Abstract
BACKGROUND:
The importance of an apolipoprotein AV (apoAV) gene for plasma triglyceride (TG) level determination has been shown on transgenic and knockout mice.
The influence of apoAV polymorphisms (T-1131/C and Ser19/Trp) on plasma TG levels was evaluated in a representative sample of 1191 men and 1368 women, in 435 patients with myocardial infarction (MI) and in 83 individuals with extreme TG levels (20.4±12.8 mmol/L).
METHODS:
ApoAV variants were analyzed using polymerase chain reaction and restriction analysis.
RESULTS:
T-1131/C variation in the apoAV gene affects plasma TG levels, showing a higher level in C-1131 carriers than in T/T-1131 homozygotes. This association has been observed both in men (P<0.05) and in women (P<0.01). TG levels were also influenced by the Ser19/Trp apoAV genotypes. In both males and females, the Trp19 carriers have higher plasma TGs (P<0.01) than do Ser19 homozygotes. In hypertriglyceridemic patients, the frequency of carriers of the T/C-1131 and C/C-1131 genotypes (32.5% versus 15.4%, P<0.0001) and Ser/Trp19 and Trp/Trp19 genotypes (30.1% versus 14.1%, P<0.0001) was much higher than in the population sample. In a group of MI patients (n=435), the frequency of the disadvantageous homozygous genotypes, with their effect of increasing the TG concentration (C/C-1131 and/or Trp/Trp19), was significantly higher than in the population sample (7.4% versus 2.0%, P<0.00001).
CONCLUSION:
Variation(s) in the apoAV gene play an important role in the genetic determination of plasma TG levels and influence the risk of MI.
Keywords: Apolipoprotein AV, Genetics, Myocardial infarction, Polymorphism, Triglycerides
Cardiovascular disease is the most common cause of death in industrialized countries, with high plasma triglyceride (TG) levels being suggested to be an independent risk factor (1,2). Plasma TGs are influenced by environmental factors (diet, exercise, etc), but little is known about genetic determination of TG levels.
The genetic predisposition to a high level of plasma TGs has been intensively analyzed in the past 15 years. Dozens of polymorphisms that could influence TG levels have been analyzed (3–5) and the most promising results are connected with the apolipoprotein (apo)AI/apoCIII/apoAIV gene cluster. The apoCIII SstI polymorphism (5) is especially believed to be an important genetic determinant of plasma TGs, but the results are not consistent (6–8) and polymorphism is located in the 3′ untranslated sequence; thus, the mechanism of the influence is not clear.
Recently, an apolipoprotein AV (apoAV) gene was identified in the same region using comparative sequencing (9–11). In mice, apoAV has been detected in very low-density lipoprotein and high-density lipoprotein fractions. The human apoAV gene consists of four exons and codes for a 369-amino acid protein that is only expressed in the liver. Transgenic and knockout mice were generated to assess the importance of this gene for plasma TG determination. The transgenic mice exhibited diminished levels of plasma TGs and the knockout mice showed elevated levels, whereas the plasma cholesterol levels were not influenced significantly.
Human DNA has been screened for apoAV polymorphisms, and, among others, two common variants (T-1131/C, originally referred to as SNP3, and C56/G=S19/W) have been detected (9,10,12).
An association between T/C-1131 polymorphisms and TG levels has been found in 501 healthy, nonsmoking individuals without a lipid-lowering medication (9) on random high-fat and low-fat diets. Additionally, the frequency of the disadvantageous allele was found to be higher (29.9% versus 4.2%) in individuals stratified according to TG level in the two extreme groups (greater than 90% and less than 10%). Surprisingly, this association was valid for men but not for women (frequencies were 9.4% and 11.3%, respectively) (9). Subsequently, the C-1131 allele was found to be associated with extreme levels of plasma TGs (13,14).
Similarly, Trp19 carriers have significantly higher plasma levels of TGs, and this association was observed in men and women with different ethnicities (Whites and Blacks but not Hispanics) (12,15). Furthemore, Trp19 allele carriers were more frequently found among patients with extreme TG levels than in members of the general population (16).
The aim of the present study was to evaluate the association between apoAV variations (T-1131/C, Ser19/Trp) and plasma TG levels in a large population-based study, and to analyze the allele frequencies of apoAV genotypes in patients with extreme TG levels and in myocardial infarction (MI) survivors.
PATIENTS AND METHODS
Population sample
The 2559 unrelated White subjects (1191 males and 1368 females, response rate of 84%) included in this study represented a three-year cohort of the selected sample of 1% of the Czech population. The individuals were recruited in 1997–1998 and reinvited to participate in the study in 2000–2001 according to the protocol used for the Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) project (17). Written informed consent was obtained from the study participants, and the local ethics committee approved the design of the study. The basic characteristics of the control subjects are summarized in Table 1.
TABLE 1.
Basic characteristics of the individuals involved in the study
| Controls | MI patients | ||
|---|---|---|---|
| Males | Females | Males | |
| N | 1191 | 1368 | 435 |
| Age, years ± SD | 49.2±10.8 | 48.8±10.6 | 55.1±7.6 |
| Cholesterol, mmol/L ± SD | 5.75±1.06 | 5.80±1.15 | 6.02±1.26 |
| TGs, mmol/L ± SD | 2.12±1.58 | 1.50±0.37 | 3.1±1.8 |
| BMI, kg/m2 ± SD | 28.2±4.0 | 27.6±5.5 | 28.0±3.7 |
| Diabetes, N/% | 72/6.0 | 60/4.4 | 109/25.0 |
| Hypertension, N/% | 489/41.1 | 457/33.4 | 187/43.0 |
| Smoking prevalence, N/% | 389/32.7 | 348/25.4 | 113/26.0 |
In myocardial infarction (MI) patients, triglyceride (TG) levels were available only for a subset of 383 patients. BMI Body mass index
MI patients
Four hundred thirty-five men under 65 years of age who had survived their first MI were included in the MI group. MI patients tend to be older and have higher plasma cholesterol levels, diabetes prevalence and smoking prevalence (expressed as current smokers), but do not differ in body mass index (BMI) or hypertension prevalence (Table 1).
Patients with extreme TG levels
The patients were selected from the database of the Prague Lipid Clinic of the Third Internal Department, which actively follows almost 2500 patients and has more than a 30-year tradition in the diagnosis and treatment of lipid metabolism disorders. The group of patients comprised 83 unrelated individuals (67 males and 16 females) aged 50.2±9.3 years with extreme lipid parameters (TGs of 20.4±12.8 mmol/L and total cholesterol of 10.4±3.7 mmol/L). For inclusion in the study, initial lipid levels measured without any lipid-lowering medication were considered.
DNA and biochemical analysis
DNA was isolated using the standard method (18).
Oligonucleotides AV6-F 5′ GAT TGA TTC AAG ATG CAT TTA GGA C and AV6-R 5′ CCC CAG GAA CTG GAG CGA AATT were used to amplify a fragment that was digested with Tru1I restriction enzyme to analyze the T-1131/C polymorphism. The oligonucleotides AV1-F 5′ TGC TCA CCT GGG CTC TGG CTC TTC and AV1-R 5′ CCA GAA GCC TTT CCG TGC CTG GGC GGC and restriction enzyme Eco52I were used to genotype the Ser19/Trp polymorphism (19).
The restriction fragments were analyzed using 10% polyacrylamide gel microtiter array diagonal gel electrophoresis (20), stained with ethidium bromide and visualized on an ultraviolet transilluminator.
The lipoprotein parameters were measured enzymatically by the World Health Organization Regional Lipid Reference Centre, Prague, Czech Republic, on the Roche COBAS-MIRA autoanalyzer (Hoffmann-La Roche, Czech Republic), using conventional enzymatic methods with reagents from Hoffmann-La Roche. BMI was calculated as weight in kilograms divided by height in metres squared.
Statistical analysis
Statistical analysis was performed using analysis of variance with repeated measures. TGs were logarithmically transformed before the analysis. Analyses of differences in apoAV genotype and haplotype frequencies between groups were performed using the χ2 test with Yates correction.
RESULTS
T-1131/C polymorphism
Distributions of the apoAV T-1131/C polymorphism genotypes are summarized in Table 2. The frequencies of the alleles and genotypes are about the same as the frequencies described previously (9,12,15).
TABLE 2.
Distribution of the genotypes and haplotypes of the T/C-1131 and Ser/Trp19 polymorphisms in the apolipoprotein AV gene in the Czech population and in myocardial infarction (MI) patients
| T/C-1131 polymorphism | |||
|---|---|---|---|
| Males, N (%) | Females, N (%) | MI patients, N (%) | |
| T/T | 1017 (85.4) | 1147 (83.9) | 366 (84.1) |
| T/C | 152 (12.8) | 203 (14.8) | 46 (10.8) |
| C/C | 22 (1.8) | 18 (1.3) | 23 (5.3) |
| Ser/Trp19 polymorphism | |||
|---|---|---|---|
| (%) | Males, N (%) | Females, N (%) | MI patients, N |
| Ser/Ser | 1037 (87.1) | 1161 (84.9) | 369 (84.8) |
| Ser/Trp | 152 (12.7) | 200 (14.6) | 56 (12.9) |
| Trp/Trp | 2 (0.2) | 7 (0.5) | 10 (2.3) |
| Genotype | Ser/Trp19 | Ser/Ser | Ser/Trp | Trp/Trp |
|---|---|---|---|---|
| T/C-1131 | N (%) Total | 2198 (85.9) | 352 (13.8) | 9 (0.3) |
| T/T | 2164 (84.6) | 1855 (72.5) | 302 (11.8) | 7 (0.3) |
| T/C | 355 (13.9) | 308 (12.0) | 45 (1.8) | 2 (0.1) |
| C/C | 40 (1.5) | 35 (1.3) | 5 (0.2) | 0 |
Male carriers of the apoAV allele C-1131 had higher plasma TGs than did T/T-1131 homozygotes (P<0.05). Female carriers of the C-1131 apoAV allele had higher and the T/T-1131 homozygotes lower (P<0.01) plasma concentrations of TGs (Table 3).
TABLE 3.
Effect of the T/C-1131 and Ser/Trp19 polymorphisms in the apolipoprotein AV gene on plasma triglycerides (TGs) (mmol/L)
| Males | Females | |||
|---|---|---|---|---|
| T/T | C/T + C/C | T/T | C/T + C/C | |
| N | 1017 | 174 | 1147 | 221 |
| TG | 2.06±1.66 | 2.40±1.63 | 1.43±0.85 | 1.57±0.88 |
| P | 0.05 | 0.01 | ||
| Ser/Ser | + Trp | Ser/Ser | +Trp | |
| N | 1037 | 154 | 1161 | 207 |
| TG | 2.07±1.60 | 2.40±1.97 | 1.43±0.82 | 1.65±1.02 |
| P | 0.01 | 0.01 | ||
Ser19/Trp polymorphism
The apoAV S19/W polymorphism genotype distributions are summarized in Table 2. The frequencies of the alleles and genotypes were similar to the frequencies described previously (12,15).
In both males and females, this polymorphism influenced plasma levels of TGs significantly – Trp19 had higher levels of TGs than did Ser19 carriers (P<0.01, Table 3).
Haplotype analysis
Because neither of the apoAV polymorphisms is in allelic association (Table 2), individuals were separated into three subgroups for haplotype analysis. The first subgroup comprised T/T-1131 and Ser/Ser19 homozygotes, the second subgroup comprised carriers of one rare allele (C-1131 or Trp19) and the third subgroup was made up of individuals with more than one rare allele.
In males, carriers of common haplotypes had lower levels of TGs (2.03±1.62 mmol/L) than did carriers of one (2.31±1.70 mmol/L) or more (2.71±2.00 mmol/L) rare apoAV alleles (P<0.005).
The effect was also significant in females (P<0.001), where individuals homozygous for the common alleles had the lowest TG levels (1.40±0.72 mmol/L), and carriers of more than one rare allele had the highest TG levels (1.77±0.94 mmol/L), with C-1131 or Trp19 heterozygotes showing an intermediate level (1.58±0.96 mmol/L).
ApoAV polymorphisms in patients with extreme TG levels
The pattern of distribution of apoAV genotypes is summarized in Figure 1. The frequencies of carriers of the C/T-1131 and C/C-1131 genotypes (32.5% versus 15.4%, respectively) and the Ser/Trp19 and Trp/Trp19 genotypes (30.1% versus 14.1%, respectively) were much higher in patients with extreme TG levels than in the population sample (P<0.0001 for both variants). Although not all hypertriglyceridemic individuals have the C-1131 or Trp19 alleles, this result supports the importance of the apoAV gene in the genetic determination of plasma TG levels. There is no linkage disequilibrium between rare alleles of both apoAV polymorphisms and, thus, the effect of both polymorphisms is independent.
Figure 1).
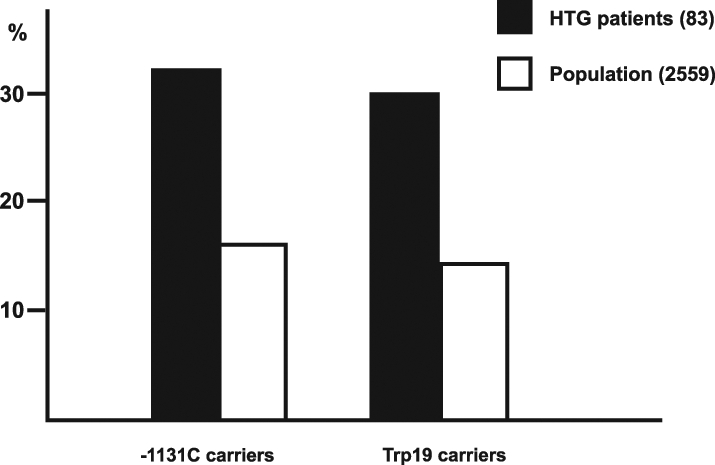
Patients with extreme triglyceride levels (HTG) were found to be carriers of the C-1131 and Trp19 alleles more frequently than were individuals in the population sample
ApoAV polymorphisms and MI
The distribution of individual genotypes did not differ significantly between MI patients and control subjects. The haplotype analysis revealed that the frequency of homozygotes for C/C-1131 and/or Trp/Trp19 in the apoAV gene was significantly higher in the MI survivors than in the male population (32 of 435 [7.4%] and 24 of 1191 [2.0%], respectively, P<0.00001, Figure 2).
Figure 2).
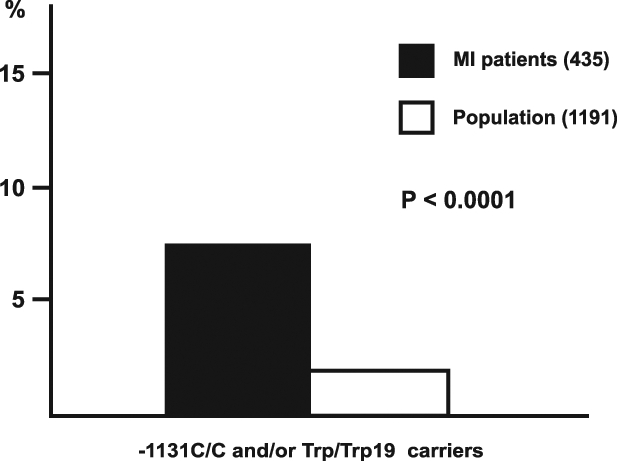
The frequency of the rare homozygotes for at least one APOAV polymorphism was significantly higher in myocardial infarction (MI) patients than in males from the population sample
Levels of plasma TGs were only available in the subset of MI patients (n=383) and, unfortunately, not from the same time after MI in all cases. Nevertheless, we have observed that Ser19 → Trp polymorphism influenced plasma TG levels similarly to the population sample (Trp/Trp19 3.47±1.84 mmol/L, Trp/Ser19 2.60±1.61 mmol/L, Ser/Ser19 2.33±1.49 mmol/L, P<0.05). No association has been found between T-1131 → C polymorphism and TG levels in MI patients (data not shown).
DISCUSSION
The present study was performed to analyze the role of common variants of the apoAV gene in the determination of plasma TGs in more than 2500 unrelated White individuals (equally distributed between sexes, with a wide age range). The results of most of the association studies undertaken are not consistent and reproducible every time. The causes of the differences are insufficient numbers of individuals, and differences in sex, ethnicity, age, BMI, diet, etc. To minimize the chance of false-positive or false-negative results, we used the protocol of the MONICA study. This protocol was prepared for World Health Organization monitoring of cardiovascular risks and is accepted as one of the best possible selection criteria for preparation of a representative population sample.
ApoAV polymorphisms have been studied in two large population studies with similar results. The study of Talmud et al (12) included 2808 White males, and associations between the rare alleles of both apoAV polymorphisms and higher plasma TGs were observed. Pennacchio et al (15) analyzed males and females from different ethnic groups (848 Whites, 1392 Blacks and 420 Hispanics). Ser/Trp19 polymorphism was associated with plasma TG levels in Whites and Blacks, but not in Hispanics. On the other hand, the impact of a T/C-1131 polymorphism was more pronounced in Hispanics than in Whites and was not detectable in Blacks.
In the present study, both men and women were included (all Whites). Although the absolute impact varied, we have found associations between TG levels and rare alleles of both apoAV polymorphisms in both sexes. Finally, although plasma TGs are substantially changed after MI, we also suggest that there is an effect of the Ser/Trp19 apoAV polymorphism on TG levels in these patients.
The importance of using large representative selected studies is obvious if we compare these three studies with the study of Ribalta et al (14). They did not find any association between T/C-1131 polymorphism and TGs; however, they used preselected population samples with a low number of participants (89 and 408) with plasma TGs much lower (~0.9 mmol/L and ~1.3 mmol/L) than is normally observed in the populations.
We have failed to find any differences between MI patients and the population when single polymorphisms have been analyzed. Because neither polymorphism is in allelic association (Table 2), we performed haplotype analysis, which showed that the frequency of the rare homozygotes was significantly different when compared with the population. Analyses using two or more genotypes in these cases are not yet common but are necessary – coronary artery disease is surely a polygenic disease and more polymorphisms will participate. This result needs to be confirmed in a large study.
When compared with other plasma apolipoproteins, apoAV plasma levels are very low. Because the exact mechanism by which apoAV influences plasma levels of TGs is unknown, it is difficult to speculate in which way analyzed variants could influence the metabolism of TGs. Nevertheless, the T/C-1131 variant is in 100% linkage disequilibrium with another variant (A/G) localized just three base pairs before the start codon. Such a change could, for example, decrease the rate of expression of the apoAV gene, but no experiments have been done so far. Computational analysis of apoAV protein suggest that Ser/Trp19 changes could result in impaired export of apoAV from the liver (10).
Results from our large population cohort represent a confirmation of an association between the common polymorphisms T/C-1131 and Ser/Trp19 in the apoAV gene and plasma TGs in the general White male population. An analysis of 83 unrelated hypertriglyceridemic individuals suggested a strong association between both T-1131/C and Ser/Trp19 polymorphisms in the apoAV gene and extreme levels of plasma TGs. Additionally, both polymorphisms influenced plasma TGs significantly, not only in men but also in women. C/C-1131 and/or Trp/Trp19 homozygosity of the apoAV gene have been recognized as a potential risk factor for MI development.
Acknowledgments
This work was partly supported by Grant No. CEZ:L17/98:00023001 from the Grant Agency of the Czech Republic, by Grant No. NB/5986-3 from the Internal Grant Agency of the Ministry of Health of the Czech Republic, and by Grant No. LN00A069 from the Ministry of Education, Youth and Sport.
REFERENCES
- 1.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–9. [PubMed] [Google Scholar]
- 2.Austin MA, King MC, Bawol RD, et al. Risk factors for coronary heart disease in adult female twins. Genetic heritability and shared environmental influences. Am J Epidemiol. 1987;125:308–18. doi: 10.1093/oxfordjournals.aje.a114531. [DOI] [PubMed] [Google Scholar]
- 3.Gehrisch S. Common mutations of the lipoprotein lipase gene and their clinical significance. Curr Atheroscler Rep. 1999;1:70–8. doi: 10.1007/s11883-999-0052-4. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JC, Vega GL, Grundy SM. Hepatic lipase: New insights from genetic and metabolic studies. Curr Opin Lipidol. 1999;10:259–67. doi: 10.1097/00041433-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Talmud PJ, Humphries SE. Genetic polymorphisms, lipoproteins and coronary artery disease risk. Curr Opin Lipidol. 2001;12:405–9. doi: 10.1097/00041433-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Hubacek JA, Waterworth DM, Poledne R, et al. Genetic determination of plasma lipids and insulin in the Czech population. Clin Biochem. 2001;34:113–8. doi: 10.1016/s0009-9120(01)00184-9. [DOI] [PubMed] [Google Scholar]
- 7.Buzza M, Fripp Y, Mitchell RJ. Apolipoprotein AI and CIII gene polymorphisms and their association with lipid levels in Italian, Greek and Anglo-Irish populations of Australia. Ann Hum Biol. 2001;28:481–90. doi: 10.1080/03014460010019777. [DOI] [PubMed] [Google Scholar]
- 8.Russo GT, Meigs JB, Cupples LA, et al. Association of the Sst-I polymorphism at the APOC3 gene locus with variations in lipid levels, lipoprotein subclass profiles and coronary heart disease risk: The Framingham offspring study. Atherosclerosis. 2001;158:173–81. doi: 10.1016/s0021-9150(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 9.Pennachio LA, Olivier M, Hubacek JA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–73. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 10.Pennacchio LA, Rubin EM. Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler Thromb Vasc Biol. 2003;23:529–34. doi: 10.1161/01.ATV.0000054194.78240.45. [DOI] [PubMed] [Google Scholar]
- 11.Šeda O, Šedová L. New apolipoprotein A-V: Comparative genomics meets metabolism. Physiol Res. 2003;52:141–6. [PubMed] [Google Scholar]
- 12.Talmud PJ, Hawe E, Martin S, et al. Relative contribution of variation within the APOC3/A4/A5 gene cluster in determining plasma triglycerides. Hum Mol Genet. 2002;11:3039–46. doi: 10.1093/hmg/11.24.3039. [DOI] [PubMed] [Google Scholar]
- 13.Horínek A, Vráblík M, Ceška R, et al. T-1131 → C polymorphism within the apolipoprotein AV gene in hypertriglyceridemic individuals. Atherosclerosis. 2003;167:369–70. doi: 10.1016/s0021-9150(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 14.Ribalta J, Figuera L, Fernandez-Ballart J, et al. Newly identified apolipoprotein AV gene predisposes to high plasma triglycerides in familial combined hyperlipidemia. Clin Chem. 2002;48:1597–600. [PubMed] [Google Scholar]
- 15.Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC. Two independent apolipoprotein AV haplotypes influence human plasma triglyceride levels. Hum Mol Genet. 2002;11:3031–8. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 16.Vráblík M, Horínek A, Ceška R, et al. Ser19 → Trp polymorphism within the apolipoprotein AV gene in hypertriglyceridemic people. J Med Genet. 2003;40:e105. doi: 10.1136/jmg.40.8.e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases: “MONICA Project.” Manual of operations, WHO/MNC 82. 2 Nov. 1983.
- 18.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for DNA extraction from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubacek JA, Škodová Z, Adámková V, Lánská V, Poledne R.The influence of apoAV polymorphisms (T-1131>C and S19>W) on plasma triglyceride levels and risk of myocardial infarction Clin Genet 2004(In press) [DOI] [PubMed] [Google Scholar]
- 20.Day IN, Humphries SE. Electrophoresis for genotyping: Microtiter array diagonal gel electrophoresis on horizontal polyacrylamide gels, hydrolink, or agarose. Anal Biochem. 1994;222:389–95. doi: 10.1006/abio.1994.1507. [DOI] [PubMed] [Google Scholar]


