000331978803900604. [DOI] [PubMed] [Google Scholar]
- 76.Lechleitner P, Genser N, Mair J, et al. Pentoxifylline influences acute-phase response in acute myocardial infarction. Clin Investig. 1992;70:755. doi: 10.1007/BF00180743. [DOI] [PubMed] [Google Scholar]
- 77.Ulus AT, Aksoyek A, Katircioglu SF, Gokce P, Koc B. Preservation of myocardial functions by pentoxyphylline cardioplegia during and after cardiopulmonary bypass. Panminerva Med. 2000;42:253–6. [PubMed] [Google Scholar]
- 78.Abraham E, Bursten S, Shenkar R, et al. Phosphatidic acid signaling mediates lung cytokine expression and lung inflammatory injury after hemorrhage in mice. J Exp Med. 1995;181:569–75. doi: 10.1084/jem.181.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Semmler J, Gebert U, Eisenhut T, et al. Xanthine derivatives: Comparison between suppression of tumour necrosis factor-alpha production and inhibition of cAMP phosphodiesterase activity. Immunology. 1993;78:520–5. [PMC free article] [PubMed] [Google Scholar]
- 80.Molnar-Kimber K, Yonno L, Heaslip R, Weichman B. Modulation of TNF alpha and IL-1 beta from endotoxin-stimulated monocytes by selective PDE isozyme inhibitors. Agents Actions. 1993;39:C77–9. doi: 10.1007/BF01972726. [DOI] [PubMed] [Google Scholar]
- 81.Bessler H, Gilgal R, Djaldetti M, Zahavi I. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J Leukoc Biol. 1986;40:747–54. doi: 10.1002/jlb.40.6.747. [DOI] [PubMed] [Google Scholar]
- 82.Hou X, Baudry N, Lenoble M, Vicaut E. Leukocyte adherence in an ischemic muscle perfused by a collateral circulation. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S119–23. doi: 10.1097/00005344-199500252-00025. [DOI] [PubMed] [Google Scholar]
- 83.Semmler J, Wachtel H, Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int J Immunopharmacol. 1993;15:409–13. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- 84.Sener G, Akgun U, Satiroglu H, Topaloglu U, Keyer-Uysal M. The effect of pentoxifylline on intestinal ischemia/reperfusion injury. Fundam Clin Pharmacol. 2001;15:19–22. doi: 10.1046/j.1472-8206.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang M, Sethi R, Xu YJ, Dhalla NS. Increased production of TNF-alpha in hearts subjected to Ca2+-Paradox. J Mol Cell Cardiol. 2003;35:A30. (Abst) [Google Scholar]
- 86.Zhang M, Xu YJ, Rathi SS, Dhalla NS. Effect of pentoxifylline on ischemia reperfusion-induced heart injury in rats. J Mol Cell Cardiol. 2002;34:A24. (Abst) [Google Scholar]
- 87.Kishi M, Tanaka H, Seiyama A, et al. Pentoxifylline attenuates reperfusion injury in skeletal muscle after partial ischemia. Am J Physiol. 1998;274:H1435–42. doi: 10.1152/ajpheart.1998.274.5.H1435. [DOI] [PubMed] [Google Scholar]
- 88.Bottiger BW, Motsch J, Braun V, Martin E, Kirschfink M. Marked activation of complement and leukocytes and an increase in the concentrations of soluble endothelial adhesion molecules during cardiopulmonary resuscitation and early reperfusion after cardiac arrest in humans. Crit Care Med. 2002;30:2473–80. doi: 10.1097/00003246-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998;179:169–87. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- 90.Kyriakides C, Austen W, Wang Y, et al. Skeletal muscle reperfusion injury is mediated by neutrophils and the complement membrane attack complex. Am J Physiol. 1999;277:C1263–8. doi: 10.1152/ajpcell.1999.277.6.C1263. [DOI] [PubMed] [Google Scholar]
- 91.Silver D, Dhar A, Slocum M, Adams JG, Shukla S. Role of platelet-activating factor in skeletal muscle ischemia-reperfusion injury. Adv Exp Med Biol. 1996;416:217–21. doi: 10.1007/978-1-4899-0179-8_35. [DOI] [PubMed] [Google Scholar]
- 92.Currie MS, Rao KM, Padmanabhan J, Jones A, Crawford J, Cohen HJ. Stimulus-specific effects of pentoxifylline on neutrophil CR3 expression, degranulation, and superoxide production. J Leukoc Biol. 1990;47:244–50. doi: 10.1002/jlb.47.3.244. [DOI] [PubMed] [Google Scholar]
- 93.McCarty MF. Nitric oxide deficiency, leukocyte activation, and resultant ischemia are crucial to the pathogenesis of diabetic retinopathy/neuropathy – preventive potential of antioxidants, essential fatty acids, chromium, ginkgolides, and pentoxifylline. Med Hypotheses. 1998;50:435–49. doi: 10.1016/s0306-9877(98)90217-1. [DOI] [PubMed] [Google Scholar]
- 94.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: A juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–34. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hammerschmidt DE, Kotasek D, McCarthy T, Huh PW, Freyburger G, Vercellotti GM. Pentoxifylline inhibits granulocyte and platelet function, including granulocyte priming by platelet activating factor. J Lab Clin Med. 1988;112:254–63. [PubMed] [Google Scholar]
- 96.Hanazawa S, Prewitt RL, Terzis JK. The effect of pentoxifylline on ischemia and reperfusion injury in the rat cremaster muscle. J Reconstr Microsurg. 1994;10:21–6. doi: 10.1055/s-2007-1006567. [DOI] [PubMed] [Google Scholar]
- 97.Bahrami S, Yao YM, Shiga H, Leichtfried G, Redl H, Schlag G. Comparison of the efficacy of pentoxifylline and albifyllin (HWA 138) on endotoxin-induced cytokine production, coagulation disturbances, and mortality. Shock. 1996;5:424–8. doi: 10.1097/00024382-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 98.Chapelier A, Reignier J, Mazmanian M, et al. Pentoxifylline and lung ischemia-reperfusion injury: Application to lung transplantation. Universite Paris-Sud Lung Transplant Group. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S130–3. [PubMed] [Google Scholar]
- 99.Savas C, Aras T, Cakmak M, et al. Pentoxifylline inhibits overflow and reduces intestinal reperfusion injury. J Pediatr Surg. 1997;32:905–10. doi: 10.1016/s0022-3468(97)90648-5. [DOI] [PubMed] [Google Scholar]
- 100.Savas C, Dindar H, Aras T, Yucesan S. Pentoxifylline improves blood flow to both testes in testicular torsion. Int Urol Nephrol. 2002;33:81–5. doi: 10.1023/a:1014469323448. [DOI] [PubMed] [Google Scholar]
- 101.Savas C, Dindar H, Bilgehan A, Ataoglu O, Yucesan S. Pentoxifylline attenuates reperfusion injury in testicular torsion. Scand J Urol Nephrol. 2002;36:65–70. doi: 10.1080/003655902317259391. [DOI] [PubMed] [Google Scholar]
- 102.Creager MA. Medical management of peripheral arterial disease. Cardiol Rev. 2001;9:238–45. doi: 10.1097/00045415-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Capron L. Pharmacologic approaches to the treatment of atherosclerotic arterial obstruction. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S40–3. doi: 10.1097/00005344-199500252-00009. [DOI] [PubMed] [Google Scholar]
- 104.Dormandy JA, Hoare E, Colley J, Arrowsmith DE, Dormandy TL. Clinical, haemodynamic, rheological, and biochemical findings in 126 patients with intermittent claudication. Br Med J. 1973;4:576–81. doi: 10.1136/bmj.4.5892.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ott E, Lechner H, Fazekas F. Hemorheological effects of pentoxifylline on disturbed flow behavior of blood in patients with cerebrovascular insufficiency. Eur Neurol. 1983;22(Suppl 1):105–7. doi: 10.1159/000115658. [DOI] [PubMed] [Google Scholar]
- 106.Chen YM, Wu KD, Tsai TJ, Hsieh BS. Pentoxifylline inhibits PDGF-induced proliferation of and TGF-beta-stimulated collagen synthesis by vascular smooth muscle cells. J Mol Cell Cardiol. 1999;31:773–83. doi: 10.1006/jmcc.1998.0910. [DOI] [PubMed] [Google Scholar]
- 107.Dosquet C, Weill D, Wautier JL. Cytokines and thrombosis. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S13–9. doi: 10.1097/00005344-199500252-00004. [DOI] [PubMed] [Google Scholar]
- 108.Wakefield PE, James WD, Samlaska CP, Meltzer MS. Tumor necrosis factor. J Am Acad Dermatol. 1991;24:675–85. doi: 10.1016/0190-9622(91)70102-8. [DOI] [PubMed] [Google Scholar]
- 109.McNamara DB, Champion HC, Kadowitz PJ. Pharmacologic management of peripheral vascular disease. Surg Clin North Am. 1998;78:447–64. doi: 10.1016/s0039-6109(05)70325-x. [DOI] [PubMed] [Google Scholar]
- 110.Muller R. Pentoxifylline – a biomedical profile. J Med. 1979;10:307–29. [PubMed] [Google Scholar]
- 111.Strano A, Davi G, Avellone G, Novo S, Pinto A. Double-blind, crossover study of the clinical efficacy and the hemorheological effects of pentoxifylline in patients with occlusive arterial disease of the lower limbs. Angiology. 1984;35:459–66. doi: 10.1177/000331978403500709. [DOI] [PubMed] [Google Scholar]
- 112.Wu CC, Liao MH, Chen SJ, Yen MH. Pentoxifylline improves circulatory failure and survival in murine models of endotoxaemia. Eur J Pharmacol. 1999;373:41–9. doi: 10.1016/s0014-2999(99)00265-4. [DOI] [PubMed] [Google Scholar]
- 113.Haustein KO. State of the art – treatment of peripheral occlusive arterial disease (POAD) with drugs vs. vascular reconstruction or amputation. Int J Clin Pharmacol Ther. 1997;35:266–74. [PubMed] [Google Scholar]
- 114.Dinn RF, Yang HT, Terjung RL. The influence of pentoxifylline and torbafylline on muscle blood flow in animals with peripheral arterial insufficiency. J Clin Pharmacol. 1990;30:704–10. doi: 10.1002/j.1552-4604.1990.tb03630.x. [DOI] [PubMed] [Google Scholar]
- 115.Hoeffner U, Aarhus LL, Katusic ZS, Vanhoutte PM. Pharmacology of pentoxifylline in isolated canine arteries and veins. J Cardiovasc Pharmacol. 1989;14:899–907. doi: 10.1097/00005344-198912000-00017. [DOI] [PubMed] [Google Scholar]
- 116.Hudlicka O, Price S. Effects of torbafylline, pentoxifylline and buflomedil on vascularisation and fibre type of rat skeletal muscles subjected to limited blood supply. Br J Pharmacol. 1990;99:786–90. doi: 10.1111/j.1476-5381.1990.tb13007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kamphuis J, Smits P, Thien T. Vascular effects of pentoxifylline in humans. J Cardiovasc Pharmacol. 1994;24:648–54. doi: 10.1097/00005344-199410000-00016. [DOI] [PubMed] [Google Scholar]
- 118.Seiffge D. Pentoxifylline: its influence on the interaction of blood cells with the vessel wall. Atherosclerosis. 1997;131(Suppl):S27–8. doi: 10.1016/s0021-9150(97)06121-2. [DOI] [PubMed] [Google Scholar]
- 119.Blayney L, Thomas H, Muir J, Henderson A. Action of caffeine on calcium transport by isolated fractions of myofibrils, mitochondria, and sarcoplasmic reticulum from rabbit heart. Circ Res. 1978;43:520–6. doi: 10.1161/01.res.43.4.520. [DOI] [PubMed] [Google Scholar]
- 120.Fredholm BB, Lindstrom K. The xanthine derivative 1-(5’-oxohexyl)-3-methyl-7-propyl xanthine (HWA 285) enhances the actions of adenosine. Acta Pharmacol Toxicol (Copenh) 1986;58:187–92. doi: 10.1111/j.1600-0773.1986.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 121.Wu PH, Barraco RA, Phillis JW. Further studies on the inhibition of adenosine uptake into rat brain synaptosomes by adenosine derivatives and methylxanthines. Gen Pharmacol. 1984;15:251–4. doi: 10.1016/0306-3623(84)90169-1. [DOI] [PubMed] [Google Scholar]
- 122.Belch JJ, Ho M. Pharmacotherapy of Raynaud’s phenomenon. Drugs. 1996;52:682–95. doi: 10.2165/00003495-199652050-00006. [DOI] [PubMed] [Google Scholar]
- 123.Chopra HK, Chopra KL, Aggarwal KK, Parashar SK. Pentoxifylline (Trental) – a new drug for the treatment of peripheral chronic occlusive arterial disease. J Med. 1988;19:89–107. [PubMed] [Google Scholar]
- 124.Chirkov YY, Chirkova LP, Sage RE, Horowitz JD. Impaired responsiveness of platelets from patients with stable angina pectoris to antiaggregating and cyclic AMP-elevating effects of prostaglandin E1. J Cardiovasc Pharmacol. 1995;25:961–6. [PubMed] [Google Scholar]
- 125.Muller R, Lebrach F. Haemorheological role of platelet aggregation and hypercoagulability in microcirculation: Therapeutical approach with pentoxifylline. Pharmatherapeutica. 1980;2:372–9. [PubMed] [Google Scholar]
- 126.Berry CN, Lorrain J, Lochot S, et al. Antiplatelet and antithrombotic activity of SL65.0472, a mixed 5-HT1B/5-HT2A receptor antagonist. Thromb Haemost. 2001;85:521–8. [PubMed] [Google Scholar]
- 127.Becker RC. The role of blood viscosity in the development and progression of coronary artery disease. Cleve Clin J Med. 1993;60:353–8. doi: 10.3949/ccjm.60.5.353. [DOI] [PubMed] [Google Scholar]
- 128.Beyreder J. Use of pentoxifylline in the treatment of acute cerebrovascular insufficiency. Eur Neurol. 1983;22(Suppl 1):116–23. doi: 10.1159/000115660. [DOI] [PubMed] [Google Scholar]
- 129.Dintenfass L. The clinical impact of the newer research in blood rheology: An overview. Angiology. 1981;32:217–29. doi: 10.1177/000331978103200401. [DOI] [PubMed] [Google Scholar]
- 130.Kwaan HC, Bongu A. The hyperviscosity syndromes. Semin Thromb Hemost. 1999;25:199–208. doi: 10.1055/s-2007-994921. [DOI] [PubMed] [Google Scholar]
- 131.Lechner H, Ott E, Bertha G. Therapeutical aspects of cerebrovascular disease. Eur Neurol. 1983;22(Suppl 1):74–7. doi: 10.1159/000115653. [DOI] [PubMed] [Google Scholar]
- 132.Levenson J, Del Pino M, Simon A. [Blood and arterial wall rheology and cardiovascular risk factors] J Mal Vasc. 2000;25:237–40. [PubMed] [Google Scholar]
- 133.Nash GB. Blood rheology and ischaemia. Eye. 1991;5:151–8. doi: 10.1038/eye.1991.29. [DOI] [PubMed] [Google Scholar]
- 134.Liu M, Counsell C, Wardlaw J. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database Syst Rev. 2000:CD000091. doi: 10.1002/14651858.CD000091. [DOI] [PubMed] [Google Scholar]
- 135.Ehrly AM. Drugs that alter blood viscosity. Their role in therapy. Drugs. 1990;39:155–9. doi: 10.2165/00003495-199039020-00001. [DOI] [PubMed] [Google Scholar]
- 136.Ely H. Pentoxifylline therapy in dermatology. A review of localized hyperviscosity and its effects on the skin. Dermatol Clin. 1988;6:585–608. [PubMed] [Google Scholar]
- 137.Bath PM, Bath FJ, Asplund K. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database Syst Rev. 2000:CD000162. doi: 10.1002/14651858.CD000162. [DOI] [PubMed] [Google Scholar]
- 138.Ernst E. Pentoxifylline for intermittent claudication. A critical review. Angiology. 1994;45:339–45. doi: 10.1177/000331979404500502. [DOI] [PubMed] [Google Scholar]
- 139.Schneider R, Schmid-Schonbein H, Kiesewetter H. The rheological efficiency of parenteral pentoxifylline (Trental) in patients with ischemic brain lesions. Preliminary results. Eur Neurol. 1983;22(Suppl 1):98–104. doi: 10.1159/000115657. [DOI] [PubMed] [Google Scholar]
- 140.Mukherjee D, Topol EJ. The role of low-molecular-weight heparin in cardiovascular diseases. Prog Cardiovasc Dis. 2002;45:139–56. doi: 10.1053/pcad.2002.127679. [DOI] [PubMed] [Google Scholar]
- 141.Nowak SN, Jaber LA. Aspirin dose for prevention of cardiovascular disease in diabetics. Ann Pharmacother. 2003;37:116–21. doi: 10.1345/aph.1C101. [DOI] [PubMed] [Google Scholar]
- 142.Sawczuk IS, Williams D, Chang DT. Low molecular weight heparin for venous thromboembolism prophylaxis in urologic oncologic surgery. Cancer Invest. 2002;20:889–92. doi: 10.1081/cnv-120005901. [DOI] [PubMed] [Google Scholar]
- 143.Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29:181–96. doi: 10.1016/s0306-3623(96)00314-x. [DOI] [PubMed] [Google Scholar]
- 144.Isbrucker RA, Peterson TC. Platelet-derived growth factor and pentoxifylline modulation of collagen synthesis in myofibroblasts. Toxicol Appl Pharmacol. 1998;149:120–6. doi: 10.1006/taap.1997.8357. [DOI] [PubMed] [Google Scholar]


