Abstract
Spinal motor neurons are specified to innervate different muscle targets through combinatorial programs of transcription factor expression. Whether transcriptional programs also establish finer aspects of motor neuron subtype identity, notably the prominent functional distinction between alpha and gamma motor neurons, remains unclear. In this study, we identify DNA binding proteins with complementary expression profiles in alpha and gamma motor neurons, providing evidence for molecular distinctions in these two motor neuron subtypes. The transcription factor Err3 is expressed at high levels in gamma but not alpha motor neurons, whereas the neuronal DNA binding protein NeuN marks alpha but not gamma motor neurons. Signals from muscle spindles are needed to support the differentiation of Err3on/NeuNoff presumptive gamma motor neurons, whereas direct proprioceptive sensory input to a motor neuron pool is apparently dispensable. Together, these findings provide evidence that transcriptional programs define functionally distinct motor neuron subpopulations, even within anatomically defined motor pools.
Keywords: motor neuron, spinal cord, transcription factors
Neuronal diversity underlies many features of central nervous system (CNS) organization and function. Neurons located within different regions of the CNS typically exhibit distinct morphologies and patterns of connectivity that help to determine their physiological functions. Within a single region, neurons that serve closely related functions can be further subdivided, both anatomically and physiologically. The retina, for example, contains multiple subclasses of ganglion and amacrine neurons that are distinguishable by position, patterns of dendritic arborization, and their role in visual processing (1, 2). Similarly, the cerebral cortex contains many local circuit interneurons, each with specialized anatomy, circuitry, and physiology (3). Little is known, however, about how such fine distinctions in CNS neuronal subtype identity and connectivity are assigned.
The spinal cord represents a region of the CNS where the diversity of neuronal subtypes has been shown to emerge as a consequence of the expression of intrinsic molecular determinants, acting in a hierarchical manner to assign subtype identities to a generic set of motor neurons (4, 5). The motor neurons that project to skeletal muscle targets can be subdivided into distinct columnar subgroups, each projecting to a different target domain—axial, body wall, and limb targets. The lateral motor column (LMC) neurons that project their axons to limb muscles can be further subdivided into divisional and pool subclasses that, together, specify the pattern of target muscle connectivity (4, 5). The sequential steps involved in controlling motor neuron subtype identities and target projections are programmed through the cell-type selectivity of transcription factor expression, notably members of the Hox, LIM, Nkx6, and ETS families (6–10). Thus combinatorial programs of transcription factor expression appear to provide the fundamental logic of spinal motor neuron diversification and connectivity to specific peripheral muscle targets.
Yet neurons within a single motor pool also exhibit further subtype distinctions. Most motor pools are composed of a mixture of fast and slow motor neurons (11)—two classes that exhibit distinct profiles of activation, produce different degrees of force during the process of muscle contraction (11), and show distinct vulnerabilities in motor neuron disease (12). Arguably the most prominent distinction between neurons within a motor pool, however, is the presence of alpha and gamma motor neurons. Alpha and gamma motor neurons differ in morphology, as well as in their peripheral and central patterns of connectivity (11). Alpha motor neurons predominate within motor pools and innervate force-generating extrafusal muscle fibers at neuromuscular junctions (13). Gamma motor neurons constitute approximately one third of all motor neurons within a pool and innervate the intrafusal muscle fibers found in muscle spindles, where they modulate the sensitivity of muscle spindles to stretch (13–16). Gamma and alpha motor neurons also differ profoundly with respect to their soma size and connectivity profile within the spinal cord. Alpha motor neurons have large cell bodies, and most receive direct group Ia–derived proprioceptive sensory input (17), whereas gamma motor neurons have small cell bodies (13) and lack direct input from proprioceptive sensory afferents (18). Because almost every motor neuron pool includes gamma and alpha motor neurons, subtype diversification at this intrapool level is conceptually different from the broad target–based distinctions that correlate with motor neuron pools.
The fundamental distinctions in alpha and gamma motor neuron connectivity and function pose the question of how this finer, intrapool, aspect of motor neuron specification is programmed. Can the principle of transcriptional specification of motor neuron subtype be extended to the distinction between gamma and alpha motor neurons, features that are independent of the identity of the target muscle group? If so, do gamma and alpha motor neurons each possess defining molecular markers, or do gamma motor neurons simply lack certain of the genes that define alpha motor neuron identity, and vice versa? How do gamma and alpha motor neurons acquire their diverse anatomical and functional properties—through cell-intrinsic programs, through the influence of peripheral signals, or by virtue of their central connectivity? Intriguingly, the muscle fiber targets of gamma and alpha motor neurons can be distinguished by transcription factor expression; the zinc-finger transcription factor Egr3 and the ETS transcription factors Pea3 and Er81 are expressed selectively by intrafusal muscle fibers (19–21). Defining molecular markers that distinguish gamma and alpha motor neurons would provide a first step in addressing the developmental specification of intrapool motor neuron subtypes.
In this study, we set out to determine whether gamma and alpha motor neurons in the spinal cord of the mouse are distinguishable on the basis of their profile of expression of transcription factors and other molecular markers. We uncovered two genes with complementary expression profiles in gamma and alpha motor neurons. Gamma motor neurons express high levels of the orphan nuclear hormone receptor Err3 (22, 23) and lack expression of neuronal DNA binding protein NeuN (24). Conversely, alpha motor neurons are characterized by low or negligible levels of Err3 and high-level NeuN expression. Mice deficient in muscle spindle differentiation exhibit a selective absence of Err3on/NeuNoff presumptive gamma motor neurons. These findings establish that gamma and alpha motor neurons are molecularly distinct, and extend the principle that spinal motor neuron subtype identity has its origins in hierarchical programs of transcription factor expression, even within a single anatomically coherent motor pool.
Results
Anatomical Identification of Presumptive Gamma and Alpha Motor Neurons.
Within the mammalian spinal cord, gamma and alpha motor neurons can be distinguished by two main anatomical features. First, the cell bodies of gamma motor neurons are significantly smaller than those of alpha motor neurons (11, 13). Second, alpha but not gamma motor neurons receive direct synaptic input from proprioceptive sensory afferents (18). To distinguish gamma from alpha motor neurons in spinal cord of the mouse, we analyzed the size of lumbar motor neurons as well as the status of proprioceptive sensory input. We visualized motor neuron cell bodies and proximal dendrites by expression of choline acetyltransferase (ChAT), the rate-limiting enzyme in acetylcholine synthesis. We identified synaptic contacts between proprioceptive terminals and motor neurons by monitoring expression of the vesicular glutamate transporter vGlut1, a selective marker of sensory terminals (25, 26).
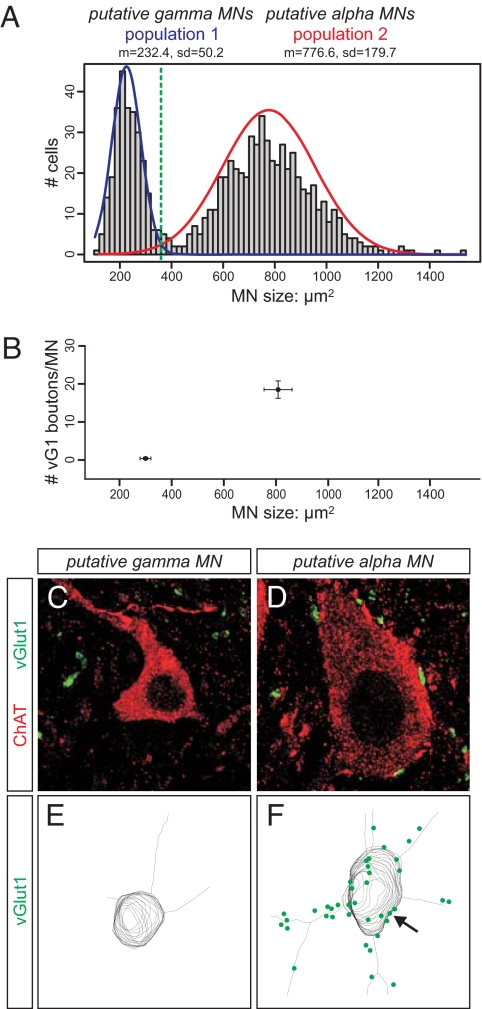
We determined the size distribution of motor neuron cell bodies in the lumbar spinal cord of p21 wild-type mice (n > 800 neurons; largest cross-sectional area), a stage when proprioceptive terminals approach their mature size. The sizes of ChATon motor neuron somata segregated into two normally distributed populations, with an optimal threshold between the two cell populations at 360 μm2 (Fig. 1A). The small neuronal population (n = 260/840; 31% of total number of motor neurons) exhibited a mean cross-sectional area of 232.4 ± 50 μm2 (SD), whereas the large neuronal population (n = 580/840; 69% of total) had a mean cross-sectional area of 776.6 ± 180 μm2 (SD) (Fig. 1A). This distinction in cell size was already evident at p14, with small (193 ± 48 μm2; SD) and large (601 ± 143 μm2; SD) motor neurons segregated at a threshold sectional area of 295 μm2 (Fig. 2C and [supporting information (SI) Fig. S1]).
Fig. 1.
Anatomical characterization of putative gamma motor neurons. (A) Cell size distribution of LMC motor neurons in the lumbar spinal cord of p21 wild-type mice. Frequency histogram depicting number of motor neurons in each size bin (y axis; binned in 20-μm2 steps), and size of motor neurons (x axis; μm2). Statistical analysis of cell cross-sectional area reveals two normally distributed cell populations (green dashed line: optimal threshold between two populations at 360 μm2; Material and Methods). (B) Analysis of the number of vGlut1on appositions per motor neuron (y axis) in the small-sized (left) and large-sized (right) cell population. Analysis includes cell body and proximal dendrite domain of individual motor neurons. Note that small-sized motor neurons exhibit no, or very low, incidence of vGlut1on inputs when compared with large-sized motor neurons. (C and D) Representative example of the largest cross-sectional area of a putative gamma (C) and a putative alpha (D) motor neuron, analyzing apposition of vGlut1on terminals (green) to ChATon motor neuron (red). (E and F) Representative examples of three-dimensional surface reconstructions of vGlut1on appositions (green) on ChATon individual gamma (E) and alpha (F) motor neurons (black). Solid arrow indicates high density of vGlut1 input to cell body of alpha motor neuron.
Fig. 2.
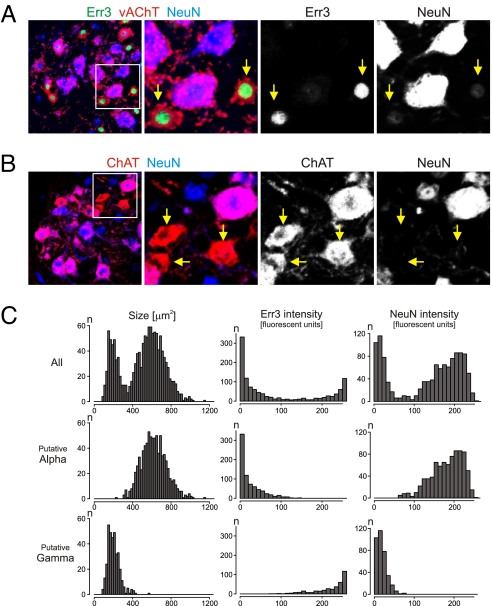
Putative gamma motor neurons display high Err3 and low NeuN expression. (A and B) Analysis of Err3 (green), vAChT (red), and NeuN (blue) (A) or ChAT (red) and NeuN (blue) (B) expression in LMC motor neurons at lumbar level of p14 mice. Box in low-resolution pictures (Left) indicates area shown at higher resolution and for split channels (Right). (C) Quantitative analysis of cell body size range, and Err3 and NeuN intensity (arbitrary fluorescent intensity units, using ImageJ measurements) for p14 lumbar LMC motor neurons analyzed (Top), or gated by size ranges to large putative alpha motor neurons (Middle) and small putative gamma motor neurons (Bottom). (See Fig. S1 for analysis of cell size populations at p14.)
We next determined the frequency of vGluton inputs to motor neurons within these two size populations. We reconstructed proprioceptive inputs to motor neurons in p21 spinal cord using high-resolution light microscopy to determine apposition of vGluton terminals to ChATon motor neuron cell bodies and the proximal dendritic domain. We found that the small ChATon motor neuron population was contacted by very few vGlut1on terminals (mean ± SEM, 0.4 ± 0.3/neuron; n = 10), whereas large ChATon motor neurons were contacted by 18.5 ± 2.3 (n = 10) vGlut1on terminals (Fig. 1 B–F). Based on these findings and prior analyses in other species, we infer that the small, sensory-sparse neurons are gamma motor neurons and that the large, sensory-supplied neurons are alpha motor neurons.
Molecular Markers of Presumptive Gamma and Alpha Motor Neurons.
To define molecular markers expressed differentially by gamma and alpha motor neurons, we reasoned that the commonality of expression of transcription factors and cell surface molecules by proprioceptive sensory neurons and motor neuron pools (27, 28) might extend to subsets of motor neurons within pools. We therefore probed proprioceptor-enriched genes identified in an Affymetrix-based screen for expression by ChATon LMC motor neurons in p14 spinal cord.
This analysis revealed that expression of the orphan nuclear hormone receptor Err3 was restricted to a population of motor neurons scattered throughout the ChATon motor neuron cohort, along the entire rostro-caudal extent of the spinal cord (Fig. 2 A and B). Quantitative analysis of nuclear Err3 protein expression intensity in lumbar ChATon motor neurons at p14 revealed two distinct peaks in the frequency histogram (Fig. 2C). Neurons with low or negligible level Err3 expression (mean ± SEM, 18 ± 1 arbitrary fluorescent intensity units [afiu], ImageJ) corresponded to large ChATon motor neurons, whereas high level Err3 expression (222 ± 1 afiu) was confined to small ChATon motor neurons (Fig. 2C). The restriction of Err3 expression to small motor neurons occurred gradually over the first 2 postnatal weeks, and was maintained into adulthood (Fig. S2). However, in the early postnatal stages Err3 was expressed by a majority of motor neurons, both small and large (Fig. S3). Together, these findings show that small putative gamma motor neurons express high levels of Err3, whereas large putative alpha motor neurons exhibit low or negligible Err3 expression.
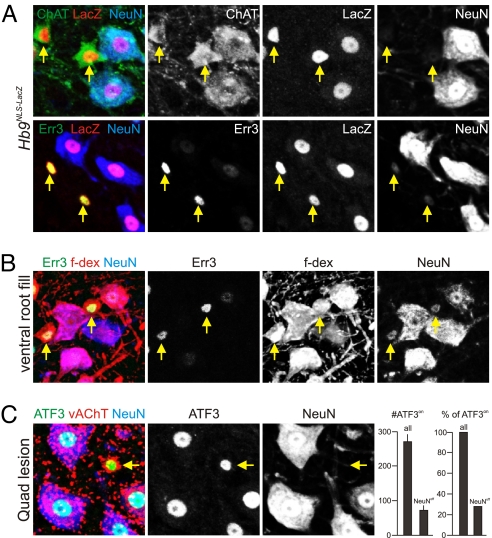
To probe whether Err3 protein was restricted to neurons in the ventral spinal cord, we correlated the expression of Err3 with that of NeuN, a widely used marker for most mammalian PNS and CNS neurons (24). Surprisingly, we found that Err3on/ChATon motor neurons lacked appreciable NeuN expression. To determine whether Err3on/NeuNoff cells are motor neurons, we analyzed the expression of Hb9, a homeodomain protein expressed by motor neurons (29, 30). We assessed the status of Hb9 expression in Err3on/NeuNoff cells using mice expressing LacZ targeted to the nucleus of Hb9on cells (29). We found that Err3on/NeuNoff and Err3off/NeuNon motor neurons in Hb9nlsLacZ mice expressed LacZ at similar levels (Fig. 3A). Another defining feature of spinal motor neurons is the projection of axons peripherally, to innervate target muscles. We therefore examined whether Err3on/NeuNoff cholinergic neurons give rise to axons that project into the ventral root, by application of fluorescently labeled dextran (f-dex) to cut lumbar ventral roots of p16 mice. We found that all labeled cells were cholinergic, and that both Err3off/NeuNon and Err3on/NeuNoff neuronal populations accumulated retrograde f-dex label (Fig. 3B). These findings show that despite their NeuNoff status, these neurons project axons into the periphery, adding another example of a neuronal subpopulation devoid of NeuN expression (24, 31). Together with their transcriptional profile, these findings indicate that cholinergic Err3on/NeuNoff cells are motor neurons.
Fig. 3.
Putative gamma motor neurons display molecular characteristics and projection phenotypes characteristic of motor neurons. (A) Analysis of Hb9 expression by virtue of LacZ expression in lumbar motor neurons of p20 Hb9NLS-LacZ mice. ChAT (green), LacZ (red), and NeuN (blue) (Upper) or Err3 (green), LacZ (red), and NeuN (blue) (Lower) expression in merged (left) or split channel configuration. Note that both putative alpha (Err3off/NeuNon) and putative gamma (Err3on/NeuNoff; yellow arrows) motor neurons express LacZ. (B) Analysis of Err3 (green), f-dex (red), and NeuN (blue) expression in merged (Left) or split channel configuration. Lumbar motor neurons were labeled retrogradely from the L4 ventral root of a p16 spinal cord. Err3on motor neurons were f-dexon (yellow arrows), indicating that they project axons through the ventral root. (C) ATF3 (green), vAChT (red), and NeuN (blue) expression (Left: merged; Middle, Right: split channels for ATF3 and NeuN) in quadriceps motor neurons 3 days subsequent to lesion of the quadriceps nerve in p20 mice. Both NeuNoff putative gamma motor neurons (yellow arrow) and NeuNon putative alpha motor neurons express ATF3. (Right) Quantitative analysis of all quadriceps motor neurons and NeuNoff putative gamma motor neurons identified by ATF3 expression (n = 4 independent experiments; ± SEM).
Complementary Expression of Err3 and NeuN in Gamma and Alpha Motor Neurons.
The observation that ChATon motor neurons with high Err3 expression levels were associated with low NeuN expression prompted us to examine in greater detail the profile of motor neuron NeuN expression and its relationship to Err3. A combined analysis of Err3 and NeuN intensity in ChATon motor neurons showed a tight inverse relationship between the two marked populations (Fig. 2A), suggesting that ChATon motor neurons with high Err3 status exhibit low NeuN expression. In support of this view, separation of motor neurons into NeuNon/Err3off and NeuNoff/Err3on populations, and analysis of the cell size profiles of these two populations, demonstrated that the NeuNon/Err3off molecular profile corresponded to large putative alpha motor neurons and that the NeuNoff/Err3on profile coincided with small putative gamma motor neurons (Fig. 2C, left). These findings demonstrate that Err3 and NeuN exhibit complementary expression patterns in ChATon motor neurons of different sizes, and suggest that the Err3on/NeuNoff and Err3off/NeuNon status of motor neurons delineates putative gamma and alpha motor neurons.
Quantitative Analysis of Putative Gamma Motor Neurons by Molecular Markers.
We next analyzed the distribution and frequency of molecularly defined putative gamma motor neurons along the rostro-caudal extent of the spinal cord. Err3on/NeuNoff cholinergic neurons comprised ≈30% of all motor neurons analyzed (n = 349/1172 lumbar LMC motor neurons) and were found to be scattered throughout the rostro-caudal extent of the spinal cord. In addition, we characterized the representation of gamma motor neurons within a group of motor neurons that innervates the quadriceps muscles of the hindlimb. Because f-dex labels only a subset of motor neurons that innervate the injected muscle, we used a nerve lesion paradigm to obtain a more accurate assessment of the number of putative gamma motor neurons within the quadriceps pool. We cut the nerve innervating the quadriceps muscles in p20 mice and assessed the number and molecular status of quadriceps motor neurons 3 days after lesion, by virtue of expression of ATF3, a transcription factor that is rapidly upregulated in the nuclei of motor neurons after nerve damage (32). We found that both NeuNon and NeuNoff quadriceps motor neurons upregulate ATF3 robustly (Fig. 3C). Quantitative analysis revealed that approximately 70% of all ATF3on quadriceps motor neurons coexpressed NeuN, whereas ≈30% showed low or negligible NeuN expression (Fig. 3C). These values are in agreement with previous studies assessing the identity of putative gamma motor neurons based on cell size criteria in mice, which have estimated 20–30% of all motor neurons to be gamma motor neurons (11, 13). We conclude that both distribution and frequency of small Err3on/NeuNoff motor neurons matches the profile expected for gamma motor neurons.
Loss of Muscle Spindles Leads to Absence of Putative Gamma Motor Neurons.
The identification of putative gamma motor neurons by molecular markers permitted us to begin to explore the mechanisms that control their specification. Muscle spindle differentiation and maturation is initiated by an inductive signal supplied anterogradely by group Ia proprioceptive afferents to nascent muscle fibers (21, 33). In turn, gamma motor neurons depend on trophic support from intrafusal fibers in muscle spindles (34, 35). We therefore reasoned that if Err3on/NeuNoff status defines gamma motor neurons, then expression of these markers should be lost in the absence of muscle spindles. To test this prediction, we used genetic methods to block muscle spindle formation in mice and examined the status of Err3 and NeuN expression by LMC motor neurons.
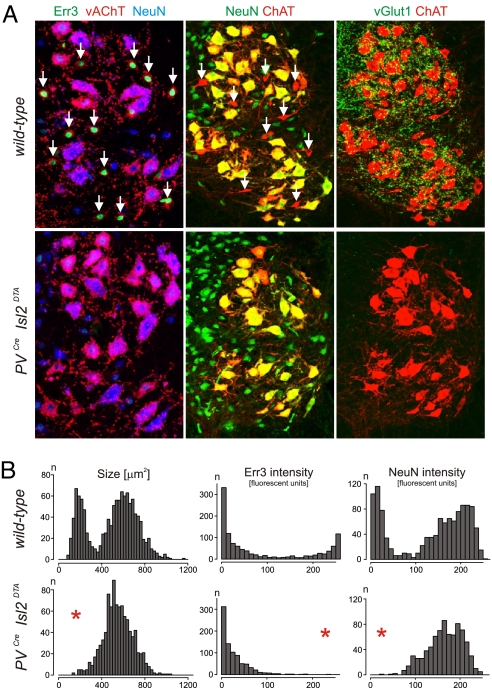
To prevent spindle differentiation we eliminated proprioceptive sensory afferents, the source of the spindle-inducing factor Nrg1 (21), using an established, diphtheria toxin–mediated cell ablation strategy (PVCreIsl2DTA) (26). PVCre Isl2DTA mice exhibit a virtually complete absence of proprioceptive input to the spinal cord (26) (Fig. 4A) as well as an absence of vGlut1on proprioceptive sensory endings in hindlimb muscles analyzed at p10–p14 (Fig. S4). In addition, we found a complete absence of muscle spindles, as assessed by loss of expression of Pea3 and Egr3 (Fig. S4), two transcription factors normally expressed selectively by intrafusal muscle fibers (19, 21).
Fig. 4.
Gamma motor neuron loss in mice lacking proprioceptive afferents. (A) Analysis of Err3 (green), vAChT (red), and NeuN (blue; left); NeuN (green), and ChAT (red; middle) or vGlut1 (green) and ChAT (red; right) in the lumbar spinal cord of p14 wild-type (left) or PVCreIsl2DTA (bottom) mice. Note that Err3on/NeuNoff putative gamma motor neurons (white arrows) are present in wild-type mice but absent in mice lacking proprioceptive sensory neurons. (B) Quantitative analysis of cell body size range, Err3 and NeuN intensity (arbitrary fluorescent intensity units, using ImageJ measurements) for p14 lumbar LMC motor neurons analyzed in wild-type (top) and PVCreIsl2DTA (bottom) mice. Red stars indicate differences from wild-type. Note that the average cell size of motor neurons in PVCreIsl2DTA mice is smaller than alpha motor neuron peak in wild-type mice, most likely because of the absence of proprioceptive input.
To determine the effect of loss of muscle spindles on spinal motor neurons, we first analyzed the cell size distribution profile in p14 wild-type and PVCre Isl2DTA mice. Whereas ChATon motor neurons in wild-type mice segregated into two separate populations (Fig. 1A), unbiased clustering analysis in PVCre Isl2DTA mice revealed only a single, normally distributed cell population (mean ± SD, 552.8 ± 137 μm2; n = 840 neurons) which overlapped with the larger population in wild-type animals. The absence of the small motor neuron population was accompanied by a 98% decrease in the number of Err3on/NeuNoff putative gamma motor neurons in the ventral horn of PVCre Isl2DTA mice, compared with wild-type littermates (Fig. 4 A and B; n = 17/840 LMC motor neurons analyzed). In contrast, the number of cholinergic Err3off/NeuNon presumptive alpha motor neurons appeared unchanged in PVCre Isl2DTA mice (Fig. 4 A and B). Thus, the absence of Err3 expression is likely to reflect the death of this subset of motor neurons subset rather than their conversion to alpha motor neurons.
We next considered whether the loss of proprioceptive afferent inputs to the spinal cord in PVCre Isl2DTA mice might contribute to the absence of Err3on/NeuNoff gamma motor neurons. To assess this, we analyzed the Err3/NeuN status of motor neurons innervating the cutaneous maximus (Cm) muscle. The Cm muscle contains muscle spindles supplied by group Ia proprioceptive afferents, yet Cm motor neurons fail to receive direct proprioceptive input (26, 36). We therefore analyzed the Err3on/NeuNoff status of the ventral-most ChATon motor neurons at cervical level c8 in wild-type mice, the location of most Cm motor neuron cell bodies (26, 36). Despite the absence of vGlut1on terminal contacts with Cm motor neurons (Fig. 5A) (36), we found that Err3on/NeuNoff cells were present (constituting ≈10–20% of all Cm motor neurons) (Fig. 5A). These results demonstrate that the establishment of proprioceptive inputs to the neurons within a motor pool is not required to direct the core molecular distinction between gamma and alpha motor neurons.
Fig. 5.
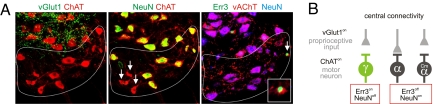
Establishment of molecular status of gamma motor neurons is independent of proprioceptive input to motor neurons. (A) Analysis of molecular markers identifying putative gamma motor neurons within the Cm motor neuron pool, in which motor neurons lack direct proprioceptive input (left: vGlut1 [green] and ChAT [red]). (Middle, right) Analysis of NeuN (green) and ChAT (red) or Err3 (green), vAChT (red), and NeuN (blue) expression reveals the presence of Err3on/NeuNoff putative gamma motor neurons in this motor neuron pool, as for motor neuron pools that receive direct proprioceptive afferent input. (B) Gamma motor neurons receive little vGlut1on proprioceptive input in the spinal cord and can be identified by their Err3on/NeuNoff status. In contrast, many alpha motor neurons receive direct proprioceptive input centrally and can be distinguished from gamma motor neurons by their Err3off/NeuNon status. In the Cm motor pool, alpha motor neurons do not receive direct proprioceptive input centrally, yet Err3off/NeuNon gamma motor neurons are present.
Discussion
We have found that gamma and alpha motor neurons can be distinguished molecularly, and that this distinction has its basis in complementary profiles of expression of DNA binding proteins (Fig. 5B). The principle that spinal motor neurons can be fractionated into functionally distinct subtypes on the basis of their profile of transcription factors therefore extends to neuronal subtypes within single motor neuron pools.
Our studies establish a molecular distinction between gamma and alpha motor neurons, but do not resolve how these two neuronal subtypes acquire their specialized molecular features. The restriction of Err3 expression to gamma motor neurons becomes apparent during the first 2 postnatal weeks (Fig. S3). The specification of gamma, and by inference, alpha motor neuron identity may therefore be a late event that occurs long after motor pool identities have been established (6, 8, 10, 27). Motor neuron pool identities are initiated by the actions of a complex Hox protein repressor network, such that specific pool fates depend critically on the precise spectrum of Hox proteins expressed by a set of motor neurons (6). The distinction between gamma and alpha motor neurons, however, is a conserved feature of all motor pools, transcending individual pool identities, suggesting that the assignment of this finer aspect of motor neuron subtype identity is achieved in a Hox-independent manner.
The restriction of Err3 expression to gamma motor neurons occurs over the period that functional sensory–motor circuits are established, raising the possibility that the gamma/alpha distinction is influenced by the peripheral or central connections of motor neurons. Could the gamma/alpha distinction be programmed by the peripheral muscle targets of motor neurons? In this view, an initially coherent pool of motor neurons may send axons to their cognate muscle target, with those axons that contact intrafusal fibers in muscle spindles exposed to spindle-derived gamma inductive signals. Because motor neuron-spindle contacts are established at embryonic stages (37), yet Err3 restriction occurs postnatally, motor neurons may be programmed to a gamma fate only gradually, over the course of days. Muscle spindle–derived signals could, for example, act to maintain Err3 expression in the face of a temporal program that directs the downregulation of Err3 expression in motor neurons.
A role for muscle spindle–derived signals in the development of gamma motor neurons has already been suggested. The neurotrophic factor glial derived neurotrophic factor (GDNF) is expressed selectively by intrafusal muscle fibers (35), and genetic studies have implicated spindle-derived GDNF signaling in the survival of gamma motor neurons (34, 35). It remains to be determined whether GDNF signaling has an early role in the specification of gamma motor neuron identity, in addition to its later gamma survival–promoting activity. Nevertheless, the idea that peripherally derived GDNF directs the transcriptional specification of motor neuron subtype identity at embryonic stages has a precedent in the induction of ETS gene expression by motor pools (8, 27, 38). Thus trophic factors such as GDNF may have sequential functions in the assignment of pool and intrapool transcriptional identities. More generally, the deployment of muscle spindle–derived inductive signals would seem to provide an effective means of superimposing intrapool identities on earlier pool-specific transcriptional programs.
In principle, the gamma/alpha distinction could have emerged as a function of differences in central sensory–motor connectivity. Gamma motor neurons are conspicuous by the absence of proprioceptive sensory contacts, even though their alpha pool siblings typically receive many sensory synapses. In this view, the absence of sensory input might be a precondition for gamma motor neuron differentiation. Two lines of evidence, however, argue against the idea that central sensory connectivity patterns instruct gamma/alpha motor neuron identity. First, in PVCre Isl2DTA mice, where all proprioceptive sensory–motor connections are eliminated, we do not detect an increase in the incidence of Err3on gamma motor neurons within specific motor pools. Second, and more telling, analysis of the gamma/alpha status of neurons in the Cm motor pool of wild-type mice reveals both Err3on gamma and NeuNon alpha motor neurons, despite the absence of proprioceptive sensory input (26, 36). Thus the program of gamma motor neuron differentiation appears sensitive to peripheral muscle–derived factors but not to the vagaries of central sensory connectivity.
The restriction of Err3 expression to gamma motor neurons provides a molecular reference point for exploring the emergence of neuronal subtype diversity within individual motor pools. We note that anatomical studies suggest that fast and slow motor neurons within a pool exhibit functional distinctions before the point of target muscle innervation (39, 40), raising the possibility that this aspect of intrapool diversification also has its origins in differential transcription factor expression.
Materials and Methods
Murine Genetics and Immunohistochemistry.
PVCre (41), Isl2DTA (42), Hb9NLSLacZ (29) mouse strains have been described previously and are maintained on a mixed genetic background (129/C57Bl6). Antibodies used in this study were as follows: mouse anti-Err3 (PPMX), mouse anti-NeuN (Chemicon), rabbit anti-tetramethylrhodamine (Invitrogen), rabbit anti-LacZ (20), rabbit anti-Pea3 (8), rabbit anti-vAChT (Sigma), rabbit anti-ATF3 (Santa Cruz), guinea pig anti-vGlut1 (Chemicon), guinea pig anti-Isl1 (20), and goat anti-ChAT (Chemicon).
Anatomical and Statistical Analysis.
For motor neuron cell size measurements, the largest cross-sectional areas were determined using ImageJ (Version 1.42g, National Institutes of Health) and Neurolucida 8 (MicroBrightField Bioscience) software. Statistical analysis of cell sizes and intensity measurements are described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We are grateful to Monika Mielich for help with the characterization of Err3 expression and to Michael Stadler and Ilir Agalliu for help with statistical analysis. A.F., D.R.L., M.S., and S.A. were supported by the Swiss National Science Foundation, National Centers of Competence in Research Frontiers in Genetics, the Kanton Basel-Stadt, European Union (EU) Framework Program 7, and the Novartis Research Foundation. J.A.K. was a Howard Hughes Medical Institute (HHMI) Research Associate and was supported by a Wellcome Trust Traveling Research Fellowship (IPTRF 064871). T.M.J. is an HHMI Investigator and is supported by National Institute of Neurological Disorders and Stroke, EU Framework Program 7, and Project ALS.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906809106/DCSupplemental.
References
- 1.Masland RH. Neuronal cell types. Curr Biol. 2004;14:R497–R500. doi: 10.1016/j.cub.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 2.Wassle H. Parallel processing in the mammalian retina. Nature Rev. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 3.Markram H, et al. Interneurons of the neocortical inhibitory system. Nature Rev. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 4.Dalla Torre di Sanguinetto SA, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr Opin Neurobiol. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 6.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- 8.Livet J, et al. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 10.De Marco Garcia NV, Jessell TM. Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity. Neuron. 2008;57:217–231. doi: 10.1016/j.neuron.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke R. Spinal cord: Ventral horn. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford Univ Press; 2004. pp. 79–123. [Google Scholar]
- 12.Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nature Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 13.Burke RE, Strick PL, Kanda K, Kim CC, Walmsley B. Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol. 1977;40:667–680. doi: 10.1152/jn.1977.40.3.667. [DOI] [PubMed] [Google Scholar]
- 14.Hunt CC, Kuffler SW. Further study of efferent small-nerve fibers to mammalian muscle spindles; multiple spindle innervation and activity during contraction. J Physiol. 1951;113:283–297. doi: 10.1113/jphysiol.1951.sp004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuffler SW, Hunt CC, Quilliam JP. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951;14:29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Hunt CC. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951;115:456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eccles JC, Eccles RM, Iggo A, Lundberg A. Electrophysiological studies on gamma motoneurones. Acta Physiol Scand. 1960;50:32–40. doi: 10.1111/j.1748-1716.1960.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 19.Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nature Genet. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- 20.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 21.Hippenmeyer S, et al. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 22.Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- 23.Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274:22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- 24.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira AL, et al. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- 26.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Lin JH, et al. Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell. 1998;95:393–407. doi: 10.1016/s0092-8674(00)81770-5. [DOI] [PubMed] [Google Scholar]
- 28.Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 29.Arber S, et al. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- 30.Thaler J, et al. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- 31.Wolf HK, et al. NeuN: A useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 32.Tsujino H, et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 33.Kucera J, Walro JM, Reichler J. Differential effects of neonatal denervation on intrafusal muscle fibers in the rat. Anat Embryol. 1993;187:397–408. doi: 10.1007/BF00185898. [DOI] [PubMed] [Google Scholar]
- 34.Gould TW, Yonemura S, Oppenheim RW, Ohmori S, Enomoto H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J Neurosci. 2008;28:2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehead J, Keller-Peck C, Kucera J, Tourtellotte WG. Glial cell-line derived neurotrophic factor-dependent fusimotor neuron survival during development. Mech Develop. 2005;122:27–41. doi: 10.1016/j.mod.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucera J, Walro JM, Reichler J. Role of nerve and muscle factors in the development of rat muscle spindles. Am J Anat. 1989;186:144–160. doi: 10.1002/aja.1001860205. [DOI] [PubMed] [Google Scholar]
- 38.Haase G, et al. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 39.Milner LD, Rafuse VF, Landmesser LT. Selective fasciculation and divergent pathfinding decisions of embryonic chick motor axons projecting to fast and slow muscle regions. J Neurosci. 1998;18:3297–3313. doi: 10.1523/JNEUROSCI.18-09-03297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson WJ, Condon K, Astrow SH. The origin and selective innervation of early muscle fiber types in the rat. J Neurobiol. 1990;21:212–222. doi: 10.1002/neu.480210114. [DOI] [PubMed] [Google Scholar]
- 41.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.