Abstract
Craving is one of the primary behavioral components of drug addiction, and cue-elicited craving is an especially powerful form of this construct. While cue-elicited craving and its underlying neurobiological mechanisms have been extensively studied with respect to alcohol and other drugs of abuse, the same cannot be said for marijuana. Cue-elicited craving for other drugs of abuse is associated with increased activity in a number of brain areas, particularly the reward pathway. This study used functional magnetic resonance imaging (fMRI) to examine cue-elicited craving for marijuana. Thirty-eight regular marijuana users abstained from use for 72 h and were presented with tactile marijuana-related and neutral cues while undergoing a fMRI scan. Several structures in the reward pathway, including the ventral tegmental area, thalamus, anterior cingulate, insula, and amygdala, demonstrated greater blood oxygen level dependent (BOLD) activation in response to the marijuana cue as compared with the neutral cue. These regions underlie motivated behavior and the attribution of incentive salience. Activation of the orbitofrontal cortex and nucleus accumbens was also positively correlated with problems related to marijuana use, such that greater BOLD activation was associated with greater number of items on a marijuana problem scale. Thus, cue-elicited craving for marijuana activates the reward neurocircuitry associated with the neuropathology of addiction, and the magnitude of activation of these structures is associated with severity of cannabis-related problems. These findings may inform the development of treatment strategies for cannabis dependence.
Keywords: cannabis, cue, fMRI, reward
The relationship between craving and drug use behavior is an integral piece of the addiction puzzle. Craving is considered the intense desire for a rewarding object or experience. Cue-elicited craving, induced by exposure to alcohol- or drug-related cues, is a particularly potent form of craving (1–3). Previous investigators have reported that subjective craving increases after exposure to cues specific to a variety of drugs of abuse, including cocaine (e.g., tactile cues, videos, i.v. administration, images, guided imagery) (4–9), heroin (e.g., images) (10, 11), alcohol (e.g., alcohol taste, images, alcohol-related words) (1, 12–17), and tobacco (e.g., visual and tactile presentations) (18, 19). Cue-elicited craving for alcohol and tobacco in particular have important clinical implications (e.g., refs. 20 and 21) and have been the focus of psychosocial and pharmacological intervention efforts (e.g., refs. 19 and 22–24).
The advent of functional neuroimaging has allowed studies of cue-elicited craving to elucidate the neurobiological mechanisms that accompany increased craving. Such neuroimaging studies have associated craving with increased activation of reward pathways (25–27). The reward circuits involve the dopamine projection from the ventral tegmental area (VTA) to striatal areas (e.g., nucleus accumbens) and the prefrontal cortex (PFC), the repeated activation of which underlies the attribution of incentive salience to otherwise neutral stimuli (28). Other reward-related areas, including the insula (29–31) and cingulate gyrus (8, 14, 15, 29, 32–36), show increased activity with the presentation of drug-related stimuli. Presentation of these stimuli is also associated with increased activity in brain structures that underlie reward and emotion regulation, such as the thalamus (9, 30, 37–40) and amygdala (32, 39).
The few published studies of cue-elicited craving for marijuana suggest that it is a reliable and valid phenomenon, analogous to cue-elicited craving for other drugs of abuse (e.g., refs. 41 and 42). Marijuana-related cues, presented in a variety of sensory modalities, elicit increases in self-reported craving. For example, auditory-presented imagery scripts induce craving in marijuana smokers, and the magnitude of this craving varies as a function of the amount of marijuana-related content presented in the script (43). Craving also increases when abstinent frequent marijuana users are exposed to an auditory script that is paired with a tactile cue, such as a used marijuana pipe or bong (41, 42). Importantly, in this paradigm, cue presentation increases craving beyond the effects induced by abstinence. Additionally, marijuana-related visual cues elicit greater craving in chronic heavy users than in controls; physiologically, users demonstrate greater skin conductance and larger late positivity of visual event-related brain potentials than controls in response to these stimuli (44).
The present study was designed to examine the effects of marijuana-related cues on the activation of reward circuitry, and to examine the relationship between these effects and the behavioral symptoms of cannabis dependence. We hypothesized that among regular marijuana users, marijuana-related cues compared with neutral cues, would elicit greater blood oxygen level dependent (BOLD) activity in reward structures (i.e., VTA, striatum, anterior cingulate, and insula). Furthermore, we hypothesized that the magnitude of this response would be associated with the number of problems related to marijuana use.
Results
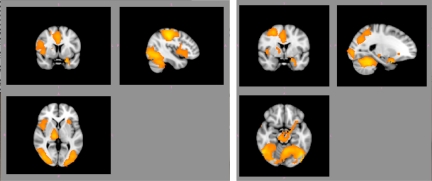
Compared with the neutral cue, presentation of the marijuana cue elicited significantly greater BOLD activation in a large cluster encompassing several areas, including the VTA, dorsal anterior cingulate cortex, cerebellum, thalamus, pre- and postcentral gyri, inferior frontal gyrus/insula, thalamus, amygdala, fusiform gyrus, pre- and postcentral gyri, inferior parietal lobe, and superior temporal gyrus (cluster-corrected z > 2.3, P < 0.05) (see Fig. 1 and Table 1).
Fig. 1.
Greater activation in several areas of interest during marijuana cues compared with neutral cues. There were significantly greater BOLD response to the marijuana pipe in reward areas such as the dorsal ACC, insula, thalamus, ventral tegmental area, and amygdala (cluster-corrected z > 2.3, P < 0.05). Right hemispheric activations are illustrated on the right side of the image.
Table 1.
Clusters of activation during marijuana cue vs. control cue contrast
| Cluster size | Anatomy | BA | Z | x | y | z |
|---|---|---|---|---|---|---|
| Cluster-corrected z > 2.3, P < 0.05 | ||||||
| 32,426 voxels | R postcentral gyrus | 2 | 6.44 | 46 | −28 | 50 |
| 2 | 5.99 | 38 | −26 | 42 | ||
| L fusiform gyrus | 19 | 5.98 | −44 | −70 | −12 | |
| L cerebellum | — | 5.86 | −28 | −52 | −28 | |
| R precentral gyrus | 3 | 5.7 | 36 | −20 | 50 | |
| L inferior parietal lobule | 40 | 5.69 | −48 | −36 | 42 | |
| 2,064 voxels | R inferior frontal gyrus | 44 | 4.03 | 54 | 14 | 16 |
| 46 | 2.87 | 54 | 28 | 16 | ||
| 46 | 2.51 | 58 | 32 | 12 | ||
| R insula | 13 | 3.43 | 44 | 6 | 2 | |
| R lateral orbitofrontal cortex | 47 | 3.34 | 46 | 20 | −10 | |
| R superior temporal gyrus | 38 | 2.51 | 58 | 10 | −14 | |
| Cluster-corrected z > 2.81, P < 0.05 | ||||||
| 6,194 voxels | L fusiform gyrus | 19 | 5.98 | −44 | −70 | −12 |
| L cerebellum | — | 5.86 | −28 | −52 | −28 | |
| L cerebellum | — | 4.82 | −4 | −58 | −18 | |
| — | 4.63 | 0 | −66 | −34 | ||
| — | 4.6 | −2 | −64 | −24 | ||
| L inferior occipital gyrus | 19 | 5.47 | −44 | −80 | −8 | |
| 6,143 voxels | R postcentral gyrus | 2 | 6.44 | 46 | −28 | 50 |
| 2 | 5.99 | 38 | −26 | 42 | ||
| R precentral gyrus | 4 | 5.7 | 36 | −20 | 50 | |
| R parietal lobe | 40 | 5.26 | 30 | −48 | 56 | |
| R middle frontal gyrus | 6 | 5.03 | 28 | −6 | 54 | |
| R inferior parietal lobule | 40 | 4.89 | 34 | −42 | 48 | |
| 4,960 voxels | R middle temporal gyrus | 37 | 5.58 | 52 | −58 | −14 |
| R cerebellum | — | 4.99 | 34 | −36 | −30 | |
| — | 4.94 | 32 | −46 | −26 | ||
| R middle occipital gyrus | 19 | 4.82 | 40 | −80 | −2 | |
| 18 | 4.76 | 34 | −84 | −4 | ||
| 18 | 4.72 | 30 | −86 | −4 | ||
| 1,887 voxels | L inferior parietal lobule | 40 | 5.69 | −48 | −36 | 42 |
| 40 | 4.89 | −36 | −42 | 40 | ||
| 40 | 4.6 | −34 | −46 | 44 | ||
| L precuneus | 7 | 3.9 | −28 | −54 | 54 | |
| 7 | 3.44 | −14 | −62 | 54 | ||
| 7 | 3.43 | −20 | −56 | 50 | ||
| 1,661 voxels | R thalamus | — | 4.8 | 14 | −20 | 4 |
| R ventral tegmental area | — | 4.61 | 10 | −24 | −10 | |
| L amygdala | 28 | 4.36 | −24 | 6 | −26 | |
| 34 | 4.14 | −12 | −6 | −16 | ||
| 34 | 3.94 | −16 | −2 | −20 | ||
| L subthalamic nucleus | — | 4.33 | −14 | −14 | −10 | |
| 1,338 voxels | Dorsal anterior cingulate gyrus | 32 | 4.31 | 2 | 12 | 44 |
| 24 | 4.12 | −2 | 12 | 30 | ||
| 24 | 4.09 | 2 | 12 | 28 | ||
| 546 voxels | R inferior frontal gyrus | 44 | 4.03 | 54 | 14 | 16 |
Significant clusters of activation are listed in descending order of cluster size based on the significance threshold of cluster-corrected z > 2.3, P < 0.05. For description purposes, the peak activations when this threshold is increased to cluster-corrected z > 2.81, P < 0.05, which breaks the large clusters into smaller clusters, are also reported. Clusters are described in terms of all local maxima within each cluster (in descending order of z-scores) with corresponding z-scores, Talairach coordinates and Brodmann areas. L = left; R = right; BA = Brodmann's area.
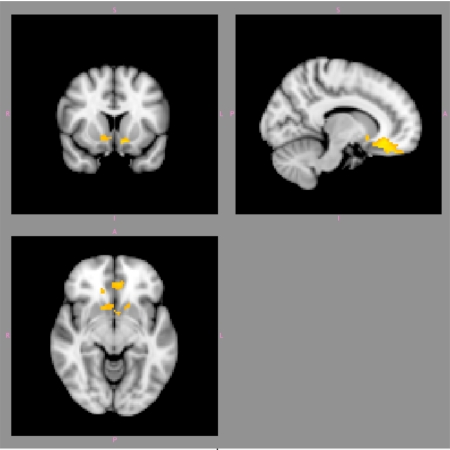
BOLD response in several of these differentially activated areas was also significantly positively correlated with total marijuana problem scale (MPS) score (cluster-corrected z > 2.3, P < 0.05). These areas included the orbitofrontal cortex (OFC) and nucleus accumbens (NAc) (see Fig. 2 and Table 2). The analyses of correlations with the Structured Clinical Interview for DSM Disorders (SCID) total symptom count, subjective urge ratings, frequency, and duration of use did not meet the significance threshold.
Fig. 2.
Significantly positive areas of correlation between BOLD response to marijuana cues (vs. neutral cues) and total MPS score in the orbitofrontal cortex and nucleus accumbens (cluster-corrected z > 2.3, P < 0.05). Right hemispheric activations are illustrated on the right side of the image.
Table 2.
Local maxima of significant cluster of activation during correlation of BOLD response to marijuana cues (vs. neutral cues) and total MPS score (cluster-corrected z > 2.3, P = 0.05)
| Anatomy | BA | Z | x | y | z |
|---|---|---|---|---|---|
| L medial orbitofrontal cortex | 47 | 3.58 | −16 | 18 | −14 |
| 11 | 3.44 | −14 | 44 | −20 | |
| R medial orbitofrontal cortex | 47 | 3.43 | 14 | 30 | −18 |
| 11 | 3.35 | 4 | 34 | −22 | |
| 11 | 3.33 | 8 | 40 | −24 | |
| R anterior cingulate | 25 | 3.32 | 12 | 32 | −14 |
Cluster size = 1,225 voxels.
Discussion
The overarching goal of the present study was to characterize the neural mechanisms that underlie cue-elicited craving in marijuana users. We hypothesized that similar to other drugs of abuse, the effects of marijuana cues on the brain involve reward areas. Our findings confirm our hypothesis and suggest that marijuana tactile cues elicit increased activation in reward-related areas of the brain in 3-day abstinent marijuana users. These findings are in concordance with the addiction literature of enhanced activation of reward areas in response to drug-related cues (1, 33, 45) and gambling (46) and do not suggest a unique mechanism for marijuana cue-elicited craving. Greater activity in the inferior frontal gyrus/insula in response to marijuana cues indicates increased motivation in the presence of the cue (e.g., refs. 47 and 48), greater activity in the dorsal anterior cingulate cortex (ACC) is inline with reward-based cognitive processes (49) and greater activity in the amygdala reflect increased emotional processing of sensory stimuli (e.g., ref. 50). Interpretations of enhanced response in these areas have been suggested in the literature. One hypothesis is that these alterations may result from the diminished ability of the PFC to process and appropriately respond to information identified as important by neurotransmission from the reward pathways (51). Nevertheless, the present findings provide evidence for similar patterns of neural response to marijuana cues as alcohol and other drug cues (e.g., cocaine and/or nicotine) (1, 29, 51).
Our findings also indicate that greater activation in reward areas such as the OFC and NAc is associated with greater number of problems related to cannabis use. Increased activation of these areas during cue-elicited craving paradigms has been associated with a greater likelihood of subsequent relapse after treatment in alcohol- and cocaine-dependent patients (8, 52). Many pharmacological treatments for addiction aim, with varying degrees of success, to reduce craving during abstinence [e.g., naltrexone (53), acamprosate (54), and topiramate (55)]. In light of this, future treatment development for cannabis dependence might assess cue-elicited brain activation at baseline as an indicator of relapse potential, or changes in activation after treatment as a marker of treatment efficacy. If cue-elicited brain activation is also related to broader symptoms of addiction (e.g., impaired control over drug use), future treatment development may be well served to focus on mechanisms beyond craving that subserve the maintenance of addiction.
Similar to other reports (e.g., refs. 1, 14, 51, and 56), we did not find significant correlations between subjective urge ratings and the BOLD response despite the fact that urge ratings during marijuana presentations were greater than those during neutral presentations. Associations between subjective craving ratings and neural response have been inconsistent in the literature. For example, others have reported significant correlations between craving and activation (29). A possible explanation for the disparity may be differences in paradigms and processes captured by the design model. For instance, it is possible that because we captured the BOLD response to the cue over a 20-second period that we may be modeling multiple processes beyond the preconscious and conscious processes of craving that may be watering down the effect. It is also possible, as others have suggested, that subjective measures are prone to error, such as social desirability (57, 58). For example, other studies and anecdotal evidence from patients suggest that the subjective experience of craving persists long after the presentation of a cue. Thus, the time course of the subjective experience and time course of the effects of reward structures are clearly different and it is counterintuitive that the 2 measures, collected with the same time course, would be significantly related. There was also an absence of an association between brain activation and total SCID symptom count, frequency, and duration of use, which may suggest that this effect is stable.
Interpretation of these findings must take into account some methodological limitations of the study. First, although the cues were consistent across participants (i.e., pipe and pencil), the participants had a wide-ranging modality of marijuana use besides our chosen cue of a marijuana pipe and, of the sample, 54% preferred the pipe as their primary mode of use. However, despite this, our primary finding of greater activation in reward areas of the brain during marijuana cue exposure compared with a neutral cue exposure was robust. Withholding possible confounding effects of using different cues per participant (i.e., associated motion, etc.), it is possible that these effects would be stronger if participant-specific cues (e.g., bong, joint, etc.) were used and should be a topic for future studies. Another caveat is the lack of a control group. However, the significantly positive correlation between functional activation and marijuana-related problems as measured by total MPS score would suggest that these findings are specific to marijuana use. Another possible caveat is that the data were collected at the end of the imaging session, which could potentially confound the findings (e.g., fatigue). However, because the task is not effortful (i.e., not a cognitively demanding task), we believe that any effects are minimal. Additionally, since the task order was consistent across all participants, and given our significant findings, we do not believe that this is a major concern. Last, we did not verify abstinence quantitatively via urine tetrahydrocannabinol (THC) metabolites. Thus, while we can say that the pattern of activation found is associated with exposure to tactile cues, we cannot presume that this response is induced by abstinence. It should be noted, however, that a study by McClernon et al. (59) in smokers, reported that brain response to cue-elicited craving is stable after short-term abstinence. Given this report, we can speculate that even if we cannot attest to our participants' abstinence, our findings would not have been different if abstinence was quantitatively verified. Regardless, these findings are consistent with studies on abstinent substance abusers (1).
To conclude, the current findings are particularly significant given the limited study of cannabis self-administration, and hence craving, in animals. These findings suggest that (i) combined marijuana visual and tactile cues elicit increased activation in reward-related brain areas in 3-day abstinent marijuana users; (ii) cue-elicited craving for marijuana is subserved by neural activation that is similar to the activation associated with such craving for drugs of abuse, suggesting the possibility of a common pathway for addiction amenable to pharmacological manipulation; and (iii) cue-elicited neural activity is related to the behavioral problems related to cannabis use, suggesting that future interventions for this disorder that target these broader may demonstrate improved efficacy over current treatment approaches.
Materials and Methods
Participants.
Thirty-eight self-reported regular marijuana users were recruited through flyers and media advertisement in the Albuquerque, NM metro area and provided informed consent to participate in this study. Of the total sample, 13 did not have marijuana problem scale data and were, therefore, excluded from the analysis that included this scale. Table 3 describes the characteristics of both the total sample and subsample. All participants were right-handed and free of MRI contraindications (i.e., no metallic implants, claustrophobia, pregnancy, etc.). Participants were compensated for their participation. The University of New Mexico Human Research Review Committee approved all procedures used. It should be noted that, to date, effect sizes of marijuana tactile cues on the BOLD response are still unknown. However, the available neuroimaging literature on the use of tactile cues in eliciting craving has been reported in a sample size of 13 in a PET study of cocaine (4). Thus, a sample size of 38 should be sufficient for the current study.
Table 3.
Characteristics of the participants included in this study
| Marijuana cues (N = 38) | MPS scores (N = 25) | |
|---|---|---|
| Age (mean ± SD) | 23.74 ± 7.25 Range = 18–46 | 22.04 ± 5.63 Range = 18–46 |
| Male (n, %) | 31, 81 | 21, 84 |
| Age when first used MJ regularly (mean ± SD) | 17.08 ± 2.3 Range = 9–22 | 17.02 ± 2.5 Range = 9–22 |
| Duration of regular MJ use in years (mean ± SD) | 7 ± 7 Range = 0.17–27 | 5.6 ± 5.37 Range = 1–23 |
| Frequency of MJ use in days per week (mean ± SD) | 6 ± 1 Range = 3–7 | 5.87 ± 1.39 Range = 3–7 |
| Frequency of MJ use per day (mean ± SD) | 3 ± 2 Range = 1–10 | 3.25 ± 2.12 Range = 1–10 |
| SCID MJ dependence (n, %) | 31, 81 | 19, 76 |
| SCID MJ abuse (n, %) | 3, 7.9 | 3, 12 |
| SCID total symptom count (total possible = 11)(mean ± SD) | 3 ± 2 Range = 0–7 | 3.28 ± 1.84 Range = 0–7 |
| Urge rating for MJ (averaged across neutral trials; total possible = 10)(mean ± SD) | 2.9 ± 2.4 Range = 0–7 | 2.43 ± 2.57 Range = 0–7.5 |
| Urge rating for MJ (averaged across marijuana trials; total possible = 10)(mean ± SD) | 4.5 ± 2.9 Range = 0–9 | 4.32 ± 2.83 Range = 0–9 |
| Marijuana problem scale (mean ± SD; total possible = 9) | — | 3.02 ± 2.37 Range = 0–9 |
This table summarizes the demographic and marijuana use characteristics of the sample included in the analyses of the main effects of marijuana cues (N = 38) and of the correlation with MPS scores (N = 25); MJ = marijuana.
Procedure.
Participants were required to be right-handed, between 18 and 50 years of age, English-speaking, and to report using marijuana at least 4 times per week over the previous 6 months. Participants were also required to be willing to abstain from marijuana use for 3 days.
Participants who met these inclusion criteria were invited to participate in the study, which took place in 2 sessions. During the first session, participants provided a saliva sample for DNA analysis, a urine sample for toxicological analysis, and completed baseline measures of marijuana use and craving. A trained research assistant administered the Substance Use Disorders and Psychotic Symptoms modules of the SCID research version IV (60). Participants were then asked to return for a second session and were instructed to abstain from marijuana use for 72 h before their appointment. Although toxicological analysis was not sufficiently sensitive to detect abstinence-induced changes in urine levels of THC metabolites, bogus pipeline procedures have demonstrated efficacy in increasing the accuracy of self-reports of drug use (e.g., ref. 61). During the second session, participants completed a battery of neuropsychological tests and self-report measures of mood and craving. Participants were then placed in the fMRI scanner. After collecting a high-resolution anatomical scan for registration and localization of the fMRI data, participants completed 2 cognitive tasks. Participants were then administered a cue-elicited craving paradigm, described below. The cue-elicited craving paradigm was the last task completed during a 105 m scanning session.
MRI images were collected using a 3T Siemens Trio. fMRI scans were collected using a gradient echo, echo-planar sequence with ramp sampling correction using the intercommissural line (AC-PC) as a reference (TR: 2.0 s, TE: 27 ms (39 ms for 1.5 T), α: 70°, matrix size: 64 × 64, 32 slices, voxel size: 3 × 3 × 4 mm3). A tilting acquisition was applied during the echo-planar imaging (EPI) sequence to compensate for the problems of B0 field spatial distortion in the OFC. Slices were acquired higher than the AC-PC, approximately perpendicular to the sinuses (62, 63). The high resolution anatomical MRI scan was collected with a multiecho MPRAGE (MEMPR) sequence with the following parameters: TR/TE/TI = 2300/2.74/900 ms, flip angle = 8°, FOV = 256 × 256 mm, slab thickness = 176 mm, matrix = 256 × 256 × 176, voxel size = 1 × 1 × 1 mm, number of echoes = 4, pixel bandwidth = 650 Hz, and total scan time = 6 min.
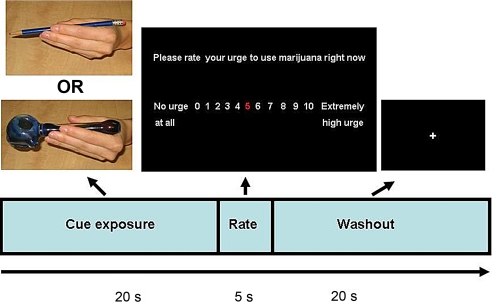
To test the main effect of marijuana cues on brain activation, we recorded the BOLD response while participants were presented with a marijuana cue-exposure paradigm. The paradigm was presented in 2 separate EPI runs of 12 pseudorandom tactile presentations of a marijuana pipe (marijuana cue × 6 trials) and a pencil (control cue × 6 trials). Each trial consisted of a 20-s cue exposure period, followed by a single 5-s urge question, and ended with a 20-s washout period to allow the hemodynamic response to return to baseline before the next trial (see Fig. 3). The total number of repetitions per run was 288 and the total task duration was 19 min and 12 s. The task was presented using a front projection to a mirror system mounted on the head coil. Responses were recorded using a fiber-optic pad. Stimulus presentations were delivered by using E-Prime (Psychology Software Tools, Inc.). The timing of the stimulus presentation was synchronized with trigger pulses from the magnet to ensure precise temporal integration of stimulus presentation and fMRI data acquisition.
Fig. 3.
Schematic of a single trial. During the exposure period, an experimenter handed the item (pipe or pencil) to the participant's left hand. The participants were explicitly instructed to hold the item as they would normally, but also so they were able to view both the item and their hand in the mirror system throughout the entire period. After the exposure period, a 5-second urge rating period followed during which participants were asked to respond on a scale of 0–10 via right-handed button press to the statement, “Please rate your level of urge to use marijuana right now.” The trial ended with a washout period during which the experimenter took the item away from the participant. The visual presentations and timing of each event (in seconds) are illustrated.
Analyses.
Preprocessing of fMRI data followed a standard procedure. First, all slices were interpolated to a common time point (i.e., slice-time correction) to correct for differences in slice acquisition. The images were realigned using INRIalign, a motion correction algorithm unbiased by local signal changes (64, 65). Five participants who had translational movement >2 mm were excluded from further analyses. Next, using FEAT (fMRI Expert Analysis Tool, v5.98), part of FSL (fMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl/), the following prestatistics processing was performed: nonbrain tissue/skull removal by using BET (Brain Extraction Tool); spatial smoothing using a Gaussian kernel of FWHM 8 mm3; mean-based intensity normalization of all volumes by the same factor; and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Time-series statistical analysis was carried out by using FILM (FMRIB's Improved Linear Model) with local autocorrelation correction. The first 7 volumes of all EPI runs were discarded to allow the MR signal to reach steady state.
Explanatory variables were created by convolving the stimulus timing files with a double gamma hemodynamic response function in FEAT. A multiple linear regression analysis was performed to estimate the hemodynamic parameters for different explanatory variables (i.e., active condition for marijuana cues, active condition for neutral cues) and a corresponding t-statistic indicates the significance of the activation of the stimulus. Contrast maps were created by contrasting marijuana active conditions vs. neutral active conditions. These maps were then registered to a high-resolution image using FLIRT (FMRIB's Linear Image Registration Tool) (66, 67). Group analyses were carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) (68, 69). Statistical maps were then registered to the Montreal Neurological Institute (MNI) template with a 2-step process. First, EPI images were registered to the high resolution MPRAGE image, which was subsequently registered to the 152 brain average MNI template. These registration steps were performed using FLIRT. After transformation of the masks into MNI space, higher-level analysis was carried out using FLAME. Z (Gaussianised T/F) statistic images were threshold by using GRF-theory-based maximum height threshold with a significance threshold of one-tailed P < 0.05 and cluster-corrected at z > 2.3. Peak loci of activation were obtained using MRI3dX v5.5 and anatomical localization was confirmed by the Talairach Daemon Database and verified by the Talairach and Tournoux brain atlas.
The effects of behavior measures of marijuana use on brain activation were also examined by adding these measures as additional covariates. We correlated behavior measures such as subjective urge data (averaged across the marijuana cue trials), SCID symptom count, and total marijuana-related problems derived from the MPS. We used the SCID research version IV (60) and counted as present any symptom that was rated as “3”, and summed across symptoms for cannabis dependence. Range of count was then 0–11. We also examined other facets of cannabis use not captured by the SCID items such as typical use per day, age of onset of regular marijuana use, and duration of regular use (in years). The MPS (70) is a 19-item measure that assesses the negative psychological, social, occupational, and legal consequences of marijuana use in the last 90 days (e.g., problems with family and significant others, missing work or losing a job, feeling bad about marijuana use). Each problem is rated from 0 (“no problem”) to 2 (“serious problem”), and the number of items endorsed as 1 or 2 is summed to create an index of the total number of problems (range = 0–19). Treatment-seeking marijuana users report an average of 9–10 problems (70, 71).
Acknowledgments.
We thank Michael Doty for his support during the paradigm development, Dr. Vincent Calhoun for development of the tilting acquisition, Jill Fries for the automated preprocessing of the data, and MRI technologists, Diana South and Cathy Smith, for their assistance during the data collection. This research was funded by the National Institute on Drug Abuse/National Institutes of Health Grants KO1 5 KO1 DA021632–02 (to FMF) and F31 DA021496 (to JPS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Filbey FM, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong DF, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 3.McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology (Berl) 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- 4.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wexler BE, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 6.Risinger RC, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Kosten TR, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 8.Sinha R, et al. Neural activity associated with stress-induced cocaine craving: A functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 9.Duncan E, et al. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Z, et al. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug Alcohol Depend. 2006;83:157–162. doi: 10.1016/j.drugalcdep.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2008;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison KE, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- 13.George MS, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 14.Heinz A, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 15.Myrick H, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 16.Wrase J, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Kunz S, Beblo T, Driessen M, Woermann F. fMRI of alcohol craving after individual cues: A follow-up case report. Neurocase. 2008;14:343–346. doi: 10.1080/13554790802366020. [DOI] [PubMed] [Google Scholar]
- 18.Tiffany ST, Carter BL, Singleton EG. Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction. 2000;95(Suppl 2):S177–S187. doi: 10.1080/09652140050111753. [DOI] [PubMed] [Google Scholar]
- 19.Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp Clin Psychopharmacol. 1999;7:250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- 20.Niaura RS, et al. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 21.Niaura R. Cognitive social learning and related perspectives on drug craving. Addiction. 2000;95(Suppl 2):S155–S163. doi: 10.1080/09652140050111726. [DOI] [PubMed] [Google Scholar]
- 22.Monti PM, et al. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- 23.Shiffman S, Khayrallah M, Nowak R. Efficacy of the nicotine patch for relief of craving and withdrawal 7–10 weeks after cessation. Nicotine Tob Res. 2000;2:371–378. doi: 10.1080/713688158. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman S, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- 25.Wise RA. The neurobiology of craving: Implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97:118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 27.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 28.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 29.Garavan H, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 30.Hommer DW. Functional imaging of craving. Alcohol Res Health. 1999;23:187–196. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GJ, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 32.Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 34.Smolka MN, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- 35.Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 38.Hermann D, et al. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 39.Franklin TR, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 40.McClernon FJ, et al. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: Results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- 41.Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology (Berl) 2009;203:511–517. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singleton EG, Trotman AJ, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the Marijuana Craving Questionnaire using imagery scripts. Exp Clin Psychopharmacol. 2002;10:47–53. doi: 10.1037//1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- 44.Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- 45.Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 46.Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26:423–428. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 48.O'Doherty J. Can't learn without you: Predictive value coding in orbitofrontal cortex requires the basolateral amygdala. Neuron. 2003;39:731–733. doi: 10.1016/s0896-6273(03)00525-7. [DOI] [PubMed] [Google Scholar]
- 49.Bush G, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 51.David SP, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grusser SM, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 53.Ray LA, et al. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: A pilot study. Psychopharmacology (Berl) 2007;193:449–456. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- 54.Richardson K, et al. Do acamprosate or naltrexone have an effect on daily drinking by reducing craving for alcohol? Addiction. 2008;103:953–959. doi: 10.1111/j.1360-0443.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- 55.Miranda R, Jr, et al. Effects of topiramate on urge to drink and the subjective effects of alcohol: A preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 56.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 57.Kimberlin CL, Winterstein AG. Validity and reliability of measurement instruments used in research. Am J Health Syst Pharm. 2008;65:2276–2284. doi: 10.2146/ajhp070364. [DOI] [PubMed] [Google Scholar]
- 58.Embree BG, Whitehead PC. Validity and reliability of self-reported drinking behavior: Dealing with the problem of response bias. J Stud Alcohol. 1993;54:334–344. doi: 10.15288/jsa.1993.54.334. [DOI] [PubMed] [Google Scholar]
- 59.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 61.Lowe JB, Windsor RA, Adams B, Morris J, Reese Y. Use of a bogus pipeline method to increase accuracy of self-reported alcohol consumption among pregnant women. J Stud Alcohol. 1986;47:173–175. doi: 10.15288/jsa.1986.47.173. [DOI] [PubMed] [Google Scholar]
- 62.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19(2 Pt 1):430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 63.Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R. Optimized EPI for fMRI studies of the orbitofrontal cortex: Compensation of susceptibility-induced gradients in the readout direction. Magma. 2007;20:39–49. doi: 10.1007/s10334-006-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. NeuroImage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- 65.Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- 66.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 67.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 68.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 69.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 70.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- 71.Stephens RS, Babor TF, Kadden R, Miller M. The Marijuana Treatment Project: Rationale, design and participant characteristics. Addiction. 2002;97(Suppl 1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]