Abstract
In a developing organism, tissues emerge from coordinated sequences of cell renewal, differentiation, and assembly that are orchestrated by spatial and temporal gradients of multiple regulatory factors. The composition, architecture, signaling, and biomechanics of the cellular microenvironment act in concert to provide the necessary cues regulating cell function in the developing and adult organism. With recent major advances in stem cell biology, tissue engineering is becoming increasingly oriented toward biologically inspired in vitro cellular microenvironments designed to guide stem cell growth, differentiation, and functional assembly. The premise is that to unlock the full potential of stem cells, at least some aspects of the dynamic three-dimensional (3D) environments that are associated with their renewal, differentiation, and assembly in native tissues need to be reconstructed. In the general context of tissue engineering, we discuss the environments for guiding stem cell function by an interactive use of biomaterial scaffolds and bioreactors, and focus on the interplay between molecular and physical regulatory factors. We highlight some illustrative examples of controllable cell environments developed through the interaction of stem cell biology and tissue engineering at multiple levels.
Introduction
Ahundred years ago, in 1907 at Johns Hopkins University, Ross Harrison conducted his landmark experiment that involved the cultivation of an embryonic tissue. He dissected neural tube fragments from tiny frog embryos and established an in vitro culture to study the outgrowth of nerve fibers.1 These experiments formed the foundation of modern nerve physiology and neurology and marked the beginning of animal cell and tissue cultures. Harrison also pioneered the techniques of organ transplantation. Many others followed his work, making further breakthroughs and developing the methods we now use to study stem cells and to engineer tissues. The “tool box” currently being developed by tissue engineers may again change the way we conduct cell and tissue culture experiments, by favoring controllable tissue models of high fidelity over two-dimensional (2D) Petri dishes. Standard protocols for the expansion of stem cells still involve cultivation in 2D settings (e.g., on tissue culture plastics or feeder layers), whereas cell differentiation commonly requires transfer into a three-dimensional (3D) setting (e.g., pellet culture and embryoid bodies). This “batch” culture (i.e., periodic exchange of medium) and limited application of regulatory signals (a bolus) seriously impair our ability to fully explore the self-renewal and differentiation of stem cells and their potential to form functional tissues. Recent studies of cancer cells in 3D cell culture models underscore the importance of cell interactions within a 3D context and suggest that the use of 3D models can provide a much needed bridge between cancer studies in cells and in whole organisms.2
The field of tissue engineering is driven by the need to provide functional equivalents of native tissues that can be used for implantation. With the aging population and increasing expectations for a high quality of life, the need continues to grow, and the field of tissue engineering is responding to it relatively slowly, due to issues such as the immense biological complexity, complex regulatory issues, and the high cost of new cell-based devices. At the same time, engineered tissues are finding new roles as models for fundamental research, studies of disease, testing of drugs, and many other applications. In all cases, the requirements are to provide the cells with the appropriate cues, to control the conditions in the cell microenvironment, and to monitor cellular responses on multiple hierarchical levels. In a developing organism, tissues emerge from coordinated sequences of cell renewal, differentiation, and assembly, within an ever-changing environment characterized by spatial and temporal gradients of multiple factors. To direct cells to differentiate at the right time, in the right place, and into the right phenotype, one needs to recreate the right environment, with biology and engineering interacting at multiple levels. One approach is to characterize the native tissue environments so that specific cues may be identified in vivo and then provided in vitro as controllable parameters that can be experimentally optimized for synergistic interactions within the cellular microenvironment.
We follow here a paradigm that a living cell is the only “tissue engineer,” and that by engineering the culture environment we can guide cell differentiation and functional assembly. In the general context of technologies for tissue engineering, we discuss the environments for guiding stem cell function, with a particular focus on two key components—biomaterial scaffolds and bioreactors. For both components, we focus on the interplay between molecular and physical regulatory factors and highlight some of the important findings toward the design of cell–scaffold–bioreactor systems for applications in tissue engineering and biological and medical research.
Biomimetic Approach
In a living organism, cells are surrounded by other cells and embedded in an extracellular matrix (ECM) that defines the architecture, signaling, and biomechanics of the cellular microenvironment. The supply of nutrients and removal of metabolites are provided by capillary networks, the density of which is dependent on the metabolic needs of the cells. Cellular processes are mediated by a variety of molecular, structural, hydrodynamic, mechanical, and electrical cues and their spatial and temporal levels and combinations. Cells respond to and remodel their immediate microenvironment, via homotypic or heterotypic interactions with neighboring cells, and with the tissue matrix. It is obvious that the biological complexity of the native cell context is not mimicked in the laboratory under standard 2D culture conditions. This is a major limitation to experiments investigating cellular responses in vitro since much of the complex interplay of mechanical and molecular factors present in vivo is absent.3 Interestingly, the first two communities to realize the significance of these differences were cancer biologists4 and tissue engineers,5,6 and then followed more recently by stem cell researchers.7,8
It has been argued that we need a new generation of 3D culture systems that would be “something between a Petri dish and a mouse,” to authentically represent a cell's environment in a living organism and be more predictive of in vivo systems.9 For stem cells in particular, to unlock their full potential and obtain biologically sound and relevant data in vitro, at least some aspects of the dynamic 3D environments that are associated with their renewal, differentiation, and assembly into tissues need to be reconstructed. A fundamental approach to the in vitro formation of engineered tissues is to direct the 3D organization of cells (via biomaterial scaffolds) and to establish the conditions necessary for the cells to reconstruct a functional tissue structure (via bioreactors). This approach is based on a premise that cells' responses to environmental factors are predictable, and that the cell function in vitro can be modulated by the same complex factors known to play a role during development and remodeling.
Biomaterial scaffolds provide structural templates for cell attachment and tissue growth, whereas bioreactors provide environmental control. Scaffolds and bioreactors also provide a multitude of regulatory signals such as cytokines (diffusing or immobilized) and physical factors (hydrodynamic shear, mechanical stretch, and electrical gradients). Both in vivo (during development and regeneration) and in vitro (for tissue engineering), the cues presented to cells are the principal determinants of the phenotypic nature and function of the resultant tissues. Hence, the design of tissue engineering systems is necessarily inspired by biology (in either the developing or adult organism). The complementary engineering principles help recapitulate the combinations of parameters in the native environments of a specific tissue or organ, to orchestrate the conversion of “collections of cells” into specific tissue phenotypes.
Tissue engineering thus opens several exciting possibilities: (i) to create functional grafts suitable for implantation and repair of failing tissues, (ii) to study stem cell behavior and developmental processes in the context of controllable 3D models of engineered tissues, and (iii) to utilize engineered tissues as models for studies of physiology and disease. Three-dimensional environments that capture the molecular, structural, and physical factors regulating cellular processes and at the same time provide control and monitoring of environmental factors are instrumental toward these goals. Moreover, essentially the same biologically inspired blueprints define the engineering design of each of these systems. We discuss key components of tissue engineering systems—biomaterial scaffolds and bioreactors—in light of the research and utilization of stem cells for tissue engineering.
Biomaterials
An important component to the stem cell microenvironment is the surrounding matrix, which includes numerous chemical and biophysical cues. In a natural setting, this environment consists of the ECM, composed of collagens, other proteins, polysaccharides, and water, whose structure is dependent on the location and function of the tissue. For instance, cartilage has a high proteoglycan content within a strong collagen network, a matrix that gives this tissue its important mechanical properties to sustain natural loading. In contrast, tendon is highly anisotropic with respect to ECM orientation, making mechanical properties directionally dependent. Various proteins and peptides in the ECM control cellular interactions and receptor binding. Also, factors such as ECM mechanics and constriction of cell shape can play a role in the differentiation of stem cells and can be engineered into synthetic matrices for directed differentiation. Advances in material synthesis and processing have opened up a range of synthetic and natural materials for use in controlled microenvironments.
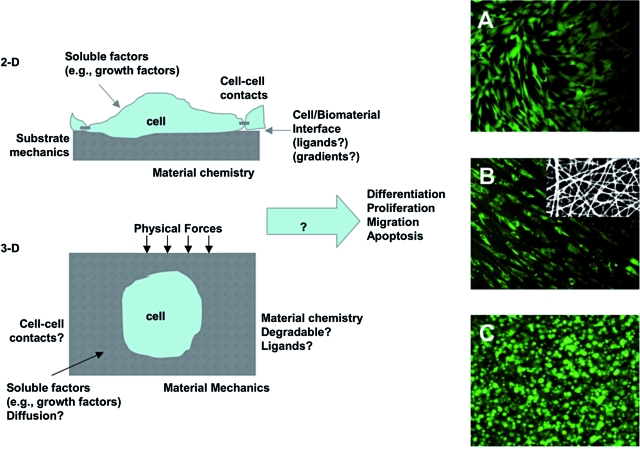
A wide variety of materials with a range of properties have been designed and utilized for interactions with stem cells.10 These materials have been fabricated into porous, fibrous, and hydrogel scaffolds.11 Porous scaffolds provide macroscopic voids for the migration and infiltration of cells, whereas fibrous scaffolds may be fabricated on a size-scale that mimics the native ECM and may be aligned to control cellular alignment.12 Alternatively, hydrogels are water-swollen polymers that may be fabricated from natural ECM components or synthetic materials and can be engineered to include capabilities of native tissues (e.g., those present during early development). Figure 1 outlines a variety of parameters that can be engineered in both 2D and 3D culture environments that can influence stem cell behavior (e.g., self-renewal, migration, and differentiation). Also, representative images of stem cell interactions on 2D surfaces, in fibrous scaffolds, and within hydrogels are shown. The morphology of the cell is distinct, based on the culture environment (i.e., spread in 2D and rounded in 3D), as found in vivo. This section will review several important advances in the design of biomaterials to control stem cell behavior, specifically material chemistry and physical properties.
FIG. 1.
Manipulating the stem cell microenvironment in 2D and 3D. Schematic of controllable parameters (e.g., matrix properties and culture environment) for altering stem cell microenvironmental behavior in both 2D and 3D. Human MSC morphology (stained with Live/Dead) when seeded in 2D on a biodegradable elastomer (A), on the surface of an electrospun fibrous scaffold from a biodegradable elastomer and gelatin (autofluoresces red) composite (B, inset is SEM of scaffold), and when encapsulated in 3D in a photocrosslinked hyaluronic acid hydrogel (C). These are examples where the biomaterial structure dictates the cellular morphology. Work by the Burdick laboratory and previously unpublished. Color images available online at www.liebertonline.com/ten.
Chemical cues to control stem cell behavior
Both natural and synthetic materials have been investigated for interactions with stem cells and to control their behavior.13 The benefits to natural materials include their ability to provide signaling to the encapsulated cells by several different mechanisms: through surface receptor interactions, by uptake in soluble form, and via degradation by cell-instructive enzymes. The limitations to such materials are that they may be difficult to process without disrupting a potentially important hierarchical structure, and that gels formed from natural materials generally have poor mechanical properties and the potential for an immune response depending on the source of the material. Alternatively, synthetic biomaterials have wide diversity in properties that may be obtained and tailored with respect to mechanics, chemistry, and degradation. Additionally, the processing of synthetic materials into desired structures may be much simpler than with natural materials. However, potential limitations to the use of synthetic materials include toxicity and a limited repertoire of cellular interactions, unless they are modified with adhesion peptides or designed to release biological molecules.
Examples of natural materials that have been used to culture stem cells include Matrigel, collagen, alginate, fibrin, and hyaluronic acid (HA).10,13 Matrigel consists of a mixture of molecules derived from natural ECM and has been investigated extensively for the culture of cells and particularly stem cells.14 The complexity and derivation from natural tissues has motivated its use in cultures, particularly for embryonic stem cells (ESCs), due to its mimicking of natural structures.15 Since collagen is abundant in native ECM and interacts with cells via integrin binding, 3D collagen gels have been widely used for stem cell encapsulation, including mesenchymal stem cells (MSCs)16 and ESCs.17 Alginate is a seaweed-derived polyanion that forms hydrogels through ionic crosslinking. Although there are no direct cellular interactions, alginate forms stable hydrogels that become soluble through the dissociation of polymer chains. ESCs have been encapsulated and differentiated in alginate hydrogels for a variety of applications both in vitro and in vivo.18,19 Additionally, fibrin has been used for the culture of murine ESCs.20
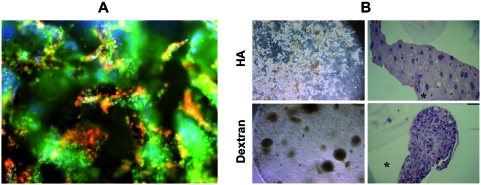
HA is a polysaccharide found in many tissues and has been modified to form hydrogels with controlled properties that allow for the encapsulation of viable cells.21,22 Cells may interact with HA through receptor binding, primarily CD44, and degrade HA with hyaluronidases. These HA hydrogels have been investigated for the culture and growth of undifferentiated human ESCs23, and an example of colonies of cells in the HA hydrogel is shown in Figure 2A. The cells were encapsulated using a photoinitiated polymerization, which is a technique that has been widely used for encapsulating viable cells in hydrogels.24 When necessary, entrapped cells can be removed from the gel with the addition of exogenous hyaluronidases and then differentiated after formation of embryoid bodies. Importantly, the colonies of ESCs remained undifferentiated in the HA hydrogels, but spontaneously differentiated in hydrogels formed from dextran, a polysaccharide with a similar structure (as shown in Fig. 2B). This example illustrates the importance of the biomaterial chemistry for controlling stem cell differentiation. Notably, the simple addition of soluble factors to these culture environments induced differentiation by entrapped cells, without the need to change the culture setting (e.g., from 2D culture on a feeder layer into the 3D embryoid body culture).23
FIG. 2.
Matrix chemistry controls stem cell differentiation. Colonies of human ESCs encapsulated in 3D hyaluronic acid hydrogels and stained for markers of undifferentiation (green, Oct4; red, SSEA4 (A) and compared using light microscopy (left) and histology (right) to human ESCs encapsulated in dextran hydrogels (B). Colonies of hESCs maintain undifferentiated in HA hydrogels and spontaneously differentiated in dextran hydrogels with a similar chemical structure, indicating the importance of biomaterial chemistry on cellular interactions. Reprinted with permission from Gerecht et al.23 Copyright (2007) National Academy of Sciences, USA. Color images available online at www.liebertonline.com/ten.
Synthetic materials investigated for stem cell cultures are widespread.10,13 Both nondegradable and materials that degrade through either hydrolytic or enzymatic mechanisms have been synthesized, and one advantage is the tunability and versatility of these physical properties.24 Poly(α-hydroxy esters) have been extensively used in the field of tissue engineering, primarily due to their history of biocompatibility and use in medicine. One composition was seeded with human ESCs for the regeneration of numerous tissues, including vascular and neural structures.25,26 Differentiation was induced through incorporation of the appropriate growth factors in culture media.
Poly(ethylene glycol) (PEG) hydrogels are one example of a synthetic material that has been investigated for the encapsulation and culture of stem cells. PEG hydrogels are inherently hydrophilic, so protein adsorption is minimal, which allows these materials to prevent nonspecific material interactions. Thus, PEG hydrogels are often modified with tethered groups, such as adhesion peptides27,28 or phosphates29 to alter cellular interactions. PEG hydrogels have been used for the culture and differentiation of stem cells toward the engineering of numerous tissues. Another hydrogel material for stem cell encapsulation includes self-assembling peptide gels. These gels form fibril nanometer–sized structures similar to the native ECM through the assembly of engineered peptides with the appropriate ionic and hydrophobic interactions.30 Self-assembling peptides have also been used for the controlled differentiation of stem cells through careful selection of the peptides. In one study, laminin-derived IKVAV peptide sequences were utilized to form gels that selectively differentiated neural progenitor cells into neurons.31
Hydrogels may also be synthetic, and still mimic many of the cues found in natural matrices. For example, Hubbell and coworkers fabricated hydrogels that incorporate adhesive and enzymatically degradable cues to support cellular migration.32 Importantly, both types of cues are necessary for cells to migrate in the hydrogels. Toward stem cell differentiation, it is important to design environments that are permissive to important cellular behaviors, such as migration and cell–cell interactions, depending on the differentiation pathway (i.e., cell type and tissue) of interest. These types of hydrogels have been used toward culture environments for the self-renewal of ESCs. As one example, poly(N-isopropylacrylamide-co-acrylic acid) (p(NIPAAm-co-AAc))–based hydrogels crosslinked with peptides and incorporating semi-interpenetrating networks of adhesive peptides have been developed for the culture of ESCs in 2D.33
Mechanical and shape cues to control stem cell differentiation
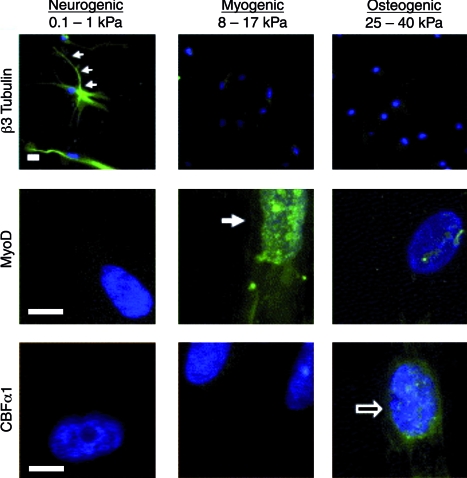
Biomaterials can be fabricated into structures that exhibit a wide range of mechanical properties with moduli from the Pa through the GPa range. Toward the differentiation of adult MSCs, hydrogels have been engineered to exhibit a wide range of mechanical properties that correlate to the mechanical properties of native tissues.34 When MSCs were seeded on the surface of these hydrogels (polyacrylamide gels modified with collagen), they showed lineage specification depending on the substrate mechanics. Importantly, MSC differentiation correlated with the in vivo matrix elasticity (i.e., soft substrates tend toward neural, intermediate substrates tend toward muscle, and stiffer substrates tend toward bone) in the absence of any known inducing soluble factors.34 Representative staining of MSCs on the gel surfaces with varied moduli is shown in Figure 3. Phenotype commitment was modified with soluble factor inclusion over the first week of culture; however, longer cultures prior to soluble factor addition were defined by matrix elasticity. The cues from matrix mechanics are thought to occur by the cell “pulling” on the matrix and then generating signals based on this force. The inhibition of nonmuscle myosin II blocked the ability of cells to differentiate based on matrix elasticity. Although this work was performed in 2D, which may limit its applicability in the fabrication of 3D tissue structures, it is anticipated that the results will correlate to a more 3D tissue-like environment where cells are surrounded on all sides by the matrix.
FIG. 3.
Matrix mechanics directs stem cell differentiation. Matrix mechanics–dependent differentiation of human MSCs stained for β3-Tubulin, MyoD, and CBFα1 as markers of neurogenic, myogenic, and osteogenic differentiation, respectively (scale bar = 5 μm). MSC differentiation correlates to tissue-specific mechanical properties (e.g., soft leads to neural differentiation, whereas stiff leads to osteogenic differentiation). Reprinted with permission from Engler et al.34 Color images available online at www.liebertonline.com/ten.
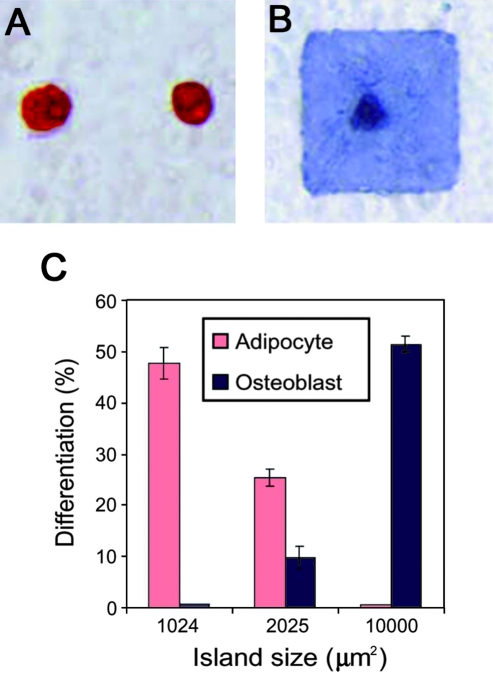
Using soft lithography techniques, researchers have investigated the influence of spatially patterned adhesion molecules on cellular behavior, including differentiation.35 These patterning tools have been used to investigate factors such as cell spreading and shape on MSC differentiation, through control over the cellular cytoskeleton. MSCs patterned on larger islands of adhesion ligands, which allowed for cell spreading tended to differentiate into osteoblasts, whereas cells on smaller islands where cells stayed rounded differentiated into adipocytes.35 Representative staining and quantification of differentiation in this system is shown in Figure 4. This work indicated that stem cell lineage commitment was regulated by RhoA and is a consideration in the design of biomaterials for stem cell cultures.
FIG. 4.
Cell spreading controls stem cell lineage specification. Human MSC adipogenic (A, Oil Red O stain) and osteogenic (B, Alkaline Phosphatase stain) differentiation in response to cell spreading through size of adhesive islands. Quantification indicates that adipogenic differentiation is favored on smaller islands, whereas osteogenic is enhanced on larger islands where more spreading is allowed (C). Reprinted with permission from McBeath et al.35 Color images available online at www.liebertonline.com/ten.
Controlled delivery of soluble factors
Soluble factors such as growth factors and cytokines are important for the initiation and control of stem cell differentiation. Regulatory molecules can be added to the culture media or secreted by the cells to induce differentiation, as, for example, for human MSCs, which can be directed to form a variety of cell lineages.36 In other cases, it is advantageous to deliver the molecules directly from the material, particularly for in vivo applications, or where spatial and temporal molecule delivery is desired. The molecules to be delivered include factors that can control differentiation, such as basic fibroblast growth factor, members of the transforming growth factor–β family, small molecules such as retinoic acid or plasmid DNA that can induce growth factor production by cells. Molecule release is typically controlled through diffusion, degradation, or combinations of these two factors, leading to a wide range of delivery profiles.37 Alternatively, regulatory molecules can be immobilized on the surface of biomaterials, which leads to enhanced spatial control, potentially through gradients that can mediate cellular migration. One recent example involves the combined application of scaffold-immobilized RGD and microencapsulated VEGF to induce vascular differentiation of human ESCs.38 Although it would be difficult to review the large amount of work that has been completed in this general area, there are studies that illustrate the importance of controlled molecule delivery. For instance, multiple growth factors have been delivered from the same scaffold sequentially based on polymer degradation rates,39,40 which opens up the possibility of controlled temporal delivery for complex signaling cascades in stem cells. In several studies, biodegradable particles have been encapsulated with stem cells in hydrogel networks to provide sustained delivery of factors to modulate stem cell differentiation.41
Bioreactors
Bioreactors are generally defined as devices in which biological processes (such as cell expansion, differentiation, or tissue formation on 3D scaffolds) occur under tightly controlled environmental conditions (such as exchange of oxygen, nutrients, and metabolites, and application of molecular and physical regulatory factors).42 In comparison with monolayer cultures, 3D systems have demanding mass-transport requirements to achieve tissue uniformity and avoid widespread necrosis in the inner regions of scaffolds. Typically, internal mass transfer relies on a combination of diffusion and convection and depends on cell density, scaffold structure, and diffusional properties. The external mass transfer is in turn a function of bioreactor hydrodynamics.
Design of a tissue engineering bioreactor should ideally incorporate quantitative understanding of the native environment and address the specific requirements for the tissue of interest. Tissue-engineering bioreactors are designed to precisely regulate the cellular microenvironment to support cell viability and 3D organization and provide spatial and temporal control of signaling. This overall requirement translates into a set of practical design objectives: (i) rapid and controllable expansion of cells; (ii) enhanced cell seeding of 3D scaffolds (at a desired cell density, high yield, high kinetic rate, and spatial uniformity); (iii) efficient local exchange of oxygen, nutrients, and metabolites; and (iv) provision of physiological stimuli.42 Current designs incorporate cascades of biological and physical stimuli to exert greater influence over cellular differentiation and development into functional tissue constructs. Bioreactors are often custom designed to account for specific mechanisms of nutrient transfer and specific physical factors inherent in the native tissue.
Bioreactors are also utilized to optimize process variables prior to actual tissue engineering applications, to investigate the physiological ranges of parameters (e.g., oxygen and shear) and their synergistic effects, in studies of disease models and drug screening. These bioreactors are often designed to be modular, mini-scaled, and multiparametric, to economize cells and reagents, and to increase the number of samples up to the high-throughput designs. In a general case, these bioreactors are not cell- or tissue-specific and are suitable to test multiple combinations of parameters to optimize conditions on small scales prior to use in tissue-engineering bioreactors, which are much less practical for conducting screening and optimization studies.
Perfusion for local control of cultured cells and vascularized tissues
One of the critical factors of cell survival and function, in vitro and in vivo, is the exchange of nutrients and waste with every cell. For tissues that are normally vascularized (e.g., muscle and bone), engineering of thick and compact grafts consisting of viable and well-differentiated cells requires some form of perfusion through the developing tissue. Otherwise, diffusional gradients of oxygen reduce the formation of viable tissue to an outer layer that is only about 100 μm thick (penetration depth of oxygen), and mostly apoptotic and dead cells in the bulk of the construct.43,44
In early studies of cardiac tissue engineering, a simple bioreactor consisting of cartridges in which the cultured tissue constructs were perfused with culture medium was developed to study the effects of hydrodynamic shear and oxygen level on the viability and function of cardiac cells.45 Subsequently, it was shown that rapid inoculation of cardiac cells into the porous scaffolds, using a thermally polymerizing hydrogel, followed by the immediate establishment of medium perfusion through the seeded scaffold substantially improved the viability and function of neonatal heart cells, but also exposed the cells to hydrodynamic shear (nonphysiologic).43,46–48 The advantages and limitations of this simple system have driven further developments of perfusion systems.
A biomimetic approach to supply oxygen to engineered cardiac tissue was established to mimic as closely as practically possible the convective-diffusive oxygen transport in native vascularized tissues.47,49 To mimic the capillary network, cells were cultured on porous scaffolds with an array of channels perfused with culture medium (to provide a separate compartment for medium flow) and the medium was supplemented with an oxygen carrier (to mimic the role of hemoglobin). This approach tends to provide in vivo–like mechanisms of oxygen supply to cultured cells and can overcome both the inherent limitations of diffusional transport in conventional culture systems and the adverse effects of shear in perfused nonchanneled tissue constructs.
The oxygen supply requirements are even more critical for cultures of stem cells, because of major effects of oxygen on cell survival and differentiation. Two types of perfused bioreactor systems have been primarily used in cultures of stem cells: bioreactors for cultivation of encapsulated or aggregated cells and bioreactors for cultivation of cells on porous 3D scaffolds. One example of the first class of bioreactors is a simple stirred flask with continuous perfusion of culture medium and monitoring of oxygen levels, which enhanced the yield and viability of the derivation of cardiomyocytes from ESCs encapsulated in agarose gel micro-drops.50,51 Another example is a similar system with medium recirculation for the expansion of neural mouse stem cells, shown to outperform several nonperfused systems, including static culture, stirred flasks, and rotating bioreactors.52
One design of a closed bioreactor with continuous perfusion of culture medium has been successfully used over long periods to derive large numbers of bone marrow–derived osteogenic cells for clinical therapies.53 Further studies are required to better characterize the effect of shear on ESCs in perfusion cultures. Microfluidic systems for cultivation of ESCs over a logarithmic range of flow rates will likely enhance these studies, as they enable tight control of hydrodynamic shear and the monitoring of cell responses by fluorescent microscopy.54
Recently, the use of perfusion bioreactors, in which the medium is pumped through the culture vessel containing cell suspensions, has been reported for the expansion of mouse ESC lines on Petri dishes with a gas-permeable base55 and for the expansion of human ESC lines on feeder layers.56 Perfusion systems yielded substantially higher cell numbers than regular static culture. Perfusion of cultures with concomitant cell retention can provide homogeneity of nutrient supplementations, waste removal, and the maintenance of cell-secreted factors.57 For stem cells to become a viable cell source for drug screening, robust and reproducible processes are needed for obtaining large numbers of cells with maintained phenotype and differentiation capacity into a range of cell types.58 Some of the bioreactors currently under research are likely to serve as precursors for the development of the new generation of technologies for stem cell expansion.
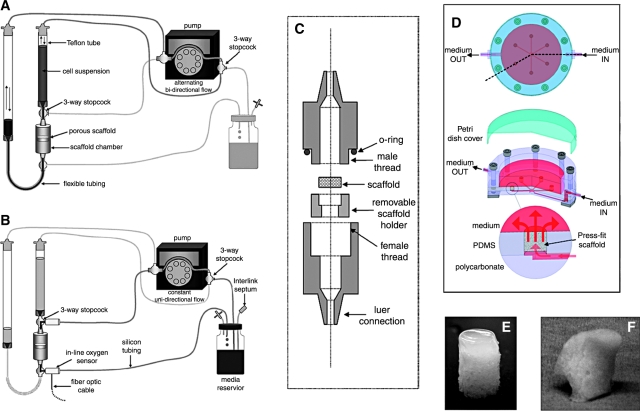
The second class of bioreactors was extensively used to culture adult human MSCs on porous biomaterial scaffolds, to facilitate cell seeding,59–61 proliferation,7 and osteogenic differentiation30,59,62–67 (please also refer to a recent review68 for more information on bioreactor designs for stem cell expansion and differentiation). Perfusing a cell suspension directly through the pores of a 3D scaffold was shown to enhance the yield and spatial uniformity of cell attachment, and to improve subsequent bone formation, presumably due to the convective transport of the culture medium throughout the entire scaffold volume.60,69 An attractive feature of the perfusion bioreactors is that the whole process occurs in one single sequence (Fig. 5, left) and that the bioreactor can be closed, which helps streamline the engineering process and reduce the safety risks associated with the transferring of constructs between bioreactors.70
FIG. 5.
Perfusion bioreactors for enhanced mass transport. Left: example of an integrated perfusion bioreactor system for cell seeding and prolonged culture of tissue constructs within a single device. (A) Cell seeding pathway: alternating bi-directional flow of cell suspension, without cell recirculation through the pump. (B) Cultivation pathway: transfer from seeding to cultivation is made by simply diverting the medium flow to a separate perfusion loop for prolonged culture. (C) Scaffold chamber: the scaffold is inserted into a removable holder and held in place by its outer 1 mm periphery. A straight region helps to fully develop the flow before reaching the construct. Right: Simple bioreactor system for tissue engineering of bone and osteochondral grafts that enables cultivation of up to six tissue constructs simultaneously, with direct perfusion and imaging capability. (D) Schematic presentation of the bioreactor system. The flow channels in the bioreactor provide an even distribution of medium flow between the six constructs (4–10 mm in diameter, up to 7 mm high) that are press-fit into the culture wells.77 (E) An example of a cartilage-bone plug from a gel layer overlaying the porous scaffold; both phases are seeded with human MSCs, and the construct is cultured in the perfusion bioreactor (D). (F) Example of an anatomically correct construct of a human temporomandibular joint condyle that was cultured in a bioreactor (D). Images (A)–(C) are reproduced with permission from Wendt et al.69 Color images available online at www.liebertonline.com/ten.
Medium perfusion through constructs also enhanced bone formation67,71–74 as a result of improved nutrient transfer and the intrinsic shear stresses associated with the medium flow. Shear forces—a physiologically relevant stimulus—have been shown to specifically influence the expression of osteogenic genes in osteoblasts,73 and to increase the deposition of mineralized matrix71,73,75 by mechanisms associated with these genes76 and further enhanced by dynamic flow.74 We recently developed a perfusion bioreactor that enables the cultivation of up to six constructs simultaneously, with medium perfusion and imaging compatibility77,78 (Fig. 5, right).
Mechanical loading for conditioning of habitually loaded tissues
Mechanical stimuli represent major regulators of the development and function of many tissues, including musculoskeletal (cartilage, bone, ligament, tendon, and skeletal muscle) and cardiovascular (myocardium, heart valve, and blood vessels) tissues. Mechanical forces regulate cell physiology under normal and pathological conditions, and modulate ECM synthesis and organization at various hierarchical levels—from molecules to whole tissues and organs. It is generally accepted that the structure and function of tissues and organs reflect the acting physical forces, with profound interactive effects. From the early days of tissue engineering, mechanical “conditioning” of cells cultured on biomaterial scaffolds was explored based on the premise that the same forces that govern tissue development and remodeling in vivo would also enhance tissue development and function in vitro. In several notable studies, stimulation protocols were designed to provide deformational loading of cartilage, mechanical tension of ligaments and muscle, and flow-induced stretch of blood vessels. The development of specialized bioreactors was a major contributing factor to these studies, which initially focused on the assembly of differentiated animal cells (e.g., bovine chondrocytes and rat cardiomyocytes) and recently have shifted toward human MSCs and ESCs.
For functional tissue engineering, bioreactors are utilized to recreate the physiologic loading environment and foster the growth of tissue constructs with mechanical properties and compositions enabling the immediate load-bearing function following implantation in vivo. For articular cartilage, deformational loading and hydrostatic pressure are primary components of the physical cell environment, and these forces have thus been employed in tissue engineering of cartilage. Most investigators utilized the regime of dynamic unconfined compressive loading between two impermeable platens, inducing the compressive and tensile strains with a minimal change in tissue volume, and giving rise to fluid flow gradients that enhance mass transport.79 The original approach, involving mechanical loading of agarose-encapsulated chondrocytes in conjunction with the supplementation of growth factors to culture medium, has been extended to human MSCs,80,81 human ESCs, other hydrogels,82 and the cultivation of osteochondral grafts79,83 (Fig. 6A).
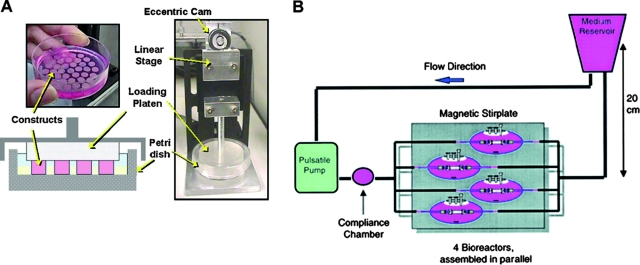
FIG. 6.
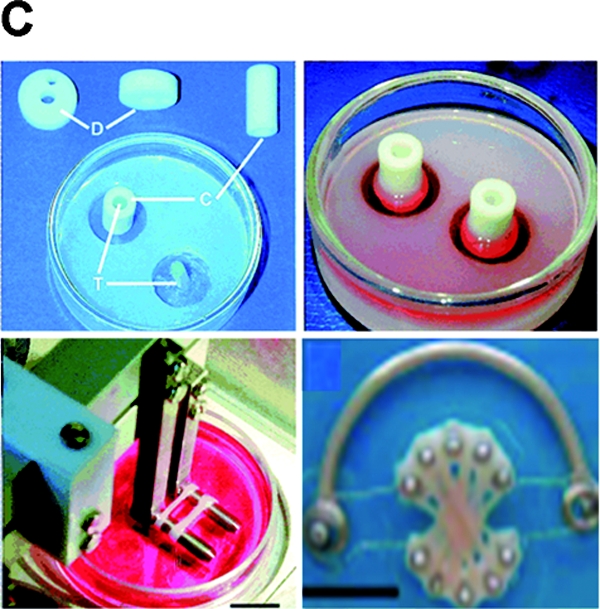
Mechanical stimulation bioreactors for functional tissue engineering. (A) Bioreactor system for dynamic deformational loading of engineered cartilage. Constructs are placed in the base of a standard Petri dish modified with a custom agarose template to maintain positioning during loading, which is carried out by applying sinusoidal deformation. Reproduced with permission from Hung et al.79 (B) Bioreactor system with pulsatile flow for engineering blood vessels. Fibrous tubular scaffolds seeded with aortic smooth muscle cells were placed over silicone tubing and subjected to pulsatile flow inducing 5% radial strain. After 8 weeks of culture, the silicone tubing was removed and a confluent layer of endothelial cells was formed in vessel lumens. Reproduced with permission from Niklason et al.89 (C) Bioreactor with mechanical stimulation for cardiac tissue engineering. Top left: Casting mold. Top right: Cells seeded into a collagen gel and cultured without stimulation for 1–4 days. Bottom left: Stretch apparatus for the application of unidirectional and cyclic stretch (10%, 2 Hz). Bottom right: Multiple rings connected into a construct for implantation studies. Reproduced with permission from Zimmermann et al.95 Color images available online at www.liebertonline.com/ten.
As compared to chondrocytes, human MSCs accumulated lower amounts of cartilaginous tissue matrix and developed lower mechanical properties over the same time periods.80 The finding that these differences are not a result of delayed differentiation suggests that the translation of these protocols from differentiated animal to undifferentiated human cells will necessitate the modifications of loading regimes or growth factor supplementation, or both. Studies by other groups indirectly support this expectation, by showing that mechanical stimulation and growth factors, applied separately or in combination, differentially regulated the expression of cartilage-specific genes in human MSCs derived from bone marrow aspirates of embryoid bodies.82,83
The same paradigm of functional tissue engineering was also applied to the in vitro creation of tendons by bioreactor cultivation of human MSCs on collagen-based scaffolds, with mechanical stimulation.84–86 In two related early studies, human ligaments were engineered by applying a combination of dynamic tension and torsion designed to mimic forces in the knee. Interestingly, mechanical loading alone, without the application of specific growth factors, induced the alignment of human MSCs and the accumulation of ligament-specific markers, in favor of alternate differentiation paths into cartilage or bone.85 In studies conducted by Butler et al., the function of engineered ligaments was substantially improved by using composite collagen scaffolds, mechanical stimulation in bioreactors, and precise measurements of forces acting in vivo.84
Native vessels develop and function in a complex mechanical environment that involves at least four different flow-induced factors: (i) hydrodynamic shear acting on endothelial cells (ECs) due to blood flow and on smooth muscle cells (SMCs) due to interstitial flow, (ii) luminal pressure, (iii) circumferential mechanical stretch, and (iv) longitudinal stretch. With a premise that the function of native vessels depends on the alignment and orientation of cells and ECM, which in turn can be mediated by mechanical forces, various bioreactors have been established to expose engineered blood vessels to mechanical stimulation. One of the designs involves the use of a mandrel to induce circumferential stretch and alignment of the cells encapsulated in the hydrogel.87 In separate studies that used a simple system with human MSCs grown on a silicone tube, combined application of the shear flow, radial stretch, and pulsatile pressure was shown to induce the expression of EC and SMC markers in cultured MSCs.88
Another meritorious design is a biomimetic perfusion system for engineering small-diameter blood vessel grafts. The system was first applied to ECs and SMCs from various species, and then the human bone marrow–derived MSCs were used as a source of SMCs.89,90 The approach involved seeding of SMCs (or their MSC-derived precursors) into tubular fibrous scaffolds and cultivation under pulsatile flow conditions using a perfusion bioreactor (Fig. 6B). Over 8 weeks of culture, vessels engineered using young bovine cells developed rupture strengths greater than 2000 mmHg, had suture retention strengths of up to 90 g, and had collagen contents of about 50% of normal.91 When vessel walls were engineered using human MSCs, the cyclic strain resulting from pulsatile flow inhibited cell proliferation independent of cell culture substrate, whereas its effects on MSC differentiation into SMCs depended on the presence of ECM and medium supplements.
Similar to blood vessels, native and tissue-engineered heart valves have been observed to respond to a combination of mechanical stimuli, including cyclic flexure, cyclic stretch, and hydrodynamic shear. The application of flexure and flow dramatically accelerated tissue formation in the bioreactor, resulting in significantly increased collagen contents and tissue stiffness.91 A novel bioreactor was developed in which these mechanical stimuli can be applied to an engineered heart valve independently or in combination.92 The bioreactor consists of two identical chambers, each holding up to 12 rectangular tissue specimens subjected to cyclic flexure, stretch, and steady laminar flow. This design provides a tool for the study of mechanical stimuli on the in vitro engineered heart valve tissue formation.
Application of cyclic mechanical stretch to neonatal rat heart cells in collagen–Matrigel gels, introduced by the Eschenhagen group, represents a significant approach to tissue engineering of well-differentiated cardiac tissue grafts with the development of contractile forces.93 This approach is also “biomimetic” in nature, as it involves the utilization of mechanical stretch as a physiologically relevant signal for cell differentiation (Fig. 6C). These constructs were suitable for implantation on infarcted rat hearts, and they delayed the thinning of the heart wall and induced functional improvement.93 However, heart cells are postmitotic and cannot be used as a source for human applications. This motivated the considerations of the applicability of the same approach to human ESCs.94 Two challenges that have been identified are the need to derive multiple cell lineages that constitute the heart tissue (cardiac myocytes, endothelial cells, smooth muscle cells, and fibroblasts) from ESCs, and to generate cell numbers that are high enough for growing clinically sized implants. The need to vascularize these tissues is also clearly recognized.
Microdevices for stem cell analysis
Microscale technologies are potentially powerful tools for addressing some of the challenges in tissue engineering.96 Micro-Electro-Mechanical Systems (MEMS) can be used to control features at length scales from 1 μm to 1 cm97 and are compatible with cells to control the cellular microenvironment in culture and miniaturize assays for high-throughput applications. Specifically, microfabrication with techniques such as soft lithography can be used to fabricate microscale devices without the use of expensive “clean rooms” and photolithographic equipment.97,98 Resolutions as low as several hundred nanometers may be achieved and used to control the topography and spatial distribution of molecules on a surface, as well as the subsequent deposition of cells.99 Soft lithographic methods can also be used to fabricate microfluidic channels and scaffolds for tissue engineering in a convenient, rapid, and inexpensive manner.100 The emergence of these tools is allowing for precise microenvironmental control of stem cells.
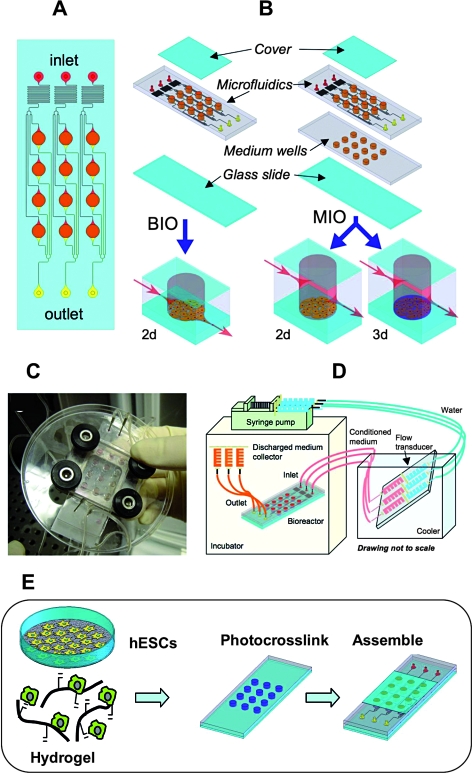
Microfluidic systems allow for controlled soluble and chemical environments (both 2D and 3D) for stem cells to probe these interactions to better understand differentiation and tools to manipulate and control their behavior. Chung et al.101 used controlled gradients of growth factors to spatially control the proliferation and differentiation of neural stem cells using MEMS devices. More complex microbioreactors have also been developed to screen soluble factor and biomaterial synergistic effects on stem cells in both 2D (attached to substrates) and 3D (encapsulated in hydrogels)102 (Fig. 7). Using automated image analysis, hESCs in these environments were assessed with respect to the expression of nuclear and cytoplasmic markers. Similarly, microdevices with microcontainers that immobilize colonies of cells have been used with rapid output analysis and illustrated good cultivation when perfused.103 The device was designed so that individual wells could be electrically stimulated to excite cells. Also, devices have been constructed that allow for the culture of cells under differential flow rates to control the soluble microenvironment and showed little proliferation of ESCs under static culture and enhanced proliferation under flow.54
FIG. 7.
Microarray bioreactors for in vitro studies. (A) The micro-bioreactor wells (3.5 mm in diameter) are arranged in an array. Each of three inlets delivers medium (red) through the flow transducers to four wells (orange) via microfluidic channels (100 μm wide); waste medium exits each bioreactor via a separate set of channels (yellow). (B) Two configurations were used: a bottom inlet/outlet (BIO) configuration, and a middle inlet/outlet (MIO) configuration (right) that allows for 3D cultivation. (C) Image of a single MBA with compression frame and fluidic connections. (D) Experimental setup. MBAs and medium collectors are placed in an incubator, and the medium reservoirs are maintained external to the incubator in an ice bath. (E) Schematic presentation of the human ESC culture in the microarray bioreactor system. Images (A)–(D) are from Figallo et al.102 and reproduced by permission of The Royal Society of Chemistry. Color images available online at www.liebertonline.com/ten.
Microengineering approaches can also be used to miniaturize cell–material interaction experiments and perform them in a highly parallel manner. This may be important in the identification of soluble factors, biomaterials, or synergy between these parameters. For example, arrays can be used to localize and track individual cells, enabling clonal analysis of stem cell fates.104 Also, clonal populations of neural stem cells were immobilized within microfabricated structures, and their progeny was tracked using real-time microscopy, yielding information about cellular kinetics and cell fate decisions in a high-throughput manner.105
Using this approach, it is possible to study the response of individual stem cells to various microenvironmental signals. Recently, robotic spotters capable of dispensing and immobilizing nanoliters of material have been used to fabricate microarrays, where cell–matrix interactions can be tested and optimized in a high-throughput manner. In these studies, synthetic biomaterial arrays have been used to test the interaction of stem cells with various extracellular signals.106 By using microarrayers, thousands of polymeric materials were synthesized and their effects on the differentiation of human ES cells106 and MSCs107 were evaluated. These interactions have led to unexpected and novel cell–material interactions. In another example, combinatorial matrices of various natural ECM were tested for their ability to maintain the function of differentiated hepatocytes and to induce hepatic differentiation from murine ES cells.108 Although these approaches were not performed in 3D settings or with interacting cell populations, they are indicative of the potential of these techniques for understanding and studying the cellular microenvironments. Finally, Demirci and Montesano109 immobilized cells in biological fluids and hydrogels using acoustic droplets to potentially investigate stem cell differentiation at the single-cell level.
Conclusions and Future Perspectives
Tissue engineering and regenerative medicine are increasingly relying on advanced scaffolds and bioreactors that provide control over multiple molecular and physical regulatory signals within sophisticated 3D culture environments. There are some general requirements applicable to any cell type or application (such as biocompatibility and degradation for a scaffold, and the control of medium composition, oxygen, and pH for a bioreactor). There are also requirements that are cell or tissue specific (such as specific structure and mechanical properties of the scaffold, cascades of signals, and need for physical stimulation of the cells). Distinctly different scaffold and bioreactor designs are now being utilized to engineer distinctly different tissues, or to study and characterize cells. In general, the utilization of 3D biomaterial scaffolds and bioreactors instead of “flat biology” or simple Petri dishes enables more rigorous experimentation under conditions that are tightly controlled and closer to those present in vivo.
The future needs outlined in the first review of the field of tissue engineering110 included (i) learning what controls cell differentiation, (ii) better techniques for cell sourcing and preservation, (iii) advanced scaffolds that combine advantages of native and synthetic materials and incorporate controlled-release techniques, (iv) vascularization, and (v) in vitro systems that can predict in vivo cellular events. Today, major advances have been made in all of these areas, but much more needs to be learned and completed before the next generation of biologically inspired technologies are possible that are sophisticated enough to elicit in vivo–like cell responses yet simple and practical enough for use in biology and medicine. In particular, further progress is needed in the identification of biological “blueprints” of engineering systems, and in the development of technologies for microenvironmental control, and these two areas are likely to progress in parallel and increasingly interact in the future.
Acknowledgments
The authors would like to acknowledge funding from the NIH (Grant K22DE015761 to J.A.B.; Grants DE16525, HL076485, EB002520, and CO21631 to G.V.N.) and a Packard Fellowship in Science and Engineering (to J.A.B.).
References
- 1.Harrison R. Observations on the living developing nerve fiber. Anat Rec. 1907;1:116–118. [Google Scholar]
- 2.Jacks T. Weinberg R.A. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111:923–925. doi: 10.1016/s0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 3.Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 4.Weaver V.M. Petersen O.W. Wang F. Larabell C.A. Briand P. Damsky C. Bissell M.J. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith L.G. Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 6.Radisic M. Park H. Gerecht-Nir S. Cannizzaro C. Langer R. Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering. Phil Trans R Soc London—B Biol Sci. 2007;362:1357–1368. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie T. Spradling A.C. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 8.Kiger A.A. Jones D.L. Shulz C. Rogers M.B. Fuller M.T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S. Beyond the Petri dish. Nat Biotechnol. 2004;22:151–152. doi: 10.1038/nbt0204-151. [DOI] [PubMed] [Google Scholar]
- 10.Dawson D. Mapili G. Erickson K. Taqvi S. Roy K. Biomaterials for stem cell differentiation. Adv Drug Del Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Nair L.S. Laurencin C.T. Biodegradable polymers as biomaterials. Prog Poly Sci. 2007;32:762–798. [Google Scholar]
- 12.Nerurkar N.L. Elliot D.M. Mauck R.L. Mechanics of orientated electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 13.Hwang N.S. Varghese S. Elisseeff J. Controlled differentiation of stem cells. Adv Drug Del Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C. Inokuma M.S. Denham J. Golds K. Kundu P. Gold J.D. Carpenter M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 15.Ruhnke M. Ungefroren H. Zehle G. Bader M. Kremer B. Fandrich F. Long-term culture and differentiation of rat embryonic stem cell-like cells into neuronal, glial, endothelial, and hepatic lineages. Stem Cells. 2003;21:428–436. doi: 10.1634/stemcells.21-4-428. [DOI] [PubMed] [Google Scholar]
- 16.Chang C.F. Lee M.W. Kuo P.Y. Wang Y.J. Tu Y.H. Hung S.C. Three-dimensional collagen fiber remodeling by mesenchymal stem cells requires the integrin-matrix interaction. J Biomed Mater Res A. 2007;80:466–474. doi: 10.1002/jbm.a.30963. [DOI] [PubMed] [Google Scholar]
- 17.Battista S. Guamieri D. Borselli C. Zeppetelli S. Borzacchiello A. Mayol L. Gerbasio D. Keene D.R. Ambrosio L. Netti P.A. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–6207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Dean S.K. Yulyana Y. Williams G. Sidhu K.S. Tuch B.E. Differentiation of encapsulated embryonic stem cells after transplantation. Transplantation. 2006;82:1175–1184. doi: 10.1097/01.tp.0000239518.23354.64. [DOI] [PubMed] [Google Scholar]
- 19.Gerecht-Nir S. Cohen S. Ziskind A. Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- 20.Liu H. Collins S.F. Suggs L.J. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Burdick J.A. Chung C. Jia X.Q. Randolph M.A. Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung C. Mesa J. Randolph M.A. Yaremchuk M. Burdick J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77A:518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. PNAS. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ifkovits J.L. Burdick J.A. Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 25.Levenberg S. Huang N.F. Lavik E. Rogers A.B. Itskovitz-Eldor J. Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. PNAS. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levenberg S. Burdick J.A. Kraehenbuehl T. Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 27.Burdick J.A. Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 28.Yang F. Williams C.G. Wang D.A. Lee H. Manson P.N. Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Nuttelman C.R. Tripodi M.C. Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68:773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X. Zhang S. Designer self-assembling peptide materials. Macromol Biosci. 2007;7:13–22. doi: 10.1002/mabi.200600230. [DOI] [PubMed] [Google Scholar]
- 31.Silva G.A. Czeisler C. Niece K.L. Beniash E. Harrington D.A. Kessler J.A. Stupp S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 32.Lutolf M.P. Raeber G.P. Zisch A.H. Tirelli N. Hubbell J.A. Cell-responsive synthetic hydrogels. Adv Mater. 2003;15:888–892. [Google Scholar]
- 33.Li Y.J. Chung E.H. Rodriguez R.T. Firpo M.T. Healy K.E. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79A:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 34.Engler A.J. Sen S. Sweeney H.L. Disher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 35.McBeath R. Pirone D.M. Nelson C.M. Bhadriraju K. Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Develop Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 36.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 37.Sokolsky-Papkov M. Agashi K. Olaye A. Shakesheff K. Domb A.J. Polymeric carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira L. Gerecht-Nir S. Shieh H. Vunjak-Novakovic G. Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen R.R. Mooney D.J. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 40.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nature Biotech. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 41.Park H. Temenoff J.S. Tabata Y. Caplan A.I. Mikos A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freshney I. Obradovic B. Grayson W. Cannizzaro C. Vunjak-Novakovic G. Principles of tissue culture and bioreactor design. In: Lanza R., editor; Langer R., editor; Vacanti J., editor. Principles of Tissue Engineering. 3rd ed. 2007. pp. 155–184. [Google Scholar]
- 43.Radisic M. Yang L. Boublik J. Cohen R.J. Langer R. Freed L.E. Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol. 2004;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 44.Radisic M. Malda J. Epping E. Geng W. Langer R. Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotech Bioeng. 2005;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 45.Carrier R.L. Rupnick M. Langer R. Schoen F.J. Freed L.E. Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175–188. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 46.Radisic M. Euloth M. Yang L. Langer R. Freed L.E. Vunjak-Novakovic G. High density seeding of myocyte cells for tissue engineering. Biotech Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 47.Radisic M. Deen W. Langer R. Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium supplemented with synthetic oxygen carriers. Am J Physiol. 2005;288:H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 48.Dvir T. Benishti N. Shachar M. Cohen S. A novel perfusion bioreactor providing a homogenous milieu for tissue regeneration. Tissue Eng. 2006;12:2843–2852. doi: 10.1089/ten.2006.12.2843. [DOI] [PubMed] [Google Scholar]
- 49.Radisic M. Park H. Chen F. Wang Y. Dennis R. Langer R. Freed L.E. Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 50.Bauwens C. Yin T. Dang S. Peerani R. Zandstra P.W. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 51.Baksh D. Zandstra P.W. Davies J.E. A non-contact suspension culture approach to the culture of osteogenic cells derived from a CD49 subpopulation of human bone marrow-derived cells. Biotechnol Bioeng. 2007;98:1195–1208. doi: 10.1002/bit.21556. [DOI] [PubMed] [Google Scholar]
- 52.Ng Y.L. Chase H.A. Novel bioreactors for the culture and expansion of aggregative neural stem cells. Bioprocess Biosyst Eng. 2007. In press. [DOI] [PubMed]
- 53.Dennis J.E. Esterly K. Awadallah A. Parrish C.R. Poynter G.M. Goltry K.L. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells. 2007;25:2575–2582. doi: 10.1634/stemcells.2007-0204. [DOI] [PubMed] [Google Scholar]
- 54.Kim L. Vahey M.D. Lee H.Y. Voldman J. Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip. 2006;6:394–406. doi: 10.1039/b511718f. [DOI] [PubMed] [Google Scholar]
- 55.Oh S.K. Fong W.J. Teo Y.W. Tan H.L. Padmanabhan J. Chin A.C.P. Choo A.B.H. High-density cultures of embryonic stem cells. Biotechnol Bioeng. 2005;91:523–533. doi: 10.1002/bit.20650. [DOI] [PubMed] [Google Scholar]
- 56.Fong W.J. Tan H.L. Choo A. Oh S.K. Perfusion cultures of human embryonic stem cells. Bioprocess Biosyst Eng. 2004;27:381–387. doi: 10.1007/s00449-005-0421-5. [DOI] [PubMed] [Google Scholar]
- 57.Thomson H. Bioprocessing of embryonic stem cells for drug discovery. Trends Biotechnol. 2007;25:224–230. doi: 10.1016/j.tibtech.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Gerecht S. Cannizzaro C. Figallo E. Elvassore N. Vunjak-Novakovic G. Bioreactors for the 3D cultivation of human embryonic stem cells. In: Masters J., editor; Palsson B., editor; Thomson J., editor. Human Embryonic Stem Cells, Human Cell Culture Series. Springer Verlag; 2007. pp. 149–172. [Google Scholar]
- 59.Zhao F. Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnol Bioeng. 2005;91:482–493. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 60.Wendt D. Jakob M. Martin I. Bioreactor-based engineering of osteochondral grafts: from model systems to tissue manufacturing. J Biosci Bioeng. 2005;100:489–494. doi: 10.1263/jbb.100.489. [DOI] [PubMed] [Google Scholar]
- 61.Timmins N.E. Scherberich A. Früh J.A. Heberer M. Martin I. Jakob M. Three-dimensional cell culture and tissue engineering in a T-CUP (tissue culture under perfusion) Tissue Eng. 2007;13:2021–2028. doi: 10.1089/ten.2006.0158. [DOI] [PubMed] [Google Scholar]
- 62.Braccini A. Wendt D. Jaquiery C. Jakob M. Heberer M. Kenins L. Wodnar-Filipowicz A. Quarto R. Martin I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23:1066–1072. doi: 10.1634/stemcells.2005-0002. [DOI] [PubMed] [Google Scholar]
- 63.Bancroft G.N. Sikavitsas V.I. Mikos A.G. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9:549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 64.Datta N. Pham Q.P. Sharma U. Sikavitsas V.I. Jansen J.A. Mikos A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. PNAS. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomes M.E. Holtorf H.L. Reis R.L. Mikos A.G. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:801–809. doi: 10.1089/ten.2006.12.801. [DOI] [PubMed] [Google Scholar]
- 66.Cartmell S.H. Porter B.D. Garcia A.J. Guldberg R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 67.Glowacki J. Mizuno S. Greenberger J.S. Perfusion enhances functions of bone marrow stromal cells in three-dimensional culture. Cell Transplant. 1998;7:319–326. doi: 10.1177/096368979800700310. [DOI] [PubMed] [Google Scholar]
- 68.King J.A. Miller W.M. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wendt D. Stroebel S. Jakob M. John G.T. Martin I. Uniform tissues engineered by seeding and culturing cells in 3D scaffolds under perfusion at defined oxygen tensions. Biorheology. 2006;43:481–488. [PubMed] [Google Scholar]
- 70.Kino-oka M. Ogawa N. Umegaki R. Taya M. Bioreactor design for successive culture of anchorage-dependent cells operated in an automated manner. Tissue Eng. 2005;11:535–545. doi: 10.1089/ten.2005.11.535. [DOI] [PubMed] [Google Scholar]
- 71.Bancroft G.N. Sikavitsas V.I. van den Dolder J. Sheffield T.L. Ambrose C.G. Jansen J.A. Mikos A.G. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteloblasts in a dose-dependent manner. PNAS. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Botchwey E.A. Pollack S.R. El-Amin S. Levine E.M. Tuan R.S. Laurencin C.T. Human osteoblast-like cells in three-dimensional culture with fluid flow. Biorheology. 2003;40:299–306. [PubMed] [Google Scholar]
- 73.Sikavitsas V.I. Bancroft G.N. Holtorf H.L. Jansen J.A. Mikos A.G. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. PNAS. 2003;100:14683. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu X.J. Botchwey E.A. Levine E.M. Pollack S.R. Laurencin C.T. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. PNAS. 2004;101:11203. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meinel L. Kareourgiou V. Fajardo R. Snyder B. Shinde-Patil V. Zichner L. Kaplan D. Langer R. Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 76.Rubin J. Rubin C. Jacobs C.R. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grayson W.L. Bhumiratana S. Cannizzaro C. Chao G.P. Lennon D. Caplan A.I. Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue engineered bone. Tissue Eng. 2008. In press. [DOI] [PMC free article] [PubMed]
- 78.Grayson W.L. Chao G.P. Marolt D. Kaplan D.L. Vunjak-Novakovic G. Engineering custom designed osteochondral tissue grafts. Trends Biotechnol. 2008;26:181–189. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hung C.T. Mauck R.L. Wang C.C. Lima E.G. Ateshian G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 80.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteo Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 82.Terraciano V. Hwang N. Moroni L. Park H.B. Zhang Z. Mizrahi J. Seliktar D. Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 83.Chao P.G. Grayson W. Vunjak-Novakovic G. Engineering cartilage and bone using human mesenchymal stem cells. J Orthop Sci. 2007;12:398–404. doi: 10.1007/s00776-007-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butler D.L. Juncosa-Melvin N. Boivin G.P. Galloway M.T. Shearn J.T. Gooch C. Awad H. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 85.Altman G. Horan R.L. Martin I. Farhadi J. Stark P.R.H. Volloch V. Richmond J.C. Vunjak-Novakovic G. Kaplan D.L. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 86.Altman G.H. Stark P. Lu H.H. Horan R.L. Calabro T. Martin I. Ryder D. Richmond J.C. Vunjak-Novakovic G. Kaplan D.L. Advanced bioreactor with multi-dimensional strain and biomimetic capability for tissue engineering. J Biomech Eng. 2002;124:742–749. doi: 10.1115/1.1519280. [DOI] [PubMed] [Google Scholar]
- 87.Isenberg B.C. Williams C. Tranquillo R.T. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 88.O'Cearbhaill E.D. Punchard M.A. Murphy M. Barry F.P. McHugh P.E. Barron V. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials. 2008;29:1610–1619. doi: 10.1016/j.biomaterials.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 89.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 90.Gong Z. Niklason L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Engelmayr G.C. Sales V.L. Mayer J.E. Sacks M.S. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: implications for engineered heart valve tissues. Biomaterials. 2006;27:6083–6095. doi: 10.1016/j.biomaterials.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 92.Engelmayr G.C. Soletti L. Vigmostad S.C. Budilarto S.G. Federspiel W.J. Chandran K.B. Vorp D.A. Sacks M.S. A novel flex-stretch-flow bioreactor for the study of engineered heart valve tissue mechanobiology. Ann Biomed Eng. 2008;36:700–712. doi: 10.1007/s10439-008-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zimmermann W.H. Melnychenko I. Wasmeier G. Didie M. Naito H. Nixdorff U. Hess A. Budinsky L. Brune K. Michaelis B. Dhein S. Schwoerer A. Ehmke H. Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 94.Zimmermann W.H. Eschenhagen T. Embryonic stem cells for cardiac muscle engineering. Trends Cardiovasc Med. 2007;17:134–140. doi: 10.1016/j.tcm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann W.H. Schneiderbanger K. Schubert P. Didié M. Münzel F. Heubach J.F. Kostin S. Neuhuber W.L. Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 96.Andersson H. van den Berg A. Microfabrication and microfluidics for tissue engineering: state of the art and future opportunities. Lab Chip. 2004;4:98–103. doi: 10.1039/b314469k. [DOI] [PubMed] [Google Scholar]
- 97.Kane R.S. Takayama S. Ostuni E. Ingber D.E. Whitesides G.M. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 98.Whitesides G.M. Ostuni E. Takayama S. Jiang X.Y. Ingber D.E. Soft lithography in biology and biochemistry. Ann Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 99.Khademhosseini A. Jon S. Suh K.Y. Tran T.N.T. Eng G. Yeh J. Seong J. Langer R. Direct patterning of protein- and cell-resistant polymeric monolayers and microstructures. Adv Mater. 2003;15:1995–2000. [Google Scholar]
- 100.Walker G.M. Zeringue H.C. Beebe D.J. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4:91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 101.Chung B.G. Flanagan L.A. Rhee S.W. Schwartz P.H. Lee A.P. Monuki E.S. Jeon N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 102.Figallo E. Cannizzaro C. Gerecht-Nir S. Burdick J. Langer R. Elvassore N. Vunjak-Novakovic G. Micro-bioreactor array for controlling cellular environments. Lab Chip. 2007;7:710–719. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 103.Gottwald E. Giselbrecht S. Augspurger C. Lahni B. Dambrowsky N. Truckenmüller R. Piotter V. Gietzelt T. Wendt O. Pfleging W. Welle A. Rolletschek A. Wobus A.M. Weibezahn K.F. A chip-based platform for the in vitro generation of tissues in three-dimensional organization. Lab Chip. 2007;7:777–785. doi: 10.1039/b618488j. [DOI] [PubMed] [Google Scholar]
- 104.Tourovskaia A. Figueroa-Masot X. Folch A. Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip. 2005;5:14–19. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 105.Chin V.I. Taupin P. Sanga S. Scheel J. Gage F.H. Bhatia S.N. Microfabricated platform for studying stem cell fates. Biotechnol Bioeng. 2004;88:399–415. doi: 10.1002/bit.20254. [DOI] [PubMed] [Google Scholar]
- 106.Anderson D.G. Levenberg S. Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–886. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 107.Anderson D.G. Putnam D. Lavik E.B. Mahmood T.A. Langer R. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 108.Flaim C.J. Chien S. Bhatia S.N. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 109.Demirci U. Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip. 2007;7:1139–1145. doi: 10.1039/b704965j. [DOI] [PubMed] [Google Scholar]
- 110.Langer R. Vacanti J.P. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]