Abstract
BACKGROUND:
Diabetes mellitus is one of the leading causes of illness and death in North America. Cardiovascular diseases are a common secondary complication in the diabetic population. One of the important risk factors identified for the development of cardiovascular disease is an elevation in the sulfur amino acid, homocysteine. Although the exact mechanism(s) that underlie the relationship between elevated plasma homocysteine levels and cardiovascular disease remain unclear, it has been suggested that endothelial dysfunction produced by modestly elevated blood homocysteine concentrations may account for an increased risk of both arterial and venous occlusive disease.
OBJECTIVES:
The present study examined the effects of three- and eight-weeks bis(maltolato)oxovanadium(IV) (BMOV) treatment on plasma concentrations of homocysteine and cysteine in both control and streptozotocin (STZ) diabetic rats.
METHODS:
Diabetes was induced in male Wistar rats by a single intravenous injection of STZ (60 mg/kg) in normal saline. Control animals received normal saline only. Animals were further randomized into treated and untreated groups. Treated animals received BMOV orally, dissolved in tap water, while untreated animals only received tap water. Three or eight weeks postinduction of diabetes, blood samples were obtained by cardiac puncture from the animals. Plasma harvested from each blood sample was used to determine glucose, insulin, homocysteine and cysteine concentrations.
RESULTS:
There was a significant decrease in plasma homocysteine levels in the diabetic (three- and eight-week study) groups compared with their respective controls (three-week study: diabetic group 3.1±0.7 μmol/L and control group 6.1±0.7 μmol/L; eight-week study: diabetic group 4.3±0.5 μmol/L and control group 6.9±1.0 μmol/L). Plasma cysteine levels were significantly decreased in the diabetic and diabetic treated groups (eight-week study) compared with their respective control groups (diabetic group 90.2±32.3 μmol/L and control group 177.9±36.7 μmol/L). BMOV treatment restored plasma homocysteine concentrations in diabetic animals to concentrations found in nondiabetic animals.
CONCLUSIONS:
Taken together, these findings suggest that STZ-induced diabetes may result in decreased plasma homocysteine and cysteine levels and that BMOV treatment may increase plasma homocysteine concentrations to nondiabetic concentrations. These results may provide further insight on how this insulin-enhancing/mimetic agent modifies plasma homocysteine metabolism.
Keywords: Bis(maltolato)oxovanadium(IV), Cysteine, Diabetes, Homocysteine
Diabetes mellitus is one of the leading causes of illness and death in North America. Cardiovascular diseases are a common secondary complication in the diabetic population (1–3). Developments of cardiovascular disease and abnormalities in heart and vascular function have been widely reported in both experimental and clinical diabetes (4–6). Cardiovascular dysfunction has a complex etiology, such as macro- (4) and microangiopathy (5), and a cardiomyopathy specific to the diabetic condition (6,7). Cardiomyopathy results in an impaired force of contraction, delayed relaxation and inappropriate ventricular filling (8,9). Studies conducted by McNeill et al (10) and others (11,12) have shown the presence of cardiovascular disease and heart dysfunction in both chemically induced and spontaneously diabetic rats similar to that observed in diabetic patients.
Homocysteine is a sulfur amino acid that has been recognized as an important risk factor for the development of cardiovascular disease (13). However, as early as 1969, McCully (14) published a report where he observed a child suffering from homocystinuria exhibited arterial lesions that were strikingly similar to those in patients with cystathionine beta-synthase deficiency. This observation led to the hypothesis that elevated plasma homocysteine concentrations found in patients with homocystinuria was responsible for the development of premature occlusive vascular disease (13,14). Since this important observation, numerous reports have been published linking an elevation in plasma homocysteine levels to the increased incidence of vascular disease (15–19).
Although the exact mechanism(s) that underlie the relationship between elevated plasma homocysteine levels and cardiovascular disease remain unclear, several possible mechanisms have been postulated. Bellamy and McDowell (20) have suggested that endothelial dysfunction produced by modestly elevated blood homocysteine concentrations may account for an increased risk of both arterial and venous occlusive disease. Specifically, they speculated that excessive blood homocysteine may impair the release and/or action of nitric oxide in response to blood flow, causing smooth muscle cell proliferation, extra-cellular matrix modification and lipoprotein oxidation; in particular, changes in cellular reduction and oxidation status. Although homocysteine does not appear to modify circulating coagulation factors, it may indirectly promote enhanced thrombin production by its effects on the endothelium.
An elevation in homocysteine levels in type I diabetic patients appears to be dependent on the presence or absence of nephropathy (21). Diabetic patients with renal dysfunction exhibit elevated plasma homocysteine levels due to the decreased renal clearance of homocysteine (22–24). Conversely, type I diabetic patients with normal renal function have lower plasma homocysteine levels (25). Jacobs and colleagues (26) reported that this decrease in plasma homocysteine levels is a result of an increase in the activities of hepatic transsulfuration enzymes (eg, cystathionine beta-synthase and cystathionine gamma-lyase) which are involved in the catabolism of plasma homocysteine. They further reported that this decrease in plasma homocysteine levels could be prevented when diabetic rats received insulin (26).
Vanadium is a group Va transition element that has been shown to improve glucose homeostasis and preserve insulin reserves in different animal models of diabetes mellitus (27–31). Bis(maltolato)oxovanadium(IV) (BMOV) is an organic vanadium compound (32) which has been shown to be two to three times more potent than inorganic vanadium (33). Previous studies have reported that BMOV treatment (0.75 mg/mL) in drinking water over a 25-week period resulted in the accumulation of vanadium in bone, kidney, liver, muscle and fat, without any mortality. BMOV treatment reduced plasma glucose, glycosylated hemoglobin, triglycerides and cholesterol in diabetic-treated rats compared with controls. More significantly, BMOV therapy prevented the development of the heart dysfunction commonly associated with streptozotocin (STZ)-induced diabetic rats (27–33).
Because abnormalities in plasma homocysteine levels are an independent risk factor for the development of cardiovacular disease and BMOV treatment appears to prevent the development of cardiomyopathy, the purpose of the present study was to examine the effects of BMOV on plasma concentrations of homocysteine and cysteine in both control and STZ diabetic rats. Based on earlier work, which showed that insulin treatment increased plasma homocyteine levels to normal in the diabetic rat (26), the present study was intended to determine the effects of BMOV under similar experimental conditions.
MATERIALS AND METHODS
Experimental protocols
Male Wistar rats, weighing 190 g to 220 g were obtained from Charles River Laboratories (Canada). All animals used in the present study were cared for in accordance with the principles and guidelines published by the Canadian Council on Animal Care and the University of British Columbia. Rats were housed individually in the treated groups and in pairs in the control groups, on a 12 h light, 12 h dark schedule and given food (PMI Feeds, USA) and fluid ad libitum.
Three weeks treatment with BMOV
Thirty-six male Wistar rats were randomly divided into two groups: control and diabetic. Experimental diabetes was induced by a single intravenous injection of STZ (60 mg/kg; Sigma Chemical Co, USA) in 0.9% normal saline into the tail vein under light halothane (Fluothane, Ayerst Laboratories, Canada) anesthesia. The control group received an equivalent volume of normal saline. Three days after STZ injection, rats with blood glucose levels higher than 14 mmol/L were considered diabetic. One week after STZ injection, control and diabetic rats were divided into further subgroups: control (C, n=8), control treated with BMOV (CT, n=8), diabetic (D, n=10) and diabetic treated with BMOV (DT, n=10). The treatment solution was prepared by dissolving BMOV in tap water. Treatment solutions were prepared fresh every two days. An initial BMOV concentration of 0.25 mg/mL in the drinking water was increased to a maximum of 1 mg/mL, in 0.25 mg/mL increments every three days. The average daily dose of BMOV was 0.25±0.01 mmol/kg/day in control animals and 1.58±0.38 mmol/kg/day in diabetic animals. Untreated animals received tap water only. Body weights and food and fluid intakes were measured daily during the study.
After three weeks of treatment with BMOV, rats were anesthetized with an overdose of pentobarbital sodium (65 mg/kg, intraperitoneal). Blood was collected by cardiac puncture for measurements of plasma glucose, insulin, homocysteine and cysteine. For each 5 mL of whole blood collected 0.2 mL of heparin 10,000 U/mL (Benson Medical, Canada) was added. Heparinized whole blood was centrifuged (10,000 g, 25 min, 4°C), plasma was recovered and stored at −20°C until assayed. Five millilitres of blood were taken to obtain adequate plasma for subsequent analysis.
Eight weeks treatment with BMOV
Thirty-six male Wistar rats were randomly divided into two groups: control and diabetic. Induction of diabetes and assessment of the diabetic state were carried out as described above. One week after STZ injection, control and diabetic rats were divided into further subgroups: control (C, n=8), control treated with BMOV (CT, n=8), diabetic (D, n=10) and diabetic treated with BMOV (DT, n=10). BMOV solution preparation treatment was as described above, except that the experimental period was increased to a total of eight weeks. The average daily dose of BMOV was 0.29±0.04 mmol/kg/day in control animals and 0.35±0.05 mmol/kg/day in diabetic animals. Untreated animals received tap water only. Terminating procedures were as described above.
Plasma parameters
Glucose and insulin quantification:
Plasma glucose (mmol/L) levels were measured using a Beckman Glucose Analyzer 2 (Beckman Instrumentals Inc, USA). Plasma insulin (pmol/L) levels were determined by radioimmunoassay using kits obtained from Cedarlane Laboratories Ltd (Canada).
Homocysteine and cysteine quantification:
Total plasma homocysteine and cysteine (μmol/L) (ie, the sum of reduced, oxidized and protein-bound forms) levels were determined using a high performance liquid chromatography methodology previously described by Fortin and Genest (34).
Statistical analysis
Plasma glucose, insulin, homocysteine and cysteine levels were compared between animal groups using a two-way analysis of variance (Number Cruncher Statistical System, USA) followed by a Newman-Keuls test. P<0.05 was taken as level of significance. Values are expressed as the mean ± SEM.
RESULTS
The general characteristics for the experimental groups are summarized in Table 1. At the end of the both studies (three weeks and eight weeks), body weights were significantly lower in the diabetic and BMOV-treated diabetic groups compared with the control group.
TABLE 1.
General characteristics of animals in different treatment groups
| Parameters | Control (C) | Control treated (CT) | Diabetic (D) | Diabetic treated (DT) | |
|---|---|---|---|---|---|
| Body weight (g) | 3 weeks termination | 408±4 | 373±4 | 345±3* | 347±6* |
| 8 weeks termination | 464±14 | 426±12 | 360±8* | 350±12* | |
| Fluid intake (mL/day) | 3 weeks termination | 65±2 | 40±2 | 323±7* | 52±4 |
| 8 weeks termination | 75±2 | 59±3 | 299±10* | 62±5 | |
| Food intake (g/day) | 3 weeks termination | 32±1 | 29±1 | 63±1* | 30±1 |
| 8 weeks termination | 34±1 | 32±1 | 60±1* | 33±1 |
Values are presented as the mean ± SEM. Statistical analysis was completed applying Newman-Keuls test.
Significantly different from control group (P<0.05)
Fluid intake was significantly higher in the diabetic groups (both studies) compared with the control groups, but was not different among the diabetic treated, control treated and control groups (both studies). In both studies, significantly higher food consumption was noted in the diabetic groups of animals compared with the control group, while diabetic treated and control treated groups were not different from the respective control groups (Table 1).
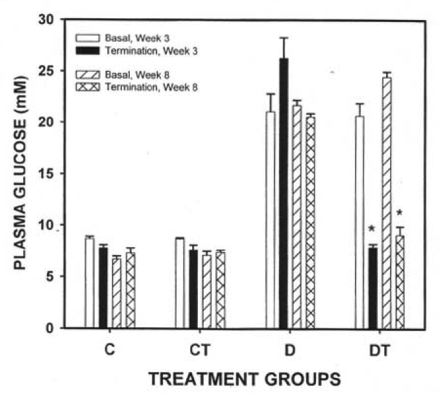
STZ injection resulted in a profound increase in plasma glucose concentration (Figure 1) and a significant decrease in plasma insulin (Figure 2) levels in Wistar rats. BMOV treatment in both the three- and eight-week diabetic groups reduced glucose to control levels (Figure 1). There was no difference in plasma glucose levels (Figure 1) in control and control treated groups (three-week study and eight-week study). Plasma insulin levels were significantly lower in all diabetic groups than in controls (Figure 2). There was no effect of duration of diabetes or BMOV treatment on plasma insulin levels in diabetic animals (Figure 2). There was a significant lowering of insulin values in the three-week control treated animals (Figure 2).
Figure 1).
Plasma glucose concentration (mean ± SEM) in three-week and eight-week control and streptozotcin diabetic rats before (Basal) and following (Termination) bis(maltolato)oxovanadium(IV) (BMOV) treatment. C Nondiabetic control; CT Nondiabetic animals treated with BMOV; D Nontreated diabetic animals; DT Diabetic animals treated with BMOV. *Different from basal values (P<0.05)
Figure 2).
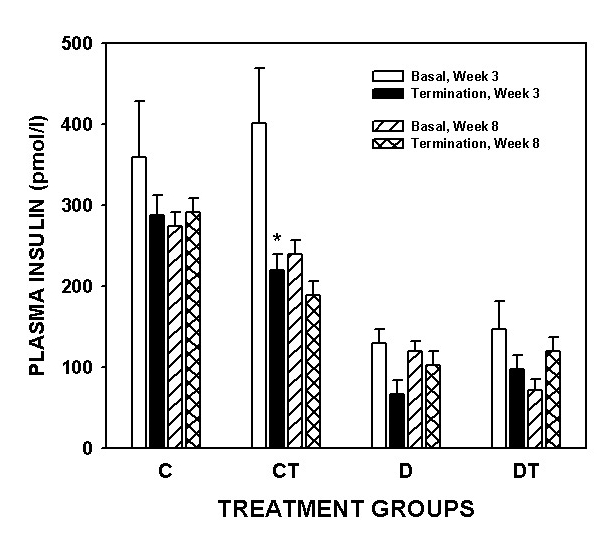
Plasma insulin concentration (mean ± SEM) in three-week and eight-week control and streptozotocin diabetic rats before (Basal) and following (Termination) bis(maltolato)oxovanadium(IV) (BMOV) treatment. C Nondiabetic control; CT Nondiabetic animals treated with BMOV; D Nontreated diabetic animals; DT Diabetic animals treated with BMOV. *Different from basal, week 3 values
There was a significant decrease in plasma homocysteine concentrations in the diabetic groups (three- and eight-week study) compared with their respective controls (Figure 3). Plasma cysteine concentrations were significantly decreased in the diabetic and diabetic treated groups (eight-week study) compared with their respective control groups (Figure 4). While there is an apparent decrease in CT cysteine levels at three weeks, there was no statistically significant difference at either three or eight weeks (Figure 4).
Figure 3).
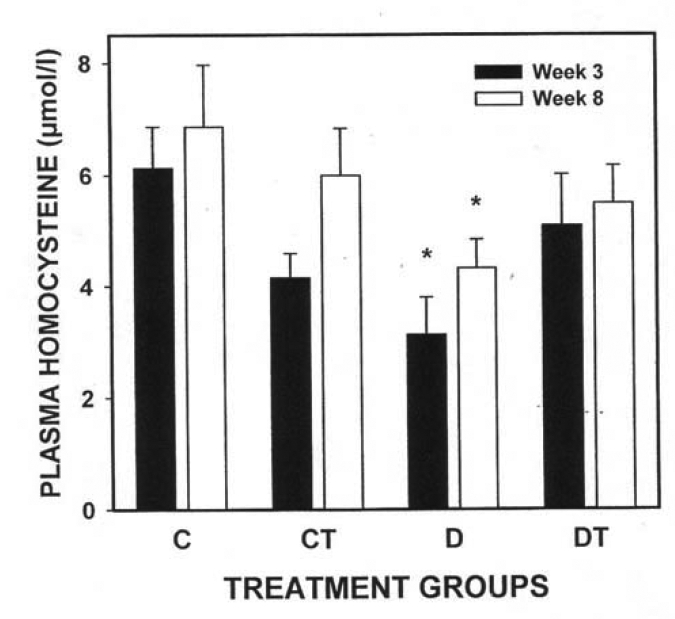
Plasma homocysteine concentration (mean ± SEM) in three-week and eight-week control and streptozotocin diabetic rats with or without bis(maltolato)oxovanadium(IV) (BMOV) treatment. C Nondiabetic control; CT Nondiabetic animals treated with BMOV; D Nontreated diabetic animals; DT Diabetic animals treated with BMOV. *Different from respective controls (P<0.05)
Figure 4).
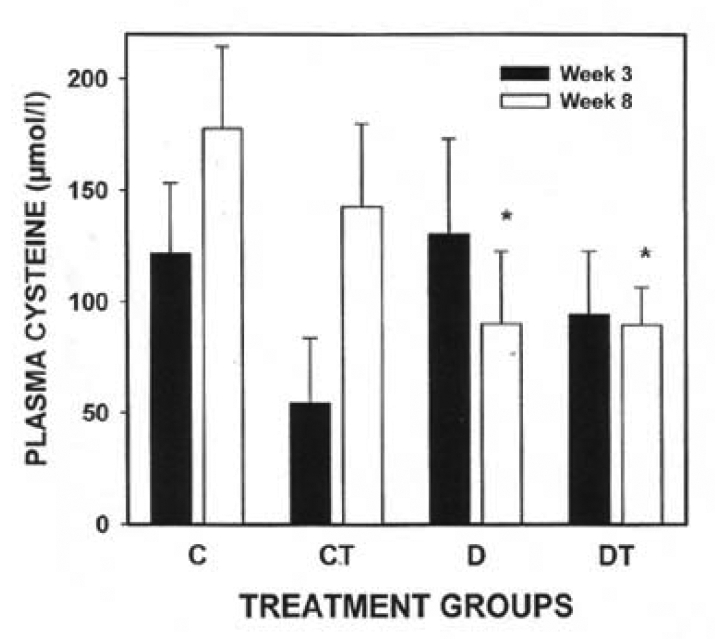
Plasma cysteine concentration (mean ± SEM) in three-week and eight-week control and streptozotocin diabetic rats with or without bis(maltolato)oxovanadium(IV) (BMOV) treatment. C Nondiabetic control; CT Nondiabetic animals treated with BMOV; D Nontreated diabetic animals; DT Diabetic animals treated with BMOV. *Different from control, week 8 values (P<0.05)
DISCUSSION
Previous studies have shown vanadium compounds improved insulin sensitivity (35). BMOV is an organic vanadium compound, which has been postulated to be an insulin mimetic/enhancing agent. The insulin-enhancing effect of BMOV (36,37) was confirmed by the significantly lower plasma glucose levels (Figure 1) in three- and eight-week diabetic treated groups. The improvement in glucose homeostasis with BMOV treatment occurred independently of any increase in plasma insulin levels (Figure 2).
BMOV treatment had no effect on body weight up to eight weeks of treatment in control animals, unlike inorganic vanadium (38,39). The three- and eight-week diabetic-induced hyperphagia and polydipsia associated with elevated plasma glucose levels were corrected with vanadium treatment. Malabu at al (40) have suggested that in STZ diabetic rats, the glucose-lowering effects of vanadium are a consequence of a reduction in food intake. However, other studies have dissociated the glucose-lowering effects of vanadium from changes in food intake in STZ diabetic rats (41–43). These studies indicate that the reductions in food and fluid intake are associated with a correction in hyperglycemia. In the three-week study, there was a large variability in plasma insulin levels in the control groups. However, extensive work done with vanadium compounds has clearly shown a significant decrease in plasma insulin levels in control animals, which has been related to an increase in insulin sensitivity in these animals (9–12).
Further studies have demonstrated that elevated plasma homocysteine concentrations are a strong indicator of cardiovascular disease (15–19). An elevation in homocysteine levels appears to be dependent on the presence or absence of nephropathy (21). Audelin and Genest (44) showed that the relative hyperfiltration secondary to renal hyperperfusion, which occurs in early diabetes, might lead to increased homocysteine catabolism. These data may explain the lower than normal plasma homocysteine levels in diabetic patients. Previous studies by our group (27–33) have suggested that BMOV exhibits insulin-mimetic and -enhancing activity. Jacobs and colleagues (26) have demonstrated that diabetic animals exhibited a similar decrease in plasma homocysteine versus controls to that observed in the present study (Figure 3). In addition, Jacobs et al (26) further reported that insulin treatment in diabetic animals increased plasma homocysteine concentrations, restoring them to nondiabetic control levels. Similarly, we observed that BMOV treatment in diabetic animals restored plasma homocysteine concentration to nondiabetic control concentrations (Figure 3). Taken together, these findings demonstrate that the effects of BMOV are due to its insulin-enhancing properties because the BMOV effect on homocysteine mimicked the effect of insulin. The findings also suggest that, in the rat, elevated homocysteine levels are not correlated to diabetic cardiomyopathy, because cardiomyopathy is known to occur at six weeks following STZ injection (9) and yet, homocysteine levels were decreased at both three and eight weeks following STZ injection. We interpret these results as the ability of BMOV (like insulin in the Jacobs study (26)) to restore plasma homocysteine levels to nondiabetic levels and not any higher.
Plasma cysteine levels were significantly decreased in the eight-week diabetic and diabetic treated group compared with controls (Figure 4). An increase in plasma cysteine levels would imply an intact transsulfuration pathway of homocysteine metabolism (20,26). Mosharov at al (45) have suggested that an increase in transsulfuration may represent a physiological response to oxidative stress because a major function of this pathway is the synthesis of the intracellular antioxidant glutathione. However, Colombatto and Grillo (46) have suggested the possibility of a direct effect of vanadium on transsulfuration, specifically the decarboxylation of S-adenosylmethionine in the presence of pyridoxal phosphate. Additional studies to further understand our findings are required.
CONCLUSIONS
Our findings confirm that STZ-induced diabetes may result in decreased plasma homocysteine and cysteine levels and that BMOV treatment may increase plasma homocysteine concentrations to nondiabetic levels. Because BMOV is an insulin-enhancing agent, this lends support to the suggestion by Jacobs et al (26) that insulin is a factor in the regulation of homocysteine levels in the rat.
Acknowledgments
Funding provided from Canadian Institutes of Health Research and the Heart and Stroke Foundation of British Columbia and Yukon (JHM and KMW).
REFERENCES
- 1.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: A statement for the healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, Agostino RBD, Levy D, Belanger H, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S89–100. doi: 10.1053/ajkd.1998.v32.pm9820468. [DOI] [PubMed] [Google Scholar]
- 4.Garcia MJ, McNamara PM, Gordon T, Kannel B. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 5.Factor SM, Okun EM, Minase T. Capillary microaneurysms in the human diabetic heart. N Engl J Med. 1980;302:384–8. doi: 10.1056/NEJM198002143020706. [DOI] [PubMed] [Google Scholar]
- 6.Ledet T, Gotzsche O, Heickendorff L. The pathology of diabetic cardiopathy: Pathogenic reflections. In: Jerrett RJ, editor. Diabetes and Heart Disease. New York: Elsevier; 1984. pp. 121–45. [Google Scholar]
- 7.Singer DE, Moulton W, Nathan DM. Diabetic myocardial infarction. Interaction of diabetes with other preinfarction risk factors. Diabetes. 1989;38:350–7. doi: 10.2337/diab.38.3.350. [DOI] [PubMed] [Google Scholar]
- 8.Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol. 1985;1:263–81. [PubMed] [Google Scholar]
- 9.McNeill JH, Tahiliani AG. Diabetes-induced cardiac changes. Trends Pharmacol Sci. 1986;7:364–7. [Google Scholar]
- 10.Vadlamudi RVS, Rodgers RL, McNeill JH. The effect of chronic alloxan- and streptozotocin-induced diabetes on isolated rat heart performance. Can J Physiol Pharmacol. 1982;60:902–11. doi: 10.1139/y82-127. [DOI] [PubMed] [Google Scholar]
- 11.Fein FS, Kornstein LB, Strobeck JE, Capasso JM, Sonnenblich EH. Altered myocardial mechanics in diabetic rats. Circ Res. 1980;47:922–33. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues B, McNeill JH. Cardiac function in spontaneously hypertensive diabetic rats. Am J Physiol. 1986;251:H571–80. doi: 10.1152/ajpheart.1986.251.3.H571. [DOI] [PubMed] [Google Scholar]
- 13.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 14.McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SS, Wong PWK, Malinow MR. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr. 1992;12:279–98. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 16.Mallinov MR. Homocyst(e)ine and arterial occlusive disease. J Intern Med. 1994;236:603–17. doi: 10.1111/j.1365-2796.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 17.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infaraction in US physicians. JAMA. 1992;268:877–81. [PubMed] [Google Scholar]
- 18.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–9. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 20.Bellamy MF, McDowell IFW. Putative mechanisms for vascular damage by homocysteine. J Inherit Metab Dis. 1997;20:307–15. doi: 10.1023/a:1005377310872. [DOI] [PubMed] [Google Scholar]
- 21.Bostom AG, Culleton BF. Hyperhomocysteinemia in chronic renal disease. J Am Soc Nephrol. 1999;10:891–900. doi: 10.1681/ASN.V104891. [DOI] [PubMed] [Google Scholar]
- 22.Hultberg B, Agardh E, Andersson A, et al. Increased levels of plasma homocysteine are associated with nephropathy, but not severe retinopathy in Type 1 diabetes mellitus. Scand J Clin Lab Invest. 1991;51:277–82. doi: 10.3109/00365519109091615. [DOI] [PubMed] [Google Scholar]
- 23.Hoogeveen EK, Kostense PJ, Beks PJ, et al. Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin-dependent diabetes mellitus: A population-based study. Arterioscler Thromb Vasc Biol. 1998;18:133–8. doi: 10.1161/01.atv.18.1.133. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann MA, Kohl B, Zumbach MS, et al. Hyperhomocyst(e)inemia and endothelial dysfunction in IDDM. Diabetes Care. 1998;21:841–8. doi: 10.2337/diacare.21.5.841. [DOI] [PubMed] [Google Scholar]
- 25.Robillon JF, Canivet B, Candito M, et al. Type 1 diabetes mellitus and homocyst(e)ine. Diabete Metab. 1994;20:494–6. [PubMed] [Google Scholar]
- 26.Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967–70. doi: 10.2337/diabetes.47.12.1967. [DOI] [PubMed] [Google Scholar]
- 27.Heyliger CE, Tahiliani AG, McNeill JH. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985;227:1474–7. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- 28.Brichard SM, Pottier AM, Henquin JC. Long term improvement of glucose homeostasis by vanadate in obese hyperinsulinemic fa/fa rats. Endocrinology. 1989;125:2510–6. doi: 10.1210/endo-125-5-2510. [DOI] [PubMed] [Google Scholar]
- 29.Battel ML, Yuen VG, McNeill JH. Treatment of BB rats with vanadyl sulfate. Pharmacol Commun. 1992;1:291–301. [Google Scholar]
- 30.Brichard SM, Bailey CJ, Henquin JC. Marked improvement of glucose homeostasis in diabetic ob/ob mice given oral vanadate. Diabetes. 1990;39:1326–32. doi: 10.2337/diab.39.11.1326. [DOI] [PubMed] [Google Scholar]
- 31.Yuen VG, Pederson RA, Dai S, Orvig C, McNeill JH. Effects of low and high dose administration of bis(maltolato)oxovanadium(IV) on fa/fa Zucker rats. Can J Physiol Pharmacol. 1996;74:1001–9. [PubMed] [Google Scholar]
- 32.McNeill JH, Yuen VG, Hoveyda HR, Orvig C. Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J Med Chem. 1992;35:1489–91. doi: 10.1021/jm00086a020. [DOI] [PubMed] [Google Scholar]
- 33.Yuen VG, Orvig JH, McNeill JH. Comparison of the glucose-lowering properties of vanadyl sulfate and bis(maltolato)oxovanadium(IV) following acute and chronic administration. Can J Physiol Pharmacol. 1995;73:55–64. doi: 10.1139/y95-008. [DOI] [PubMed] [Google Scholar]
- 34.Fortin LJ, Genest J. Measurement of homocysteine in the prediction of arteriosclerosis. Clin Biochem. 1995;28:155–62. doi: 10.1016/0009-9120(94)00073-5. [DOI] [PubMed] [Google Scholar]
- 35.Brichard SM, Okitolonda W, Henquin JC. Long term improvement of glucose homeostasis by vanadate treatment in diabetic rats. Endocrinology. 1988;123:2048–53. doi: 10.1210/endo-123-4-2048. [DOI] [PubMed] [Google Scholar]
- 36.Gil J, Miralpeix M, Carreras J, Bartrons R. Insulin-like effects of vanadate on glucokinase activity and fructose 2,6-bisphosphate levels in the liver of diabetic rats. J Biol Chem. 1988;263:1868–71. [PubMed] [Google Scholar]
- 37.Rodriguez-Gil JE, Gomez-Foix AM, Fillat C, Bosch F, Guinovart JJ. Activation by vanadate of glycolysis in hepatocytes from diabetic rats. Diabetes. 1991;40:1355–9. doi: 10.2337/diab.40.10.1355. [DOI] [PubMed] [Google Scholar]
- 38.Pederson RA, Ramanadham, Buchan AMJ, McNeill JH. Long-term effects of vanadyl treatment on streptozotocin-induced diabetes in rats. Diabetes. 1989;38:1390–5. doi: 10.2337/diab.38.11.1390. [DOI] [PubMed] [Google Scholar]
- 39.Ramandahman S, Mongold JJ, Browsney RW, Cros GH, McNeill JH. Oral vanadyl sulfate in treatment of diabetes mellitus in rats. Am J Physiol. 1989;257:H904–11. doi: 10.1152/ajpheart.1989.257.3.H904. [DOI] [PubMed] [Google Scholar]
- 40.Malabu UH, Dryden S, McCarthy HD, Kilpatric A, Williams G. Effects of chronic vanadate administration in the STZ-induced diabetic rat. The antihyperglicemic action of vanadate is attributable entirely to its suppression of feeding. Diabetes. 1994;43:9–15. doi: 10.2337/diab.43.1.9. [DOI] [PubMed] [Google Scholar]
- 41.Cam MC, McNeill JH. Glucose lowering effect of oral vanadyl sulfate in the streptozotocin diabetic rat. Clin Invest Med. 1990;13:B25. [Google Scholar]
- 42.Cam MC, Pedersen RA, Brownsey RW, McNeill JH. Long-term effectiveness of oral vanadyl sulfate in streptozotocin-diabetic rats. Diabetologia. 1993;36:218–24. doi: 10.1007/BF00399953. [DOI] [PubMed] [Google Scholar]
- 43.Yuen VG, Orvig C, McNeill JH. Effects of bis(maltolato)oxovanadium(IV) are distinct from food restriction in STZ-diabetic rats. Am J Physiol. 1997;272:E30–5. doi: 10.1152/ajpendo.1997.272.1.E30. [DOI] [PubMed] [Google Scholar]
- 44.Audelin MC, Genest J. Homocysteine and cardiovascular disease in diabetes mellitus. Atherosclerosis. 2001;159:497–511. doi: 10.1016/s0021-9150(01)00531-7. [DOI] [PubMed] [Google Scholar]
- 45.Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–11. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 46.Colombatto S, Grillo MA. Effect of vanadate and pyridoxal phosphate on S-adenosylmethionine. Int J Biochem. 1985;17:657–60. doi: 10.1016/0020-711x(85)90301-5. [DOI] [PubMed] [Google Scholar]