Cell-surface receptor mediated signaling is mechanistically complex. Hetero- and homo-multimerization of receptors appears to occur naturally and is a significant regulatory component of signal transduction.[1] Additionally, exogenous entities, such as cells and viruses, can interact with multiple heterologous receptors and induce clustering.[2] Such multivalent interactions are characterized by enhanced affinities (avidities) relative to monovalent binding and enhanced specificities with heteromultivalent interactions. For example, polymers containing α-MSH (α-melanocyte stimulating hormone) ligands bind with higher affinity to melanoma cells compared to monovalent α-MSH ligands.[3–5] Recapitulation of these natural phenomena using synthetic multivalent agents has been proposed for many years.[3–9] Although homomultivalent agents are known, there is little precedent for synthetic heteromultivalent targeting of cell-surface receptors. Herein we detail the synthesis and bioevaluation of heterobivalent ligands (htBVLs) targeted to two heterologous cell-surface receptors.
Bivalent ligands display two copies of ligands connected by linkers or attached to a scaffold. Linker structure is important because it must present ligands simultaneously to their cognate receptors with minimal entropic penalty. Although rigid linkers have low internal entropy, they may not be conformationally ideal.[10,11] Conversely, high flexibility may present a high conformational entropic cost. In addition, linkers must have good physicochemical properties (e.g. solubility, low nonspecific binding affinity) and low toxicity. Hence, we chose short flexible ethylene glycol (PEGO) spacers in combination with semirigid Pro-Gly (PG) repeats as linkers for bivalent ligands. Both elements fit well into a modular solid-phase synthesis scheme wherein ligands and linker elements are added systematically. The combination of PEGO and Pro-Gly linkers allows for linkers to be constructed with various lengths and rigidities. The Pro-Gly linker was a reasonable choice as it occurs naturally in scaffolds, such as collagen, which contains a repetitive Pro-Hyp-Gly motif, and in silk, which is characterized by Gly-Gly-X or Gly-Pro-Gly-X-X repeats.[12,13]
Recently, we have described the solid-phase synthesis and bioevaluation of homobivalent MSH ligands connected with short rigid phenyl, biphenyl, and terphenyl linkers,[10,11] with flexible tetraethylene glycol (PEGO) linkers,[7,14] and with Pro-Gly oligomers.[8] From this earlier work, we determined that binding affinities of bivalent ligands with short (<20Å) linkers were enhanced relative to monomers and that this was mainly due to increased “statistical” binding, that is, increased local concentration of ligand in close proximity to the same receptor, and not to receptor crosslinking. Modeling of GPCR (G-protein coupled receptor) dimers estimated the distance between two adjacent binding sites to be in the range of 25–50 Å and possibly longer, up to approximately 100 Å in the case of loosely packed receptors with associated lipids.[10] Similar results have been proposed by others.[6,15,16] Consistent with this, homobivalent α-MSH ligands connected by long (up to 100 Å) semirigid linkers bound with more than tenfold higher affinities compared to monovalent MSH(7).[8] Although these data suggest that homomultivalent agents can bind multivalently and simultaneously to more than one receptor, it is impossible to discriminate noncovalent cross-linking from statistical effects. Documentation of receptor crosslinking requires agents with two distinct binding moieties so that each receptor can be interrogated independently.
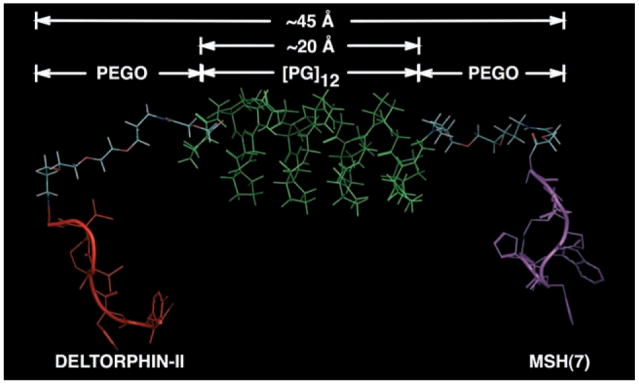
The linkers and ligands were modeled in the Macro-Model9.1 program using Monte Carlo conformational searches and Molecular Dynamic (MD) simulations (Figure 1). Initially, Ac-[Pro-Gly]n-NH2, where n = 3, 6, 9, or 12, were modeled for low-energy conformations using the Monte Carlo algorithm embedded in the MacroModel program. The study revealed a predominant helical structure in its low-energy conformations with the characteristic triangular shape (viewed down the radial axis) typical of Type II polyproline (PPII) helices. However, the poly(Pro-Gly) linker was found to be broader with a pitch of 5 Å per 7 residues in its lowest energy conformation. MD simulations showed that the linker retained a helical shape in the majority of the sampled population, although the pitch could extend up to 12 Å per turn. In addition, some partial random-coil structures were also observed in the populations at relatively higher energies. This situation suggests that the Pro-Gly repeats might have a population of low-to-medium energy conformers that could broaden the helical structure to reach longer distances per helical turn. Therefore, the Pro-Gly repeats were projected to be stabilized in a semirigid conformation. The secondary structure of the Ac-[PG]3-NH2 repeat was confirmed by circular dichroism (CD) spectroscopy (not shown). A negative band around 215 nm and a small positive band at 243 nm were observed, slightly red-shifted from the characteristic band pattern of a type II polyproline helix.[17] A similar analysis of the PEGO structure revealed the expected flexibility of polyethylene glycol-based linkers. The PEGO linker (20 atoms long) exhibited highly random conformations that uniformly covered a broad distance range of 4–18 Å. Based upon these models, the lengths spanned by the linkers were estimated and are shown in Table 1.
Figure 1.
Computational study of the heterobivalent ligand 7 (scheme 1; Delt-II-PEGO-[PG]12-PEGO-MSH(7)-NH2); Deltorphin-II ligand (Delt-II; red), MSH(7) ligand (magenta), linker composed of [Pro-Gly]12 (green) flanked by PEGO spacers. Only one of the conformations in the MD set with appropriate distance span of linker regions is shown.
Table 1.
Cumulative data for binding of heterobivalent ligands to MC4R- δ-OR dual expressing cells in the presence or absence of a heterologous blocking agent
| Compound | Linker | IC50 [nm] of MSH(7) b | IC50 [nm] of Delt-IIb | |||||
|---|---|---|---|---|---|---|---|---|
| Max. linker lengtha [Å] | hMCR4d | Dual expressione | Fold increasec | hδ-ORf | Dual expressiong | Fold increasec | ||
| 1 | -[PG]3- | ≤13 | 110 ± 9 | 180 ± 30 | 0.6 | 150 ± 8 | 110 ± 40 | 1.3 |
| 2 | -[PG]6- | ≤25 | 110 ± 330 | 39 ± 14 | 2.9 | 330 ± 110 | 240 ± 120 | 1.3 |
| 3 | -[PG]9- | ≤35 | 260 ± 160 | 9.7 ± 3.3 | 27 | 210 ± 130 | 100 ± 50 | 2.1 |
| 4 | -[PG]12- | ≤45 | 150 ± 70 | 3.1 ± 1.1 | 47 | 430 ± 200 | 300 ± 90 | 1.5 |
| 5 | -[PG]15- | ≤55 | 160 ± 50 | 3.3 ± 1.8 | 48 | 500 ± 90 | 230 ± 70 | 2.2 |
| 6 | -PEGO-[PG]6-PEGO- | ≤56 | 90 ± 40 | 2.1 ± 0.4 | 44 | 90 ± 40 | 70 ± 3 | 1.4 |
| 7 | -PEGO-[PG]12-PEGO- | ≤76 | 120 ± 60 | 2.5 ± 1.1 | 47 | 100 ± 30 | 290 ± 80 | 0.3 |
| 8 | -PEGO-[PG]18-PEGO- | ≤96 | 150 ± 40 | 3.5 ± 1.3 | 43 | 200 ± 90 | 110 ± 40 | 1.8 |
Maximal linker length estimated from modeling studies.
IC50 is a concentration of compound at 50% specific binding. Monomeric hexapeptide ligand Ac-MSH(7)-NH2 exhibits modest specific binding affinity to the hMC4R, IC50 = 39 ± 4.1 nM, monomeric Deltorphin-II showed IC50 = 0.42 ± 0.02 nM to the δ-OR.
Fold increase is the ratio of IC50 values between monovalent and bivalent binding modes. For each heterobivalent ligand, binding was assessed by four different competitive binding assays using the dual-receptor expressing cells.
hMC4R monovalent binding mode; at hMC4R alone by competing Eu-NDP-αMSH in the presence of a saturating concentration of 10 μM Naloxone, a δ-OR blocking agent.
hMC4R heterobivalent binding mode; at the hMC4R with both receptors available for binding by competing Eu-NDP-αMSH.
At the δ-OR alone using Eu-DPLCE and a saturating concentration of 10 μM NDP-αMSH, which blocks the hMC4R.
At the δ-OR with both receptors available using Eu-DPLCE.
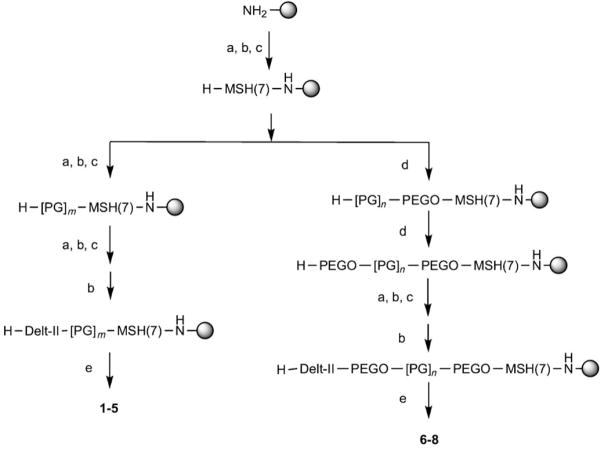
Heterobivalent ligands were constructed of MSH(7) and Delt-II binding moieties connected with PEGO-[Pro-Gly]x-PEGO and [Pro-Gly]x linkers to crosslink two different GPCRs: the melanocortin-4 (hMC4R) and δ-opioid (δ-OR) receptors. These ligands were prepared as a parallel series with linkers identical to those used in reported MSH homobivalent ligands.[8] Synthesis of ligand dimers 1–8, consisting of the MSH(7) ligand H-Ser-Nle-Glu-His-DPhe-Arg-Trp-NH2 at the C-terminal and the Deltorphin-II sequence, H-Tyr-DAla-Phe-Glu-Val-Val-Gly, at the N-terminal connected by PEGO and/or Pro-Gly linkers, is depicted in Scheme 1. Compounds 1–8 were purified by gel filtration, reverse-phase C18 preparative HPLC and were characterized by ESI-MS, MALDI-TOF, or FT-ICR mass spectrometry.
Scheme 1.
Solid-phase synthesis of heterobivalent ligands. a) Fmoc-AA-OH, HOBt, DIC; b) Fmoc-AA-OH, HBTU, lutidine; c) Piperidine in DMF; solid-phase cycle (i–iii); d) 1) Diglycolic anhydride in DMF, 2) CDI in DMF, 3) 4,7,10-trioxa-1,13-tridecanediamine in DMF; e) TFA-scavengers cocktail. Fmoc =(9H-fluoren-9-ylmethoxy)carbonyl, AA =amino acid, HOBt =1-hydroxy-1H-benzotriazole, DIC =N,N′-diisopropylcarbodiimide, HBTU =(1H-benzotriazol-1-yloxy)bis(dimethylamino) methylium hexafluorophosphate, CDI =N,N′-carbonyl-diimidazole.
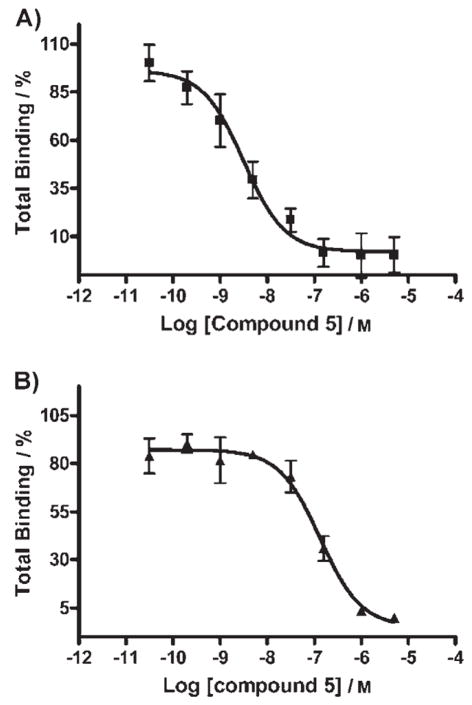
To evaluate binding affinities, we developed a cell system that expressed both the hMC4R and the δ-OR. Saturation studies using Europium-labeled ligands detected by time-resolved fluorescence[18] determined that the δ-OR and the hMC4R were present on the cell at a ratio of 6:1 (104000 ± 8600 δ-OR and 18000 ± 3300 MC4R per cell).[19] Using these cells, monovalent ligand binding to hMC4R was determined by a competitive binding assay for the heptapeptide Ac-MSH(7), revealing an IC50 = 39 ± 4.1 nM. Monovalent binding of Deltorphin-II to δ-OR was also determined with an IC50 = 0.42 ± 0.02 nM. Using the same cells, heterobivalent ligand binding was assessed by evaluating the ability of each ligand to compete with receptor-specific labeled ligand (Eu-NDP- αMSH for the hMC4R and Eu-DPLCE for the δ-OR) in the presence and absence of saturating concentrations of blocking agent for the heterologous receptor.[18,19] Representative competitive binding curves for compound 5, Delt-II-PEGO-[PG]15-PEGO-MSH(7)-NH2, are shown in Figure 2, and the complete calculated data set is presented in Table 1.
Figure 2.
Representative competitive binding curves for compound 5, Delt-II-PEGO-[PG]15-PEGO-MSH(7)-NH2. A) Compound 5 competed with 10 nM Eu-NDP-α-MSH in CHO/MC4R/δOR cells in the absence of 10 μM Naloxone, with an IC50 of 3.2 nM (R2 =0.83). B) Compound 5 competed with 10 nM Eu-NDP- α-MSH in CHO/MC4R/δOR cells in the presence of 10 μM Naloxone, with an IC50 of 134 nM (R2 =0.93).
In this series, compounds 4, 5, and 7 displayed the highest binding enhancement. Affinities were approximately 48-times higher when both receptors were available, compared to binding when only the hMC4R receptor was available, that is, in the presence of Naloxone, a potent δ-OR blocking agent. Compounds 1 and 2, connected with [Pro-Gly]3 and [Pro-Gly]6 linkers displayed no significant enhancements of binding affinity, presumably because the linkers are too short. With increasing linker length, the affinity enhancement increased significantly until a maximum of about 48-fold was reached for compounds 4, 5, and 7. Notably, the presence of the flexible PEGO did not appear to significantly affect the enhancement or affinity. This result is consistent with our earlier observations using homobivalent ligands.[8] For compounds bearing PEGO-[PG]n-PEGO linkers, maximal enhancement was observed for all compounds in this series, suggesting that there are degrees of freedom allowed in linker length. As predicted, when only a single receptor was available for binding, that is, when a saturating concentration of blocking agent against the other receptor is added, the binding affinities decreased with increasing ligand molecular weight. In hMC4R monovalent binding mode (Table 1 column 4), the shortest ligands 1, [Pro-Gly]3, and 6, PEGO-[Pro-Gly]6-PEGO exhibited higher affinities, IC50 = 113 nM and 90 nM respectively, compared to the high molecular weight compounds 5, [Pro-Gly]15, and 8, PEGO-[Pro-Gly]18-PEGO with IC50 = 160 nM and 150 nM, respectively. A similar tendency was observed in the δ-OR monovalent binding mode (hMC4R blocking agent is added). The binding affinities decreased as ligand molecular weight increased (for short linkers 1 and 6, IC50 = 150 nM and 90 nM respectively; and for long linkers 5 and 8, IC50 = 500 nM and 200 nM, respectively). This situation is in sharp contrast with what was observed for dual-receptor binding. Compound 5 exhibited an IC50 = 160 nM in hMC4R monovalent binding mode, while in dual-receptor binding an IC50 = 3.3 nM, a 48-fold enhancement. Alternatively, with δ-OR blocking agent present, hMC4R binding decreased 1.4-fold from compound 1 to 5 (IC50 = 110 nM, to IC50 = 160 nM), while dual receptor binding increased 55-fold (1, IC50 = 180 nM to 5, IC50 = 3.3 nM. Although considerable enhancement in binding was observed for these ligands at the hMC4R, no enhancement in binding to the δ-OR was observed in this system. In other words, once the low-abundance receptors were saturated by bivalent interactions, the remaining high-abundance receptors are only available to bind the ligands as monomers, which occur only weakly. This finding is in agreement with a mathematical model which predicts that the absolute number of receptors on the target cell is critical to achieving specificity and enhancement of binding affinity.[20]
In summary, a modeling study predicted that an optimal linker length of 20 to 50 Å would be required to span two well-packed GPCRs, and this hypothesis generally fits with the enhancement of binding affinities observed herein. Six out of eight heterobivalent ligands bound with greater affinity to hMC4R when both receptors were available for binding, compared to when only the hMC4R receptor was available for binding. Compounds 4 to 8 bind to the hMC4R in a dual-receptor assay with high binding affinity in the range of 2.5 to 3.5 nM IC50, which was approximately 15-times higher than binding observed for the parent monomer ligand (Ac-MSH(7)-NH2 IC50 = 39 nM). The distance between receptors spanned by active ligands in compounds 4, 5, and 7 was estimated to be 20–40, 25–50, and 28–76 Å respectively, and had a maximal enhancement of about a 48-fold increase in binding affinity to the less-abundant receptor (hMC4R) in a dual receptor assay. We have demonstrated actual cross-linking of multiple receptors using our heterobivalent ligand system and, to our knowledge, this is the first time this has been accomplished in a synthetic system.
Footnotes
We thank Ms. Lucinda Begay for technical assistance. The work was supported by grants R33 CA 95944 and RO1 CA 97360 from the National Cancer Institute and the National Institute of Health.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Yarden Y, Sliwkowski MX. Nat Rev Mol Cell Biol. 2001;2:127. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Mammen M, Choi SK, Whitesides GM. Angew Chem. 1998;110:2908. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2754. [Google Scholar]
- 3.Sharma SD, Jiang JW, Hadley ME, Bentley DL, Hruby VJ. Proc Natl Acad Sci USA. 1996;93:13715. doi: 10.1073/pnas.93.24.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma SD, Granberry ME, Jiang JW, Leong SP, Hadley ME, Hruby VJ. Bioconjugate Chem. 1994;5:591. doi: 10.1021/bc00030a015. [DOI] [PubMed] [Google Scholar]
- 5.Kessler H, Schudok M, Haupt A. Peptides. 1988:663. doi: 10.1111/j.1399-3011.1988.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 6.Kiessling LL, Gestwicki JE, Strong LE. Angew Chem. 2006;118:2408. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:2348. [Google Scholar]
- 7.Vagner J, Handl HL, Gillies RJ, Hruby VJ. Bioorg Med Chem Lett. 2004;14:211. doi: 10.1016/j.bmcl.2003.09.079. [DOI] [PubMed] [Google Scholar]
- 8.Handl HL, Sankaranarayanan R, Josan JS, Vagner J, Mash EA, Gillies RJ, Hruby VJ. Bioconjugate Chem. 2007;18:1101. doi: 10.1021/bc0603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handl HL, Vagner J, Han HY, Mash EA, Hruby VJ, Gillies RJ. Expert Opin Ther Targets. 2004;8:565. doi: 10.1517/14728222.8.6.565. [DOI] [PubMed] [Google Scholar]
- 10.Monguchi Y, Vagner J, Handl HL, Jana U, Begay LJ, Hruby VJ, Gillies RJ, Mash EA. Tetrahedron Lett. 2005;46:7589. [Google Scholar]
- 11.Vagner J, Handl HL, Monguchi Y, Jana U, Begay LJ, Mash EA, Hruby VJ, Gillies RJ. Bioconjugate Chem. 2006;17:1545. doi: 10.1021/bc060154p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timpl R. Eur J Biochem. 1989;180:487. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi CY, Lewis RV. Science. 2000;287:1477. doi: 10.1126/science.287.5457.1477. [DOI] [PubMed] [Google Scholar]
- 14.Bowen ME, Monguchi Y, Sankaranarayanan R, Vagner J, Begay LJ, Xu L, Jagadish B, Hruby VJ, Gillies RJ, Mash EA. J Org Chem. 2007;72:1675. doi: 10.1021/jo062276g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith BR, Allen BL, Rapraeger AC, Kiessling LL. J Am Chem Soc. 2004;126:1608. doi: 10.1021/ja037646m. [DOI] [PubMed] [Google Scholar]
- 16.Kiessling LL, Gestwicki JE, Strong LE. Curr Opin Chem Biol. 2000;4:696. doi: 10.1016/s1367-5931(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 17.Ladokhin AS, Selsted ME. Biochemistry. 1999;38:12313. doi: 10.1021/bi9907936. [DOI] [PubMed] [Google Scholar]
- 18.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Anal Biochem. 2004;330:242. doi: 10.1016/j.ab.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Anal Biochem. 2005;343:299. doi: 10.1016/j.ab.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Caplan MR, Rosca EV. Ann Biomed Eng. 2005;33:1113. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]