Abstract
OBJECTIVE:
Connexin 43 (Cx43), a membrane protein involved in the control of cell-to-cell communication, is thought to play a role in physiological processes such as tissue homeostasis, growth regulation and development. The aim of the present study was to investigate the change of Cx43 expression in aged myocardium.
METHODS AND RESULTS:
Sixteen male Sprague-Dawley rats (adult: 10 weeks old, n=8; aged: two years old, n=8) were used in the present study. In an isolated rat heart Langendorff model, hearts were perfused for 10 min with a modified Krebs-Henseleit bicarbonate buffer. Contractile functions were measured and all hearts were stained with anti-Cx43 antibody for fluorescence microscopic examinations. There were no significant differences observed in heart rate (234±8.2 beats/min versus 231±15.6 beats/min), left ventricular developed pressure (112.5±6.3 mmHg versus 107.2±2.5 mmHg), first derivative of the left ventricular pressure (1450.4±165.1 mmHg/s versus 1384.6±95.4 mmHg/s) and coronary flow (17.4±0.7 mL/min versus 21.3±1.8 mL/min) between adult and aged rats, respectively. However, significant differences were observed in left ventricular weight (adult versus aged; 0.639±0.108 g versus 1.124±0.257 g, P=0.04) and in fluorescence examinations where there was reduced distribution of Cx43 in aged myocardium compared with adult myocardium.
CONCLUSIONS:
These results demonstrated that the role of Cx43 may be more important than previously reported, and that this protein is partially responsible for the maintenance of cellular structure in myocardial development.
Keywords: Aging, Connexin 43, Gap junction
Gap junctions are composed of narrow membrane channels that permit the passage of ions and small molecules between the cytoplasmic compartments of abutting cells. Intracellular communication via these gap junctional channels has been proposed to contribute to a diverse range of physiological processes such as tissue homeostasis, growth regulation and development (1). Morphogenetic signals are thought to be among the small molecules that diffuse intercellularly via the gap junction. In the neonate and adult heart, developmental changes in myocardial gap junction distribution have been reported (2). Disruptions of the distribution and tissue content of connexin 43 (Cx43) gap junctions leading to impaired intracellular conduction have been reported to predispose the heart to reentry arrhythmias in infarct border zones (3) and in reversibly ischemic myocardium. Abnormal impulse propagation resulting from changes of the gap junction may contribute to the electromechanical dysfunction associated with hibernation (4). The aim of the present study was to characterize gap junction organization in aged ventricular myocardium and to establish whether abnormalities exist in the myocardium of aged hearts (5).
MATERIALS AND METHODS
The investigation was performed in conformance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No 85–23, revised 1985).
Experimental preparation
Sixteen male Sprague-Dawley rats (adult: 10 weeks old, n=8; aged: two years old, n=8) were anesthetized with an intraperitoneal injection (80 mg/kg) of pentobarbital sodium (Nembutal sodium, Abbot, Canada) and heparin (500 IU/kg) was given intravenously. Following thoracotomy, the hearts were excised and placed in an ice-cold perfusion buffer. The aorta was then cannulated and the heart perfused using the Langendorff method at a constant perfusion pressure of 100 cm of water. The perfusion medium consisted of a modified Krebs-Henseleit bicarbonate buffer (NaCl 118 mM, NaHCO3 24 mM, KCl 4.7 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, CaCl2 1.7 mM and glucose 10 mM), gassed with 95% O2/5% CO2, pH 7.4 and at 37°C.
Experimental design
All hearts were on the perfusion apparatus for a total of 10 min. Baseline contractile function was measured. The hearts were then rapidly frozen in liquid nitrogen and stored at −70°C for fluorescence microscopy and Western blotting. Heart rate, left ventricular pressure and the first derivative of the left ventricular pressure were measured with a fluid-containing complaint balloon connected through fluid-filled polyethylene tubing to a pressure transducer (P23ID, Gould Inc, USA) in the left ventricle through the mitral valve. Coronary flow rate was measured by timed collection of the coronary effluent. Developed pressure was measured as the difference between peak systolic pressure and end diastolic difference between peak systolic pressure and end diastolic pressure.
Fluorescence microscopy
Frozen myocardial samples were cut in cross section at a thickness of 5 μm; and the sections were thawed on albumin-coated glass slides. The sections were then stained with anti-Cx43 antibody. To avoid heterogeneity in staining intensity, all samples were processed and stained together. Fluorescent images for quantitative analysis were obtained from fields of view in which the cardiomyocytes (approximately 20 per field) appeared in cross section, and photomicrographs were generated with an automatic photography system (PM-30, Olympus Co, Japan). The fluorescent images were graded in terms of overall density of fluorescence and the pattern of fluorescence on the outer cardiomyocyte membranes (NIH Image version 1.55, USA). First, the density of fluorescent particles against the background was graded on a scale of 0 to 255. The density was measured at three to four points in each myocyte and adult and aged myocardium densities were compared. Lastly, each particle area was measured (the density of particles per area [μm2]).
Statistical analysis
The values for myocardial function are expressed as the mean ± SEM. Data were examined using a two-way repeated measures Analysis of Variance. For the quantitative scores from fluorescence microscopy, all samples were compared using the Friedman nonparametric repeated measures test. Differences were analyzed using Scheffe’s t test. P<0.05 was accepted as statistically significant.
RESULTS
The results from measures of cardiac function from the rats on the day of the experiments are shown in Table 1. There were no significant differences in heart rate, left ventricular developed pressure, first derivative of the left ventricular pressure or coronary flow rate between the Adult and Aged groups. However, the two groups of animals differed significantly in left ventricular weight.
TABLE 1.
Characteristic data
| Group | HR (beats/min) | LVDP (mmHg) | max LV dP/dt (mmHg/s) | CF (mL/min) | LV weight (g) |
|---|---|---|---|---|---|
| Adult | 234±8.2 | 112.5±6.3 | 1450.4±165.1 | 17.4±0.7 | 0.639±0.108* |
| Aged | 231±15.6 | 107.2±2.5 | 1384.6±95.4 | 21.3±1.8 | 1.124±0.257 |
Adult versus Aged, P=0.04. CF Coronary flow rate; HR Heart rate; LVDP Left ventricular developed pressure; LV weight Left ventricular weight; Max LV Dp/dt First derivative of the left ventricular pressure
Fluorescence microscopy
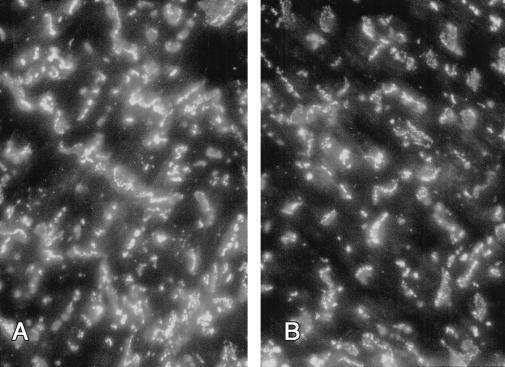
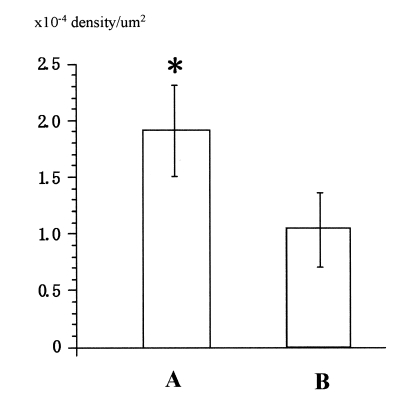
The staining pattern observed (Figure 1) was essentially the same as that described previously in contractile cells. All samples obtained from the aged group demonstrated a reduced intensity of Cx43 fluorescence (6). This was confirmed with quantitative analysis (Figure 2); the mean density of Cx43 was 1.93×10−4±0.36×10−4 particles/μm2 versus 1.12×10−4±0.23×10−4 particles/μm2 (P=0.03) in adult and aged rats, respectively.
Figure 1).
Immunofluorescence labelling with mouse monoclonal antibodies located connexin 43 at intercalated disks throughout left ventricular myocardium. Obvious difference in the number and pattern of these bands was observed between adult (A) and aged (B) rats
Figure 2).
This bar graph shows the quantitative density scores for samples from adult (A) and aged (B) rats. The fluorescence density in aged myocardium was significantly higher than adult myocardium. *P=0.04
DISCUSSION
The results of the present study demonstrate that throughout the myocardium in aged rats, progressive and substantial changes occur in the distribution pattern of Cx43 gap junctions. Many functional differences between adult and aged myocardium are directly related to changes in aged myocardial tissue components, a factor contributing to the limited ability of the aged heart to cope with various acute metabolic and hemodynamic stresses. Previous reports demonstrated that the myocardial gap junction was associated with electrical condition and arrhythmogenesis (7). It has been suggested that alterations in the number or the distribution of gap junctions could affect the distribution and, possibly, even the function of sarcolemmal ion channels or the functions of Ca2+ regulatory proteins in the sarcoplasmic reticulum that would affect excitation-contraction coupling and mechanical function. Therefore, although we did not find any differences in cardiac function in the present study, the reduced Cx43 in aged myocardium may affect electronical and mechanical function (8).
The molecular mechanism leading to the pressure-induced increase in expression of the Cx43 gene remains to be elucidated. The presence of multiple promoters in the 5′ untranslated region of this gene (9) raises the possibility that distinct transcription factors control its tissue-specific regulation. However, the negative finding suggests that Cx43 is not involved in the myocardial adaptation that accompanies the hypertension-induced increase in heart load, despite the fact that hypertensive animals featured signifcantly thicker left ventricular walls (10). It is still unclear whether any of the other mechanisms that regulate Cx43 at myocardial gap junctions and in endothelial cells function under several other conditions; thus, further studies are necessary to understand the mechanisms of reduced Cx43 in aged myocardium (11).
An agreement was found between the intensity of the fluorescent labelling and Western blot analysis. Direct measurements of antibody fluorescence intensity showed a significant difference between adult and aged myocardium. However, because the exact composition of the tissue is unknown when using both of these techniques, these results lack a reference value. This problem is avoided when using direct measurements in microscopy because the areas of interest can be identified. We cannot rule out the possibility that the changes observed in Cx43 levels with microscopy in the aged myocardium may simply reflect cancellation of decreased protein levels in some regions by increased protein levels in others. However, these results were obtained with sufficient reference suggesting that Cx43 in myocardium does change with aging.
CONCLUSIONS
The present study demonstrated that Cx43, a gap junctional protein, changes with age in rat myocardium. On the basis of our results, we believe that the role of Cx43 may be more important than previously reported, and that this protein is partially responsible for the maintenance of cellular structure in myocardial development.
REFERENCES
- 1.Fraser SE, Green CR, Bode HR, Gilula NB. Selective disruption of gap junctional communication interferes with a patterning process in hydra. Science. 1987;237:49–55. doi: 10.1126/science.3037697. [DOI] [PubMed] [Google Scholar]
- 2.Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–25. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- 3.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–96. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 4.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–75. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 5.Kaprielian RR, Gunning M, Dupont E, et al. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation. 1998;97:651–60. doi: 10.1161/01.cir.97.7.651. [DOI] [PubMed] [Google Scholar]
- 6.Severs NJ, Dupont E, Kaprielian RR, Yeh HI, Rothery S. Gap junctions and connexins in the cardiovascular system. In: Yacoub MH, Carpentier AF, Pepper JR, Fabiani JN, editors. Annual of Cardiac Surgery. London: Rapid Science Publishers; 1996. pp. 31–44. [Google Scholar]
- 7.Kanno S, Saffitz JE. The role myocardial gap junctions in electrical condition and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–77. doi: 10.1016/s1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 8.Saffitz JE, Yamada KA. Do alterations in intercellular coupling play a role in cardiac contractile dysfunction? Circulation. 1998;97:630–2. doi: 10.1161/01.cir.97.7.630. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZQ, Lefebvre DL, Bai XH, Reaume A, Rossant J, Lye SJ. Identification of two regulatory elements within the promoter region of the mouse connexin 43 gene. J Biol Chem. 1995;270:3863–8. [PubMed] [Google Scholar]
- 10.Andersen HR, Maeng M, Thorwest M, Falk E. Remodeling rather than neointimal formation explains luminal narrowing after deep vessel wall injury: Insights from porcine coronary (re)stenosis model. Circulation. 1996;93:1716–24. doi: 10.1161/01.cir.93.9.1716. [DOI] [PubMed] [Google Scholar]
- 11.Haefliger JA, Castillo E, Waeber G, et al. Hypertension increases connexin 43 in a tissue-specific manner. Circulation. 1997;95:1007–14. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]