Abstract
Purpose
Breast cancer screening by mammography and clinical breast exam are commonly used for early tumor detection. Previous cost-effectiveness studies considered mammography alone or did not account for all relevant costs. In this study, we assessed the cost-effectiveness of screening schedules recommended by three major cancer organizations and compared them with alternative strategies. We considered costs of screening examinations, subsequent work-up, biopsy, and treatment interventions after diagnosis.
Methods
We used a microsimulation model to generate women’s life histories, and assessed screening and treatment impacts on survival. Using statistical models, we accounted for age-specific incidence, preclinical disease duration, and age-specific sensitivity and specificity for each screening modality. The outcomes of interest were quality-adjusted life years (QALYs) saved and total costs with a 3% annual discount rate. Incremental cost-effectiveness ratios were used to compare strategies. Sensitivity analyses were performed by varying some of the assumptions.
Results
Compared to guidelines from the National Cancer Institute and the U.S. Preventive Services Task Force, alternative strategies were more efficient. Mammography and clinical breast exam in alternating years from ages 40 to 79 was a cost-effective alternative compared to the guidelines, costing $35,500 per QALY saved compared with no screening. The American Cancer Society guideline was the most effective and the most expensive, costing over $680,000 for an added QALY compared to the above alternative.
Conclusion
Screening strategies with lower costs and benefits comparable to those currently recommended should be considered for implementation in practice and for future guidelines.
Keywords: breast cancer, mammography, clinical breast exam, cost-effectiveness, screening
Background
Breast cancer remains the most common malignancy affecting women in North America. Early detection and improved treatment options have contributed to the persistent decline in the breast cancer mortality rate between 1990-2000[1, 2]. Mammography (MM) and clinical breast exam (CBE) are conventional screening modalities recommended by cancer societies in the U.S.
Currently, there is no consensus on the optimal approach to screening, including frequency, starting age, and examination modality. The American Cancer Society (ACS) recommends annual MM for women ages 40 and older, and CBE triennially beginning at age 20, and annually beginning at age 401. The National Cancer Institute (NCI) recommends MM every 1-2 years2, and the U.S. Preventive Services Task Force (USPSTF) recommends MM, with or without CBE, every 1-2 years for women ages 40 and older3. Because of the controversy over the recommendations, evaluating these screening strategies and other feasible alternatives to determine the optimum in terms of the tradeoff between costs and benefits is critical.
Although two of the major guidelines suggest CBE in combination with MM, almost all cost-effectiveness studies have focused on MM alone[3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14], but not the combined use of MM with CBE. Recent studies have shown that periodic CBE combined with MM improves the overall screening sensitivity compared with MM alone[15, 16, 17, 18]. As part of the annual well-woman examination, CBE is easy to administer and cheaper than MM, making it a sensible complement to MM. Biennial MM coupled with annual CBE was cost-effective compared to 47 alternative screening strategies, in an analysis using only the cumulated cost of screening examinations[19].
To date there is little research evaluating the cost-effectiveness of breast cancer screening programs combining MM and CBE while incorporating costs other than screening examinations, including costs of diagnostic follow-up due to abnormal examinations, treatment, and post-treatment costs after diagnosis. While some studies have included treatment costs subsequent to diagnosis[9, 10, 11, 3, 8, 13, 20], they have often been limited to specific age cohorts or subgroups (e.g. women older than 65). Few studies have considered costs for biopsy or work-ups, or directly addressed the issue of false-positive examinations, and of those that have, none have combined the modalities of MM and CBE.
We conducted a comprehensive microsimulation analysis investigating ten breast cancer screening strategies combining MM and CBE that encompass a variety of realistically feasible programs, including recommendations from the NCI, ACS and USP-STF. We included the cumulated cost of screening exams and subsequent medical costs, including diagnostic follow-up with potential biopsy examination, and treatments during three different phases after diagnosis for each investigated strategy. Our measure of benefits was expected quality-adjusted life years (QALYs).
Models and Data Input
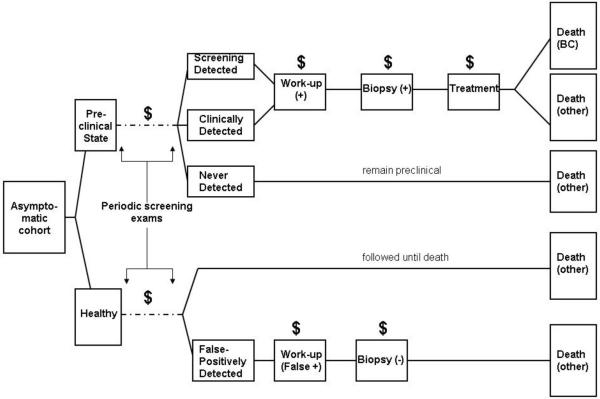
We used Monte Carlo simulation to generate each woman’s life history, following a previously published model structure[21]. We generalized the models to incorporate costs of work-up procedures due to false-positive examinations and treatments (Figure 1). Data inputs for each component in the model were estimated from published studies or randomized breast cancer screening trials. We describe new components to the model in detail below, and briefly discuss existing components; further details may be found in the literature[19, 21].
Figure 1.
Model structure for evaluating costs of screening, work-up, biopsy, and treatment for breast cancer. “$” represents accrual of costs, and “+” or “-” represents a positive or negative test result.
We considered ten screening strategies, plus a strategy of no screening. These strategies focus on realistic screening intervals, and include the recommended strategies from the ACS, NCI, and USPSTF. We varied time intervals between exams in different age cohorts to accommodate the age-dependency of incidence, sensitivity, and sojourn time.
The outcomes were expected QALYs and expected total medical costs per woman, each discounted at 3% annually beginning at age 20. We compared screening strategies using incremental cost-effectiveness ratios in an incremental analysis[22, 23]. Screening strategies were rank-ordered by increasing cost, and we used simple dominance to rule out strategies that are more costly but less effective than an alternative. The incremental cost-effectiveness ratio (ICER) was calculated for each strategy by dividing the difference in cost by the difference in benefit compared with the next least-expensive strategy. Strategies with lower effectiveness and higher ICER were ruled out by extended dominance and the ICER was recalculated after their elimination[23]. ICERs for the strategies not ruled out by dominance (efficient or cost-effective strategies) are interpreted as the ratio of additional cost per QALY saved compared to the next least-expensive alternative.
Natural History Model
We generate a birth cohort of 500,000 women by Monte Carlo simulation, where the cohort size is chosen so that the standard errors of the gain in QALYs compared to no screening is less than 0.2 days for all ten strategies. Each woman’s natural history is simulated independently. Prevalent cases are simulated according to age-specific incidences of breast cancer. Among women who develop breast cancer, we generate their natural histories of the disease over time. In the natural history model, we assume four relevant states of the progressive disease, as described by Zelen and Feinleib[24]: disease-free or asymptomatic state (H); detectable preclinical state (P); clinical state (C); and death state (D). For women who have the disease, we simulate their preclinical durations and ages at onset of clinical disease based on an assumed distribution and published data, respectively; and survival time, based on simulated age and tumor characteristics at detection.
The latent ages at onset of preclinical disease must be derived given age-specific incidence of clinical disease and preclinical sojourn time. We may easily obtain the age-specific incidences of breast cancer which are observable and well-documented, and we use age-specific estimates from an analysis by Moolgavkar et al.[25]. On the other hand, transition into the preclinical state is an unobservable event. We must therefore numerically derive the age-specific incidences of preclinical disease through a deconvolution approach described in Parmigiani[26] given the age-specific incidence of clinical disease and the sojourn time distribution.
We use the commonly used exponential distribution with an age-dependent component where the mean sojourn time, μ, depends on age at onset of preclinical disease. Uncertainty is incorporated through an inverse gamma prior for μ with scale and shape parameters that match the estimated mean and standard deviation from the CNBSS trials, where the mean sojourn time (and standard deviations) for ages ≤ 50 was 1.9 (1.2) years, and 3.1 (0.94) years for ages > 50 [15]. Thus, a random sojourn time is simulated for each subject depending on her age at onset of preclinical disease.
We modeled tumor growth by tumor volume-doubling time under the exponential growth model[27]. We assume that the threshold diameter for a tumor to be detectable by screening is 0.5cm[28], and that the diameter at which breast cancer becomes clinically manifested is 2cm or more, based on data from the CNBSS trials[29]. Depending on the number of tumor volume doublings between the minimum detectable tumor volume and the clinically symptomatic tumor volume, we calculate the doubling time (DT) for each woman as a quotient of the woman’s sojourn time and the number of doublings. We then obtain each woman’s tumor volume at diagnosis (TV), a random quantity which depends on the woman’s individual time spent in the preclinical state at the time of detection (time in P): TV = (minV ol) * 2#doublings, where minV ol is the minimum detectable tumor volume and .
Predicted survival times were based on age, tumor characteristics at diagnosis, and treatment. We used actuarial tables of a 1960 birth cohort from the U.S. Census Bureau database to determine whether a woman would die from breast cancer or from a competing risk.
Screening Impacts and Diagnostic Procedures
Evidence indicates that MM sensitivity depends on tumor size and age at screening[15, 30, 31, 28, 32, 18]. We modeled such dependence using a logit model, where the coefficients are determined using published age- and tumor size-dependent sensitivity estimates[32] and similarly, age-dependent false-positive rate estimates for MM[33]. Because insufficient evidence indicates that CBE sensitivity depends on age or tumor size, we used average values with uncertainty for CBE sensitivity and specificity[34]. We accounted for random variation in sensitivity and specificity within the cohort, consistent with previous screening trials[15, 34].
According to the National Comprehensive Cancer Network Breast Cancer Screening and Diagnosis Guidelines, women with positive or abnormal screening examinations are recalled for further work-up4. A recalled woman receives a diagnostic MM or ultrasound, then biopsy if the diagnostic test is positive. The recall rate after a positive initial screening examination ranges from 1-17%[35, 36]. We used diagnostic MM as the form of work-up, with sensitivity and specificity estimates from the Breast Cancer Surveillance Consortium5. For a woman whose tumor is detected symptomatically, a diagnostic MM and breast biopsy are also given to confirm the disease status. Women in the preclinical state who are never diagnosed are assumed to have died of other causes. For women who receive a false-positive MM, a follow-up MM will be provided 6 months after screening.
With the given data input and model assumptions, our simulated average sensitivities, specificities, and recall rates by screening modality and age group were within the range of reported estimates. The overall sensitivity and false-positive rate using both MM and CBE were calculated assuming the independence of the two modalities[37].
Treatment and Prediction of Survival
Treatments were provided according to general guidelines from the National Institutes of Health given tumor characteristics at diagnosis[38]. The number of nodes at diagnosis was predicted by a Poisson linear model given age and tumor size using Surveillance, Epidemiology, and End Results (SEER) registry data[39]. A truncated Poisson distribution was then used to constrain the number of nodes involved in a screen-detected case to be less than or equal to that of the same case at the expected time of clinical manifestation. Due to limited knowledge of the connection of tumor estrogen receptor (ER) status with other risk factors, we simulated ER status independently, allowing 70% to be ER-positive, according to the general population6.
Given tumor size and number of nodes, stage of disease was determined using the tumor-node-metastasis staging system[40]. According to the treatment guidelines, patients with stage I to IIIA breast cancer should receive breast conserving surgery (BCS) or mastectomy with or without radiation. We simulated surgery and radiation procedures according to recent studies, given disease stage at diagnosis [41, 42]. Administration of tamoxifen, chemotherapy, or a combination was simulated in accordance with observed U.S. dissemination patterns based on age, stage of disease, and ER status at diagnosis[43]
To estimate survival, measured from the time of diagnosis, we used a Cox regression model on age, ER status, primary tumor size, and number of nodes at diagnosis. We estimated covariate coefficients in the predictive survival model based on a combined analysis of four Cancer and Leukemia Group B trials[26, 44, 45, 46]. We used hazard reduction estimates due to treatment effects[43] and quality of life adjustments according to the type of adjuvant treatment[26, 47] (Table 1).
Table 1.
Age-specific reduction in mortality hazard ratio and quality-of-life adjustment factors due to treatment effects
| Treatment | Hazard Reduction by Age | Quality-of-life Adjustment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 50 | 50 - 59 | ≥ 60 | Months 1-6 |
Months 7-12 |
Year 2 |
Years 3-5 |
Remainder | |||
| None | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Tamoxifen | ER+ ER- |
0.28 0 |
0.28 0 |
0.28 0 |
ER+/- | 0.99 | 0.99 | 0.99 | 0.99 | 1 |
| Chemotherapy | 0.27 | 0.14 | 0.08 | 0.60 | 0.90 | 0.99 | 0.99 | 0.99 | ||
| Tamoxifen+Chemo | ER+ ER- |
0.47 0.27 |
0.38 0.14 |
0.34 0.08 |
ER+/- | 0.59 | 0.89 | 0.98 | 0.98 | 0.99 |
Costs
Total medical costs (Table 2) included costs of screening, including MM and CBE, and diagnostic MM[48, 49]. It should be noted that the cost used for CBE reflects the cost of the screening procedure alone, and not the total cost of a comprehensive well-woman examination. Additional costs include biopsy and treatments, where the cost of biopsy was a weighted average of costs of common biopsy procedures[48]. We included costs of primary surgery (BCS or mastectomy with or without radiation), and adjuvant chemotherapy with or without tamoxifen as part of the initial phase of care, followed by continuing care, and terminal phase[10, 50]. The initial phase included the first twelve months after diagnosis, and the terminal phase covered the last twelve months before death. The continuing-care phase included the duration between the end of the initial phase and beginning of the terminal phase, or a maximum of 25 years if the woman dies from competing risks. We included indirect costs from lost productive time through lost wages by age[51] for women who die prematurely from breast cancer. We converted all costs to year 2004 U.S. dollars using the medical care component of the Consumer Price Index7.
Table 2.
Direct costs due to breast cancer screening, diagnosis (work-up and biopsy), and treatment according to stage and type of surgery (year 2004 dollars). The initial phase of care includes any adjuvant chemotherapy
| Cost Components | Cost ($) | Ref. | |||
|---|---|---|---|---|---|
| Screening-related Costs | |||||
| Mammography (bilateral) | 83 | [48] | |||
| Clinical Breast Exam | 47 | [49] | |||
| Diagnostic Mammography (unilateral) | 78 | [48] | |||
| Breast Biopsy | 832 | [48] | |||
| Treatment-related Costs | |||||
| Tamoxifen/5yr. | 7,553 | [10] | |||
| Adjuvant Chemotherapy/1yr. (initial phase) | 5,618 | [10] | |||
| Monthly Costs by Phase and Stage | [10] | ||||
| Treatment Phase | BCS | BCS +rad. |
Mast. | Mast. +rad. |
|
| Initial Phase | |||||
| I | 1,611 | 2,688 | 2,228 | 3,228 | |
| II | 2,636 | 3,088 | 2,895 | 3,895 | |
| III/IV | - | 3,391 | 3,119 | 4,119 | |
| Continuing-Care Phase | |||||
| I | 287 | 185 | 250 | 250 | |
| II | 344 | 197 | 252 | 252 | |
| III/IV | - | 681 | 233 | 233 | |
| Terminal Phase (BC death) | |||||
| I | 3,904 | 3,071 | 3,945 | 3,945 | |
| II | 2,936 | 3,810 | 3,247 | 3,247 | |
| III/IV | - | 3,773 | 3,315 | 3,315 | |
| Monthly Terminal Phase Costs (non-BC) | 2,775 | [50] | |||
Ref = reference number corresponding to cost estimate;BCS = breast conserving surgery; Mast. = mastectomy; +rad. = (a primary treatment) plus radiation therapy; BC = breast cancer
Sensitivity Analysis
We conducted two 1-way sensitivity analyses. Because average sensitivity and specificity values for community-based CBE are different from estimates obtained in randomized clinical trials, we used a lower sensitivity and a higher specificity according to community-based estimates[34]. In a separate sensitivity analysis, we predicted survival using age- and stage-specific estimates calculated from the SEER database, used in the Cancer Intervention and Surveillance Modeling Network models[52]. In contrast to the original model, detailed tumor characteristics do not individually contribute to the survival estimate.
Results
The ten screening strategies and results are listed in Table 3. Strategy A, for example, gives MM and CBE every two years, and strategy B gives MM and CBE in alternating years, both from ages 40-79. The results showed that although every screening strategy extended life expectancy compared to no screening, some were more efficient with a lower cost per QALY saved than the alternatives: strategies A, D, F, I, and J, in order of increasing expense. Among them, biennial MM and CBE in alternating years from ages 40-79 (A) saved about 13 days of life for an additional $1,300, equivalent to $35,500 to save a year of life compared to no screening. The next cost-effective alternative was strategy D, with biennial MM and annual CBE from ages 40-79, which saved 1.8 additional days of life for $400, compared to strategy A. By replacing biennial with annual CBE in strategy A, it costs $90,100 to save an additional year of life compared to strategy A. The most expensive strategy J saved only a half day for an additional $5,500 compared to strategy I, with a very high ICER of over $3.9 million per QALY saved. Other strategies were eliminated by simple or extended dominance because of their inefficiency.
Table 3.
Results of cost-effectiveness analysis including screening, biopsy, and treatment costs. Costs and QALYs are discounted at 3%. Dominated strategies are indicated with ’—’
| Strat | MM intv(age) | CBE intv(age) | Total Cost ($) | QALYs (years) | Incremental QALYs gained | ICER |
|---|---|---|---|---|---|---|
| X | — | — | 13,100 | 27.3954 | ||
| A | 2(40-79) | 2(41-79) | 14,400 | 27.4311 | 0.0357 | 35,500 |
| B | 2(40-79) | 2(40-79) | 14,400 | 27.4272 | — | — |
| C | 1(40-79) | 14,700 | 27.4330 | — | — | |
| D | 2(40-79) | 1(40-79) | 14,800 | 27.4360 | 0.0049 | 90,100 |
| E | 1(40-49), 2(50-79) | 1(40-79) | 15,100 | 27.4361 | — | — |
| F | 1(40-59), 2(60-79) | 1(40-79) | 15,300 | 27.4388 | 0.0028 | 169,500 |
| G | 2(40-49), 1(50-79) | 1(40-79) | 15,300 | 27.4381 | — | — |
| H | 1(40-69), 2(70-79) | 1(40-79) | 15,500 | 27.4390 | — | — |
| I | 1(40-79) | 1(40-79) | 15,600 | 27.4396 | 0.0007 | 367,100 |
| J | 1(40-79) |
3(20-39) 1(40-79) |
21,100 | 27.4410 | 0.0014 | 3,939,000 |
MM=Mammography; CBE=Clinical breast exam; intv=time interval between examinations (in years)
Total Cost: Mean total cost per woman in the complete cohort, rounded to the nearest $100
QALYs: Mean total expected quality-adjusted life years per woman from age 20 in the complete cohort
ICER: Incremental cost-effectiveness ratio (incremental cost/incremental QALYs gained compared to next least-expensive strategy)
Current recommended strategies are shown in boldface: A=NCI/USPSTF; C=NCI; I=NCI/USPSTF; J=ACS
The tradeoff between total costs and expected QALYs for each screening strategy is visualized in a tradeoff plot (Figure 2), corresponding to the results in Table 3. Dominated strategies fall below the line connecting the non-dominated alternatives, representing the efficiency frontier. The most expensive (and effective) strategy J, on the upper right corner of the efficiency frontier, is the current recommendation from the ACS. Compared to the alternatives, the gain in QALYs is small considering the large cost difference.
Figure 2.
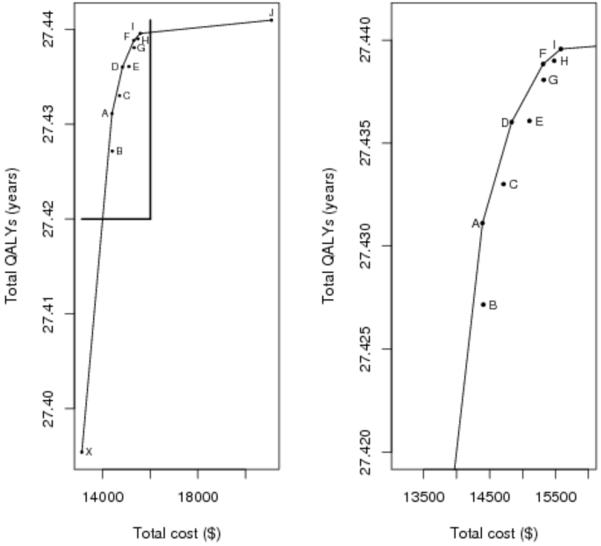
Tradeoff plot for strategies A-J (left) and excluding strategy J (right). X-axis is mean total cost in U.S. dollars. Y-axis is mean quality-adjusted life-years. Dominated strategies (B,C,E,G,H) fall below the line of efficiency connecting the non-dominated alternatives (A,D,F,I,J). The plot on the right allows better visualization of strategies A-I.
Sensitivity Analysis
When alternative sensitivity and specificity values were used for CBE, the results showed lower overall gains in QALYs and costs compared to the original analysis (Table 4). These changes may be explained by a delay in disease detection caused by the lower exam sensitivity, which leads to shorter survival. The reduction in medical costs is a result of fewer unnecessary work-ups and biopsy procedures due to the higher specificity. It is not surprising that strategy C, which does not use CBE and was not cost-effective before, became more cost-effective, since using CBE as a complement to MM is less effective when the sensitivity of CBE is low. The intensity of MM becomes more important in strategies complemented by CBE, which explains why strategies D and G, which were cost-effective in the original analysis, become dominated.
Table 4.
Results of sensitivity analyses assuming lower sensitivity and higher specificity for CBE, and assuming an alternative treatment distribution. Costs and QALYs are discounted at 3%. Dominated strategies are indicated with ’—’
| Assuming lower sensitivity and higher specificity for CBE |
Assuming alternative survival model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strat | Total Cost ($) |
QALYs (years) |
Incremental QALYs gained |
ICER | Strat | Total Cost ($) |
QALYs (years) |
Incremental QALYs gained |
ICER |
| X | 13,100 | 27.3944 | X | 13,000 | 27.4158 | ||||
| B | 14,300 | 27.4239 | — | — | A | 14,300 | 27.4427 | 0.0270 | 46,900 |
| A | 14,300 | 27.4265 | 0.0321 | 36,900 | B | 14,300 | 27.4396 | — | — |
| D | 14,700 | 27.4288 | — | — | C | 14,600 | 27.4448 | 0.0021 | 152,100 |
| C | 14,700 | 27.4331 | 0.0067 | 58,600 | D | 14,700 | 27.4450 | — | — |
| E | 14,900 | 27.4317 | — | — | E | 15,000 | 27.4449 | — | — |
| F | 15,100 | 27.4335 | — | — | F | 15,200 | 27.4459 | — | — |
| G | 15,100 | 27.4345 | — | — | G | 15,200 | 27.4466 | 0.0018 | 339,400 |
| H | 15,300 | 27.4357 | — | — | H | 15,300 | 27.4465 | — | — |
| I | 15,400 | 27.4359 | 0.0028 | 250,200 | I | 15,500 | 27.4470 | 0.0005 | 611,800 |
| J | 20,800 | 27.4376 | 0.0016 | 3,293,300 | J | 21,000 | 27.4480 | 0.0009 | 5,847,100 |
MM=Mammography; CBE=Clinical breast exam; intv=time interval between examinations (in years)
Total Cost: Mean total cost per woman in the complete cohort, rounded to the nearest $100
QALYs: Mean total expected quality-adjusted life years per woman from age 20 in the complete cohort
ICER: Incremental cost-effectiveness ratio (incremental cost/incremental QALYs gained compared to next least-expensive strategy)
Current recommended strategies are shown in boldface: A=NCI/USPSTF; C=NCI; I=NCI/USPSTF; J=ACS
Under the alternative survival model, the gain in QALYs compared to no screening appears smaller than that in the original analysis. Because disease stages do not capture detailed tumor characteristics, this model may not predict survival as accurately as the original model, which may explain the observed difference in survival. It is likely for a woman to be defined in the same disease stage at screening and clinical detection, despite any progression in tumor size or nodal status, making the survival estimates in both cases more similar. Compared to the original analysis, strategies C and G shifted and became cost-effective, while strategies D and F were not.
Discussion
This study is the first to comprehensively evaluate the cost-effectiveness of the combined use of MM with CBE in breast cancer early detection while accounting for costs of screening, work-up, biopsies due to true or false-positive examinations, and treatments. We assessed current recommended guidelines from three major cancer organizations and compared them with other realistic strategies that combine MM and CBE with different starting ages and intervals.
Compared to the alternatives, two of the recommended strategies are cost-effective in general: the NCI/USPSTF recommendation of annual MM and CBE from ages 40-79, and the most effective but expensive recommendation from the ACS that begins CBE at age 20, followed by MM and CBE from ages 40-79. The NCI/USPSTF recommendation of annual MM alone from ages 40 to 79 is cost-effective when the sensitivity of CBE is low, according to community-based settings. The NCI/USPSTF guideline of MM with CBE every two years was not an efficient strategy. A more cost-effective alternative is to provide MM and CBE in alternating years, which leads to more savings in QALYs with similar costs. Alternating exam years allows for annual examinations with one of the two screening modalities.
The strategy recommended by the ACS was the most expensive and effective. If society is willing to pay the required costs to save an additional QALY, this strategy is favorable. Alternatively, the cheapest but also effective alternative would be to offer biennial MM and CBE alternatively from ages 40-79 (strategy A).
Among all the strategies, only strategy A fell under a commonly accepted cost-effectiveness threshold of $50,000/QALY[53, 20]. Although this strategy is not as life saving as some other alternatives, the incremental gains in QALYs for the other efficient strategies D, F, I, and J, are not very large in comparison (1.8, 2.8, 3.1, or 3.6 days), with extra costs of $400, $900, $1,200, or $6,700 compared to strategy A. Compared to A, strategy J costs over $680,000 for an added QALY. The large cost difference is explained by the early accumulation of costs and discounting beginning at age 20. Under realistic monetary constraints, we must consider this large added expense when cheaper but still effective strategies exist. This issue is debatable for ethical reasons and depends on how much society is willing to pay to save an additional year of quality-adjusted life.
Our model does not make any assumptions on the effectiveness of CBE on mortality reduction, but relies on estimates of its sensitivity and specificity. The role of CBE in combination with MM depends on these estimates. The sensitivity analyses showed that higher specificity for CBE leads to lower patient recall rates, which decreases unnecessary work-ups and biopsies. However, the lower sensitivity of CBE increases the false-negative rate and delays diagnosis. The performance of screening exams affects the timing of diagnosis, recall rate, and intensity of treatment, which all affect overall costs and survival. The choice of survival model also has significant impact on the results. In a survival model where small changes in tumor size or nodal status have little effect on survival, differences in years gained due to screening may be small. Regardless of these changes in model assumptions, only three strategies remained more effective for a lower cost per QALY saved compared to the alternatives: 1) A: biennial MM and CBE in alternating years from ages 40-79, 2) I: annual MM and CBE from ages 40-79, and 3) J: the most expensive strategy of screening beginning at age 20.
Our analysis includes both direct costs of screening and treatment, and indirect costs for lost wages for women who die prematurely of breast cancer. Although it may be contended that the inclusion of lost wages in such an analysis as this may result in the double-counting of losses, we argue that our quality-of-life adjustments are due to treatment only. Adjustments beyond the duration of treatment are minimal and thus may not fully account for wages that may be lost due to early death from breast cancer.
There are several limitations to our study. First, we considered average medical costs as constants and did not take into account the variation across institutions. However, we believe our cost inputs are sufficient for this comparative analysis. Second, the surgery pattern used may not represent the general population. However, it has been shown that long-term costs for mastectomy and BCS are not notably different[42]. Third, the treatment options do not include recent changes such as third-generation endocrine therapies and axillary or sentinel lymph node dissection, because these treatment patterns for the general population were not available in the literature. Other treatment options may be included in a future analysis.
While we considered quality-of-life adjustments due to treatments, our analysis did not account for physical and emotional effects of screening, unnecessary work-ups, or biopsies. It is difficult to assign monetary values to such effects. Finally, our simulation model assumed full compliance of participants in a screening and treatment plan, which allowed us to evaluate the potential effectiveness of a screening program.
In summary, several alternative cost-effective strategies were found to be more efficient than the recommended guidelines, or to have lower costs with minimal loss of benefit. In place of current recommendations, biennial MM and CBE in alternating years from ages 40-79 was the cheapest cost-effective alternative. If enough funds are available to add annual CBEs to a screening program, the next cost-effective alternative is to offer biennial MM and annual CBE from ages 40-79. Breast cancer screening strategies with lower costs and benefits comparable to those currently recommended should be considered for implementation in practice and for future guidelines.
Acknowledgement
We thank Giovanni Parmigiani, PhD, Departments of Oncology, Biostatistics and Pathology at the Johns Hopkins University for providing his computer simulation code for our use and reference.
This research supported in part by National Institutes of Health (R01-07466)
Footnotes
Preliminary work was presented at a poster session at the Joint Statistical Meetings, 2007, Salt Lake City, UT.
References
- [1].Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- [2].Glass AG, Lacey JV, Carreon JD, Hoover RN. Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- [3].Salzmann P, Kerlikowske K, Phillips K. Cost-effectiveness of extending screening mammography guidelines to include women 40 to 49 years of age. Ann Intern Med. 1997;127:955–65. doi: 10.7326/0003-4819-127-11-199712010-00001. [DOI] [PubMed] [Google Scholar]
- [4].Carter R, Glasziou P, van Oortmarssen G, et al. Cost-effectiveness of mammographic screening in Australia. Aust J Public Health. 1993;17:42–50. doi: 10.1111/j.1753-6405.1993.tb00103.x. [DOI] [PubMed] [Google Scholar]
- [5].Eddy DM. Screening for breast cancer. Ann Intern Med. 1989;111:389–99. doi: 10.7326/0003-4819-111-5-389. [DOI] [PubMed] [Google Scholar]
- [6].Elixhauser A. Costs of breast cancer and the cost-effectiveness of breast cancer screening. Int J Technol Assess Health Care. 1991;7:604–15. doi: 10.1017/s0266462300007169. [DOI] [PubMed] [Google Scholar]
- [7].Lindfors KK, Rosenquist CJ. The cost-effectiveness of mammographic screening strategies. JAMA. 1995;274:881–4. [PubMed] [Google Scholar]
- [8].Rosenquist CJ, Lindfors KK. Screening mammography beginning at age 40 years. Cancer. 1998;82:2235–40. doi: 10.1002/(sici)1097-0142(19980601)82:11<2235::aid-cncr19>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [9].Kattlove H, Liberati A, Keeler E, Brook RH. Benefits and costs of screening and treatment for early breast cancer: development of a basic benefit package. JAMA. 1995;273:142–8. [PubMed] [Google Scholar]
- [10].Mandelblatt J, Schechter C, Yabroff K, et al. Benefits and costs of interventions to improve breast cancer outcomes in African American women. J Clin Oncol. 2004;22:2554–66. doi: 10.1200/JCO.2004.05.009. [DOI] [PubMed] [Google Scholar]
- [11].Mandelblatt J, Schecter C, Yabroff K, et al. Toward optimal screening strategies for older women. J Gen Intern Med. 2005;20:487–96. doi: 10.1111/j.1525-1497.2005.0116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98:774–82. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- [13].Boer R, deKoning H, Threlfall A, et al. Cost-effectiveness of shortening screening interval or extending age range of NHS breast screening programme: computer simulation study. BMJ. 1998;317:376–9. doi: 10.1136/bmj.317.7155.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mandelblatt J, Saha S, Teutsch S, et al. The cost-effectiveness of screening mammography beyond age 65 years: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2003;139:835–42. doi: 10.7326/0003-4819-139-10-200311180-00011. [DOI] [PubMed] [Google Scholar]
- [15].Shen Y, Zelen M. Screening sensitivity and sojourn time from breast cancer early detection trials: mammograms and physical examinations. J Clin Oncol. 2001;19:3490–9. doi: 10.1200/JCO.2001.19.15.3490. [DOI] [PubMed] [Google Scholar]
- [16].Barton MB, Harris R, Fletcher SW. Does this patient have breast cancer? The screening clinical breast examination: should it be done? How? JAMA. 1999;282:1270–80. doi: 10.1001/jama.282.13.1270. [DOI] [PubMed] [Google Scholar]
- [17].Bobo J, Lee N, Thames SF. Findings from 752081 clinical breast examinations reported to a national screening program from 1995 through 1998. J Natl Cancer Inst. 2000;92:971–6. doi: 10.1093/jnci/92.12.971. [DOI] [PubMed] [Google Scholar]
- [18].Oestreicher N, Lehman CD, Seger DJ, Buist DSM, White E. The incremental contribution of clinical breast examination to invasive cancer detection in a mammography screening program. Am J Roentgenol. 2005;184:428–32. doi: 10.2214/ajr.184.2.01840428. [DOI] [PubMed] [Google Scholar]
- [19].Shen Y, Parmigiani G. A model-based comparison of breast cancer screening strategies: mammograms and clinical breast examination. Cancer Epidemiol Biomarkers Prev. 2005;14:529–32. doi: 10.1158/1055-9965.EPI-04-0499. [DOI] [PubMed] [Google Scholar]
- [20].Kerlikowske K, Salzmann P, Phillips KA, Cauley JA, Cummings SR. Continuing screening mammography in women aged 70 to 79 years: impact on life expectancy and cost-effectiveness. JAMA. 1999;282:2156–63. doi: 10.1001/jama.282.22.2156. [DOI] [PubMed] [Google Scholar]
- [21].Shen Y, Parmigiani G. Optimization of breast cancer screening modalities. In: Nikoulina M, Commenges D, Huber C, editors. Probability, Statistics, and Modelling in Public Health. Springer Science and Business Media, Inc.; USA: 2006. pp. 405–20. [Google Scholar]
- [22].Petitti D. Meta-analysis, decision analysis, and cost-effectiveness analysis. 2nd ed. Oxford University Press, Inc.; New York, New York: 2000. [Google Scholar]
- [23].Hunink M, Glasziou P, Siegel J, et al. Decision making in health and medicine: integrating evidence and values. Cambridge University Press; New York, New York: 2001. [Google Scholar]
- [24].Zelen M, Feinleib M. On the theory of screening for chronic diseases. Biometrika. 1969;56:601–14. [Google Scholar]
- [25].Moolgavkar SH, Stevens RG, Lee JAH. Effect of age on incidence of breast cancer in females. J Natl Cancer Inst. 1979;62:493–501. doi: 10.1093/jnci/62.3.493. [DOI] [PubMed] [Google Scholar]
- [26].Parmigiani G. Modeling in medical decision making. John Wiley and Sons, Ltd; West Sussex, England: 2002. [Google Scholar]
- [27].Peer P, vanDijck J, Hendriks J, Holland R, Verbeek A. Age-dependent growth rate of primary breast cancer. Cancer. 1993;71:8547–51. doi: 10.1002/1097-0142(19930601)71:11<3547::aid-cncr2820711114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [28].Peer P, Verbeek A, Straatman H, Hendriks J, Holland R. Age-specific sensitivities of mammographic screening for breast cancer. breast Cancer Res Treat. 1996;38:153–60. doi: 10.1007/BF01806669. [DOI] [PubMed] [Google Scholar]
- [29].Shen Y, Yang Y, Inoue LYT, Munsell M, Miller A, Berry D. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97:1195–203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- [30].Cong XJ, Shen Y, Miller AB. Estimation of age-specific sensitivity and sojourn time in breast cancer screening studies. Stat Med. 2005;24:3123–38. doi: 10.1002/sim.2178. [DOI] [PubMed] [Google Scholar]
- [31].Kerlikowske K, Carney PA, Geller B, et al. Performance of screening mammography among women with and without a first-degree relative with breast cancer. Ann Intern Med. 2000;133:855–63. doi: 10.7326/0003-4819-133-11-200012050-00009. [DOI] [PubMed] [Google Scholar]
- [32].Kolb T, Lichy J, Newhouse J. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- [33].Elmore J, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false-positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089–96. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]
- [34].Elmore J, Armstrong K, Lehman C, Fletcher S. Screening for breast cancer. JAMA. 2005;293:1245–56. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yankaskas BC, Cleveland RJ, Schell MJ, Kozar R. Association of recall rates with sensitivity and positive predictive values of screening mammography. Am J Roentgenol. 2001;177:543–9. doi: 10.2214/ajr.177.3.1770543. [DOI] [PubMed] [Google Scholar]
- [36].Gur D, Sumkin JL, Hardesty LA, et al. Recall and detections rates in screening mammography. Cancer. 2004;100:1590–4. doi: 10.1002/cncr.20053. [DOI] [PubMed] [Google Scholar]
- [37].Shen Y, Wu D, Zelen M. Testing the independence of two diagnostic tests. Biometrics. 2001;57:1009–17. doi: 10.1111/j.0006-341x.2001.01009.x. [DOI] [PubMed] [Google Scholar]
- [38].NIH Consensus Conference Treatment of Early-Stage Breast Cancer. JAMA. 1991;265:391–5. [PubMed] [Google Scholar]
- [39].Ries LAG, Eisner MP, Kosary CL, et al. SEER cancer statistics review. National Cancer Institute; Bethesda, MD: 19752002. [Accessed 2007]. Available from: http://seer.cancer.gov/csr/1975_2002/ [Google Scholar]
- [40].Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–36. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- [41].Shen Y, Dong W, Esteva FJ, Kau S, Theriault RL, Bevers T. Are there racial differences in breast cancer treatments and clinical outcomes for women treated at M.D. Anderson Cancer Center? Breast Cancer Res Treat. 2006;102:347–56. doi: 10.1007/s10549-006-9337-2. [DOI] [PubMed] [Google Scholar]
- [42].Barlow WE, Taplin SH, Yoshida CK, Buist DS, Seger D, Brown M. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst. 2001;93:447–55. doi: 10.1093/jnci/93.6.447. [DOI] [PubMed] [Google Scholar]
- [43].Mariotto AB, Feuer EJ, Harlan LC, Abrams J. Dissemination of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975-1999. J Natl Cancer Inst Monogr. 2006;36:7–15. doi: 10.1093/jncimonographs/lgj003. [DOI] [PubMed] [Google Scholar]
- [44].Wood W, Weiss R, Tormey D, Holland J, Henry P, Leone L. A randomized trial of CMF versus CMFVP as adjuvant chemotherapy in women with node-positive stage II breast cancer: a CALGB study. World J Surg. 1985;9:714–8. doi: 10.1007/BF01655185. [DOI] [PubMed] [Google Scholar]
- [45].Perloff M, Norton L, Korzun A, Wood W, Carey R, Gottlieb A. Postsurgical adjuvant chemotherapy of stage II breast carcinoma with or without crossover to a non-cross-resistant regimen: a cancer and leukemia group B study. J Clin Oncol. 1996;14:1589–98. doi: 10.1200/JCO.1996.14.5.1589. [DOI] [PubMed] [Google Scholar]
- [46].Wood W, Budman D, Korzun A, Cooper M, Younger J, Hart R. Dose and dose intensity of adjuvant chemotherapy for stage II node-positive breast carcinoma. N Engl J Med. 1994;330:1253–9. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- [47].Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18:3302–17. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- [48].Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295:2374–84. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- [49].MAG Mutual Healthcare Solutions’ 2004 physicians’ fee and coding guide. MAG Mutual Healthcare Solutions, Inc.; 2003. [Google Scholar]
- [50].Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries’ costs of care in the last year of life. Health Aff. 2001;20:188–95. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- [51].Day JC, Newburger EC. The big payoff: educational attainments and synthetic estimates of work-life earnings. US Census Bureau; 2002. Available from: http://data.bls.gov/cgi-bin/surverymost/ [Google Scholar]
- [52].Cronin KA, Mariotto AB, Clarke LD, Feuer EJ. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr. 2006;36:26–9. doi: 10.1093/jncimonographs/lgj005. [DOI] [PubMed] [Google Scholar]
- [53].Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716–7. doi: 10.1046/j.1525-1497.1998.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]