Abstract
Strategies for delaying pest resistance to genetically modified crops that produce Bacillus thuringiensis (Bt) toxins are based primarily on theoretical models. One key assumption of such models is that genes conferring resistance are rare. Previous estimates for lepidopteran pests targeted by Bt crops seem to meet this assumption. We report here that the estimated frequency of a recessive allele conferring resistance to Bt toxin Cry1Ac was 0.16 (95% confidence interval = 0.05–0.26) in strains of pink bollworm (Pectinophora gossypiella) derived from 10 Arizona cotton fields during 1997. Unexpectedly, the estimated resistance allele frequency did not increase from 1997 to 1999 and Bt cotton remained extremely effective against pink bollworm. These results demonstrate that the assumptions and predictions of resistance management models must be reexamined.
The common soil bacterium Bacillus thuringiensis (Bt) produces crystals containing proteins that are toxic to certain insects but are harmless to most other organisms including people, wildlife, and most beneficial insects (1). Genes encoding Bt toxins have been incorporated into and expressed by crop plants, thus providing environmentally benign control of insect pests (2). In particular, Bt cotton produces Bt toxin Cry1Ac, which kills larvae of some major lepidopteran pests and decreases the need for conventional insecticides. However, widespread adoption of Bt crops increases the chances that pests will evolve resistance (2–4).
A critical and widely held assumption of strategies for delaying pest resistance is that genes conferring resistance are rare (2–4). Although data are limited, previous estimates for lepidopteran pests targeted by Bt crops seem to meet this assumption. Gould et al. (5) estimated that before widespread use of Bt cotton that produces toxin Cry1Ac, the frequency of a major recessive Bt resistance allele was 1.5 × 10−3 in tobacco budworm (Heliothis virescens). Andow et al. (6, 7) found no individuals of European corn borer (Ostrinia nubilalis) with alleles conferring resistance to Bt corn in 279 isofemale lines (1,116 haplotypes) from two populations.
To estimate the frequency of resistance to Bt cotton in field populations of the pink bollworm (Pectinophora gosypiella), a worldwide lepidopteran pest of cotton (8), we used two independent methods: a direct approach and an indirect approach. The direct approach was based on laboratory bioassays of susceptibility to Cry1Ac of field-derived strains of pink bollworm. The indirect approach was based on the relative abundance of live pink bollworm in field-collected bolls of Bt cotton and non-Bt cotton. Results from both approaches show that, in Arizona field populations of pink bollworm, the frequency of an allele conferring resistance to Bt cotton was surprisingly high in 1997. Yet, both approaches also indicate that the frequency of resistance did not increase as expected in 1998 or 1999.
Materials and Methods
Direct Estimates of Resistance Allele Frequency.
We estimated the frequency of resistance directly by collecting 300 to 2,000 cotton bolls from each of 10 cotton fields in Arizona from August to November in 1997 and from September to December in 1998, and from each of 13 cotton fields from August to December in 1999 (Fig. 1) (9, 10). With three exceptions, pink bollworm larvae were too scarce in Bt cotton to obtain sufficient numbers to initiate strains, and thus bolls were collected from non-Bt cotton fields adjacent to Bt cotton fields to initiate field-derived strains. At Eloy in 1997, we collected bolls from a Bt cotton field (Eloy Bt) and an adjacent non-Bt cotton field (Eloy non-Bt). At two locations (Coolidge and Mohave Valley) in 1999, we collected bolls from a Bt cotton field and an adjacent non-Bt cotton field. For each non-Bt cotton site analyzed, we obtained at least 100 individuals from bolls to initiate a field-derived strain. For the three Bt cotton sites analyzed, the numbers of individuals used to initiate field-derived strains were 41 for Eloy Bt, 9 for Coolidge Bt, and 24 for Mohave Valley Bt. Unless specified otherwise, larvae were reared on wheat germ diet without Cry1Ac (9, 10).
Figure 1.
Field sites where strains of pink bollworm were derived in 1997–1999.
To determine the susceptibility of each field-derived strain, neonates of one or more generations from the unselected F1 through F7 generations were tested in laboratory bioassays on wheat germ diet containing 0 (control), 3.2, or 10 μg of Cry1Ac per ml of diet (9, 10). For strains that were tested in more than one generation, data were pooled across generations. The susceptible APHIS-S strain, which had been reared in the laboratory for many generations without exposure to toxins, was also tested in bioassays. The source of Cry1Ac was MVPII (Dow), which is a liquid formulation containing protoxin expressed in and encapsulated by Pseudomonas fluorescens. Diet was shredded and dispensed into 33-ml cups. One neonate was transferred into each cup with a fine brush (9). Each replicate consisted of 10 to 25 individually tested neonates, with each concentration replicated at least twice for each strain. After 21 days in darkness at 29°C (+/−2°C), live fourth instar larvae and pupae were scored as survivors. Adjusted survival was calculated as survival (%) on treated diet divided by survival (%) on untreated diet for each strain, which is equivalent to correcting for control mortality with Abbott's method and calculating adjusted survival as 100% − adjusted mortality. In addition to the bioassays in which neonates were tested individually as described above, in 1999 only, we conducted mass bioassays in which one or more groups of up to 200 neonates from each of eight field-derived strains were tested in 530-ml cups containing diet.
The Arizona pooled resistant (AZP-R) strain was created by pooling survivors from the initial bioassays and selecting with Cry1Ac as follows. For the first round of selection, we pooled from the 1997 survey all survivors of exposure to 10 μg of Cry1Ac per ml of diet (16/500) and 3.2 μg of Cry1Ac per ml of diet (106/1061) with survivors of exposure to 1 μg of Cry1Ac per ml of diet from the Eloy non-Bt strain (37/215). These 159 survivors were the parents of the AZP-R strain. The F1-F4 progeny of these survivors were reared without exposure to Cry1Ac. In the second round of selection, approximately 100,000 F5 larvae were exposed to 10 μg of Cry1Ac per ml of diet. After these two rounds of selection, the median lethal concentration (LC50) for the AZP-R strain was 162 μg of Cry1Ac per ml of diet (95% fiducial limits 138–191), which is 300 times higher than the LC50 of the susceptible APHIS-S strain (0.53 μg of Cry1Ac per ml of diet, 95% fiducial limits 0.20–0.82; ref. 10). In the third selection, larvae of the F7 generation were exposed to 100 μg of Cry1Ac per ml of diet.
To determine the mode of inheritance of resistance, we tested F1 offspring from each of the reciprocal crosses between the resistant AZP-R strain and the susceptible APHIS-S strain. We determined the sex of pupae visually. For one cross, we pooled 68 APHIS-S female pupae and 56 AZP-R male pupae. For the other cross, we pooled 64 AZP-R female pupae and 60 APHIS-S male pupae. We tested neonates of F1, APHIS-S, and AZP-R on diet containing 0 (control) and 10 μg of Cry1Ac per ml of diet. A total of 40 neonates were tested in 8 bioassay cups at each Cry1Ac concentration for each strain and each of the two sets of reciprocal F1 offspring.
To determine whether a major locus conferred resistance, we conducted two independent replicates in which we tested F2 offspring from reciprocal backcrosses between F1 and AZP-R. For each replicate of each reciprocal cross, at least 28 pupae of each type (F1 and AZP-R) were included for mating and progeny production. In each replicate, 40 neonates from AZP-R, APHIS-S, and from each reciprocal cross were tested on diet containing 0 (control) and 10 μg of Cry1Ac per ml of diet. If a major locus with two alleles (R, resistant and S, susceptible) confers resistance to Cry1Ac, half of the F2 backcross larvae (from F1 × AZP-R) are expected to be resistant homozygotes (RR), and half are expected to be heterozygotes (RS). Thus, we would expect survival of the F2 backcross larvae to be the mean survival of the resistant strain (putative RR) and F1 hybrid progeny (putative RS; ref. 11). We used a χ2 test to determine whether F2 survival differed significantly from the mean survival of the AZP-R and F1 (12).
Indirect Estimates of Resistance Allele Frequency.
We based indirect estimates of resistance on the relative abundance of live pink bollworm in Bt cotton and non-Bt cotton from 1995 to 1999. For 1995 to 1998, previously published reports provided data on the number of live pink bollworm larvae per boll of Bt cotton (PBT) and non-Bt cotton (PNBT) in paired fields where Bt cotton was adjacent to non-Bt cotton (10, 13, 14). For 1999, we used previously unpublished data that were collected using similar methods. To calculate indirect estimates of resistance allele frequency from these data, we assumed that the number of eggs laid per boll and mortality unrelated to Bt were equal for Bt cotton and non-Bt cotton. We also assumed that all survivors in Bt cotton bolls were homozygous for resistance. Our greenhouse bioassays suggest that adjusted survival of homozygous resistant individuals was 40% in Bt cotton bolls. Thus, we estimated the frequency of homozygous resistant individuals as (PBT/PNBT)/0.40. Assuming Hardy–Weinberg equilibrium, the frequency of the resistance allele was calculated as the square root of the frequency of homozygous resistant individuals. We used the bootstrap method with 10,000 repetitions to estimate the 95% CI of the mean resistance allele frequency for each year.
If bolls that are designated as Bt cotton bolls do not produce enough Cry1Ac to kill susceptible and heterozygous larvae, the indirect approach could overestimate the frequency of resistance. To reduce this potential bias, we excluded three pairs of fields (one in 1995, 1997, and 1998) for which the original reports indicated that one or a few bolls designated as Bt cotton bolls were suspected to contain deficient levels of Cry1Ac. This suspicion was based on finding one or a few sampled bolls with large numbers of live pink bollworm larvae while nearly all other bolls sampled had none. For 1995 to 1997, we obtained one estimate of resistance allele frequency from each pair of Bt and non-Bt cotton fields. Compared with 1995–1997, the 1998 and 1999 data included more fields, but fewer bolls of non-Bt cotton per field. Thus, to increase reliability of the 1998 and 1999 estimates, we pooled data from two sets of paired fields (nearest neighbors) to obtain each of the 16 estimates of resistance allele frequency from the 64 fields sampled in 1998 and each of the 17 estimates of resistance allele frequency from the 68 fields sampled in 1999. Because the indirect approach requires more assumptions, we consider it less reliable than the direct approach. Moreover, the indirect estimates of resistance allele frequency for 1995 and 1996 must be interpreted cautiously because they cannot be checked against direct estimates.
Greenhouse Bioassays.
Greenhouse bioassays (15) with the resistant AZP-R strain and the heterogeneous strain derived from Safford in 1997 were replicated twice, with a cumulative total of 3,276 neonates infesting 87 bolls on 13 Bt cotton plants (Deltapine 50B) and 13 non-Bt cotton plants (Deltapine 50). The first and second replicates were done, respectively, with the F8 and F9 generations of AZP-R and the F10 and F12 generations of Safford. AZP-R had been selected with Cry1Ac three times as described above. After 5 weeks, bolls were dissected. Larvae that exited bolls or were alive and had reached the fourth instar within bolls were scored as live. For each strain, adjusted survival on Bt cotton was calculated as survival on Bt cotton divided by survival on non-Bt cotton.
Results
Direct Estimates of Resistance Allele Frequency.
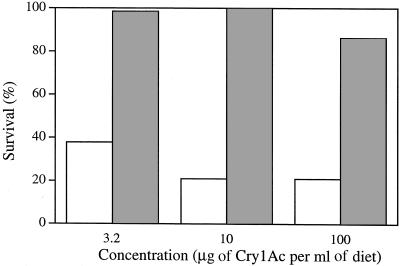
The bioassay results from 1997 show variation in pink bollworm susceptibility to Cry1Ac (Table 1). The rapid response to selection with Cry1Ac indicates that this variation was genetically based (Fig. 2).
Table 1.
Direct estimates of resistance (R) allele frequency from responses of pink bollworm larvae to Bt toxin Cry1Ac
| Strain | 3.2 μg of Cry1Ac per ml of
diet
|
10 μg of Cry1Ac per ml of diet
|
Estimated R allele frequency† | ||||
|---|---|---|---|---|---|---|---|
| Live | Total | Survival (%)* | Live | Total | Survival (%)* | ||
| APHIS-S | 10 | 170 | 6.4 | 0 | 140 | 0 | 0 |
| Coolidge | 11 | 110 | 15.5 | 0 | 40 | 0 | 0 |
| Eloy Bt | 23 | 175 | 19.3 | 4 | 50 | 11.8 | 0.34 |
| Eloy non-Bt | 1 | 140 | 1.2 | 0 | 70 | 0 | 0 |
| Marana | 0 | 95 | 0.0 | 0 | 70 | 0 | 0 |
| Mohave Valley | 15 | 100 | 19.2 | 7 | 40 | 22.4 | 0.47 |
| Paloma | 12 | 70 | 22.2 | 0 | 70 | 0 | 0 |
| Parker Valley | 5 | 106 | 6.2 | 0 | 40 | 0 | 0 |
| Safford | 12 | 80 | 19.4 | 1 | 50 | 2.6 | 0.16 |
| Solomon | 20 | 105 | 23.0 | 2 | 20 | 12.0 | 0.35 |
| Stanfield | 7 | 80 | 14.0 | 2 | 50 | 6.4 | 0.25 |
APHIS-S is a susceptible laboratory strain; the other 10 strains were derived from Arizona cotton fields in 1997.
Larvae that had not reached the fourth instar after 21 days were considered dead. Adjusted survival was calculated as survival on treated diet divided by survival on untreated diet.
Square root of the adjusted frequency of survivors at 10 μg of Cry1Ac per ml of diet.
Figure 2.
Response of pink bollworm strain AZP-R to selection with Bt toxin Cry1Ac. Adjusted survival after one round of selection (open bars, n = 50 larvae per concentration) and after two rounds of selection (shaded bars, n = 80 larvae per concentration).
The progeny of the survivors of the second round of selection, which entailed exposure to 10 μg of Cry1Ac per ml of diet (see Materials and Methods), showed 100% adjusted survival at 10 μg of Cry1Ac per ml of diet (Fig. 2). If resistance at this concentration was not recessive, some heterozygous individuals would have survived and produced some susceptible progeny in the subsequent generation. Thus, the results show that resistance to 10 μg of Cry1Ac per ml of diet was recessive, with only homozygous resistant individuals surviving.
Results of a cross between the resistant AZP-R strain and the susceptible APHIS-S strain confirmed that resistance to 10 μg of Cry1Ac per ml of diet is recessive. Exposure to 10 μg of Cry1Ac per ml of diet killed 99% of the F1 hybrid progeny tested (n = 80), all of the APHIS-S larvae tested (n = 40), and none of the AZP-R larvae tested (n = 40). Results did not differ between the F1 hybrid progeny from the two reciprocal crosses (APHIS-S female X AZP-R male and AZP-R female X APHIS-S male), indicating autosomal inheritance. Adjusted mortality of progeny from the F1 X AZP-R backcross was 48% (n = 160) at 10 μg of Cry1Ac per ml of diet, which is consistent with the major locus hypothesis (χ2 test, X2 = 0.36, P = 0.55).
Based on results from the selection and crossing experiments described above, we inferred that survivors of 10 μg of Cry1Ac per ml of diet were homozygous for an autosomal recessive resistance allele. Assuming Hardy–Weinberg equilibrium, we estimated the frequency of this allele in field-derived samples as the square root of the adjusted survival frequency at this concentration (Table 1). For the 10 strains derived from the field in 1997, the mean estimated resistance allele frequency is 0.16 (95% CI estimated by the bootstrap method with 10,000 repetitions = 0.05–0.26).
In bioassays of 10 strains derived from the field in 1998 (Fig. 1), pooled survival at 10 μg of Cry1Ac per ml of diet was only 1 of 1,100 compared with 16 of 500 in the strains derived in 1997 (Table 1). Whereas strains derived in 1997 from Mohave Valley and Safford had survivors at 10 μg of Cry1Ac per ml of diet (Table 1), strains derived in 1998 from these areas did not. The strain from Casa Grande (not sampled in 1997) was the only Arizona strain derived from the field in 1998 that showed survival at 10 μg of Cry1Ac per ml of diet (1/240; adjusted survival = 0.49%). Assuming that this survivor was homozygous resistant, the mean estimated resistance allele frequency for 1998 is 0.0070 (95% CI = 0–0.017).
In bioassays of 13 strains derived in 1999 from two Bt cotton fields and 11 non-Bt cotton fields (Fig. 1), none of the 1,179 individually tested neonates survived exposure to 10 μg of Cry1Ac per ml of diet. Further, survival was also 0% in mass bioassays of 5,549 neonates from eight strains derived from the field in 1999. These results yield an estimated resistance allele frequency of 0 for 1999.
Indirect Estimates of Resistance Allele Frequency.
Indirect estimates of resistance allele frequency based on the relative abundance of pink bollworm larvae in bolls of Bt and non-Bt cotton in the field (Table 2) confirm that the resistance allele frequency did not increase from 1997 to 1999. The indirect estimates of mean resistance allele frequency were 0.13 (95% CI = 0.055–0.24) for 1997, 0.050 (0–0.16) for 1998, and 0.11 (0.045–0.18) for 1999. For 1997 and 1998, the 95% confidence intervals for the indirect estimates overlap with those of the direct estimates, indicating agreement between the two approaches for both years. For 1999, the indirect estimate is greater than the direct estimate. In general, the direct estimate is more reliable because it entails fewer assumptions. In particular, as reported above, bioassays of strains derived in Bt cotton fields in 1999 showed that the progeny of survivors from Bt cotton from two field sites (Coolidge Bt and Mohave Valley Bt) were not resistant to Cry1Ac. These results suggest that the indirect estimate for 1999 overestimates the resistance allele frequency.
Table 2.
Indirect estimates of resistance (R) allele frequency from the relative abundance of live pink bollworm larvae in bolls from paired Bt cotton and non-Bt cotton fields in Arizona
| Year | Fields | Bolls | Relative abundance* | Efficacy,† % | Estimated mean R allele frequency (95% CI)‡ |
|---|---|---|---|---|---|
| 1995 | 8 | 39,800 | 1.1 × 10−4 | 99.99 | 0.017 (0 − 0.033) |
| 1996 | 12 | 67,200 | 2.0 × 10−3 | 99.80 | 0.070 (0.0050 − 0.12) |
| 1997 | 14 | 70,750 | 6.9 × 10−3 | 99.31 | 0.13 (0.055 − 0.24) |
| 1998 | 64 | 40,400 | 9.9 × 10−4 | 99.90 | 0.050 (0 − 0.16) |
| 1999 | 68 | 29,800 | 5.2 × 10−3 | 99.48 | 0.11 (0.045 − 0.18) |
Relative abundance, pink bollworm larvae per boll of Bt cotton/pink bollworm larvae per boll of non-Bt cotton. The mean relative abundance was calculated from arcsine-square root transformed data and was back-transformed for reporting in the Table.
Efficacy = (1 − relative abundance) × 100%.
See Materials and Methods.
Greenhouse Bioassays.
To determine whether the observed resistance confers increased survival on Bt cotton, we tested the AZP-R strain and the 1997-derived Safford strain on Bt cotton and non-Bt cotton plants in the greenhouse. Survival for AZP-R was 3.1% (28/897) on Bt cotton and 7.8% (43/551) on non-Bt cotton. Survival for Safford was 0.12% (1/801) on Bt cotton and 7.6% (78/1027) on non-Bt cotton. Thus, adjusted survival on Bt cotton was 40% for AZP-R and 1.6% for Safford. The proportion of larvae surviving was higher for AZP-R than for Safford on Bt cotton (2-tailed Fisher's exact test, P < 0.0001) but did not differ between strains on non-Bt cotton (P = 0.92). Because the Safford strain had an estimated frequency of 2.6% resistant homozygotes (Table 1), we suspect that the sole survivor on Bt cotton from this strain was homozygous for resistance. Survivors on Bt cotton were pooled as adults and produced viable progeny.
Discussion
The results imply that resistant pink bollworm larvae capable of surviving on Bt cotton were not rare in some Arizona cotton fields in 1997. The estimates of mean resistance allele frequency for 1997 were 0.16 (95% CI 0.05–0.26) from the direct approach and 0.13 (95% CI = 0.055–0.24) from the indirect approach. These estimates are roughly 100 times higher than those for other lepidopteran pests that attack Bt crops (5–7). Because Bt cotton accounted for more than half of the >100,000 hectares of cotton in Arizona in 1997, 1998, and 1999, predictions from models led us to expect that the resistance allele frequency would increase, but this did not occur. Because the observed pattern contradicts expectations, we carefully checked our methods and considered effects of potential deviations from our assumptions.
To check the possibility that the direct estimate of resistance allele frequency did not increase because of changes in our bioassay between tests of the strains derived in 1997 and those derived in 1998, we retested three of the heterogeneous 1997-derived strains concommitantly with tests of the 1998-derived strains. We retested the strains derived in 1997 from Safford and the Mohave Valley, and the substrain AZP, which had been separated from AZP-R after the first round of selection. After 1 year of rearing on untreated diet, adjusted survival at 10 μg of Cry1Ac per ml of diet (n = 40–80 larvae per test) was 21.6% for 1997-derived Safford (F21) vs. 2.6% initially (Table 1), 11.0% for 1997-derived Mohave Valley (F16–F24) vs. 22.4% initially (Table 1), and 17.5% for AZP (F18) vs. 21.4% initially (Fig. 2). Overall, mean adjusted survival increased 1.2%, which is not significantly different from zero (paired t test, t = 0.13, P = 0.91). Because the frequency of resistance did not decline significantly in the three retested strains, we conclude that changes in the bioassay were not responsible for the lack of increase in the estimated resistance allele frequency from 1997 to 1998. These results also demonstrate that, in the laboratory, major fitness costs were not associated with resistance to Cry1Ac. However, these laboratory results do not rule out fitness costs in the field, such as costs affecting overwintering (15, 16), larval development on non-Bt cotton plants, and mate location.
To estimate the frequency of the resistance allele, we assumed that the resistance was conferred by a recessive allele at a single locus. Recessive inheritance of resistance to 10 μg of Cry1Ac per ml of diet is a robust result seen in both the selection and crossing experiments reported here. Further, the same pattern was observed with the independently derived APHIS-98R strain of pink bollworm (17). Inheritance of resistance to Bt cotton is also recessive in the AZP-R and APHIS-98R strains (15). Although results from a backcross test are consistent with the major locus hypothesis, we cannot exclude the possibility that resistance to Cry1Ac in field populations of pink bollworm is conferred by more than one allele. If mutations at more than one locus are required for resistance, the frequency of resistance at each locus could be substantially higher than our estimates, which are based on a one-locus model. To estimate the frequency of the resistance allele from the frequency of resistant homozygotes, we assumed Hardy–Weinberg equilibrium. Although substantial deviations from Hardy–Weinberg equilibrium could alter our quantitative estimates of resistance allele frequency, they would not alter the conclusion that the resistance allele frequency did not increase from 1997 to 1999. Perhaps more importantly, the finding that the frequency of resistant individuals did not increase from 1997 to 1999 does not depend on assumptions or inferences about the dominance of resistance, the number of loci, or Hardy–Weinberg equilibrium.
Examination of 140,950 bolls of Bt cotton and non-Bt cotton collected statewide shows that the efficacy of Bt cotton remained extremely high in Arizona during 1997–1999 (Table 2). In addition, a well publicized Rapid Response Team implemented in collaboration with the Arizona Cotton Growers Association (9) found no efficacy problems in these 3 years. Factors that might delay evolution of resistance in the field include refuges of non-Bt cotton, slower development of resistant pink bollworm on Bt cotton relative to non-Bt cotton (15), and fitness costs associated with resistance in the field, such as reduced overwintering ability (15, 16). The magnitude of such fitness costs might vary as a function of the weather. Further, in contrast to the typical assumption of 100% adjusted survival of resistant insects on Bt crops (3), adjusted survival of pink bollworm larvae on Bt cotton was only 37% for the APHIS-98R strain (15) and 40% for the AZP-R strain studied here. Nonetheless, our data show that genes conferring resistance to Cry1Ac and significantly increased ability to survive on Bt cotton were not rare in some Arizona field populations of pink bollworm during 1997. Contrary to predictions from models, they also indicate that the frequency of resistance does not necessarily increase from one year to the next, even when a Bt crop occupies vast areas.
Acknowledgments
We thank S. Meyer, M. Sims, M. Whitlow, and the staffs of the Extension Arthropod Resistance Management Laboratory and the Arizona Cotton Research and Protection Council for technical assistance; we also thank F. Gould, D. Andow, P. Ellsworth, J. Ferré, D. Heckel, M. Kidwell, R. Roush, and D. Wheeler for helpful comments on the manuscript. This work was supported by the University of Arizona, Cotton Incorporated, the Arizona Cotton Research and Protection Council, the Cotton Foundation, and Monsanto and USDA-NRI grant 99-35302–8300.
Abbreviations
- Bt
Bacillus thuringiensis
- AZP-R
Arizona pooled resistant
References
- 1.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Microbiol Mol Biol Rev. 1998;62:755–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frutos R, Rang C, Royer M. Crit Rev Biotechnol. 1999;19:227–276. [Google Scholar]
- 3.Gould F. Annu Rev Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 4.Tabashnik B E. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 5.Gould F, Anderson A, Jones A, Sumerford D, Heckel D G, Lopez J, Micinski S, Leonard R, Laster M. Proc Natl Acad Sci USA. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andow D A, Alstad D N, Pang Y-H, Bolin P C, Hutchison W D. J Econ Entomol. 1998;91:579–584. [Google Scholar]
- 7.Andow D A, Olson D M, Hellmich R L, Alstad D N, Hutchison W D. J Econ Entomol. 2000;93:26–30. doi: 10.1603/0022-0493-93.1.26. [DOI] [PubMed] [Google Scholar]
- 8.Ingram W R. In: Insect Pests of Cotton. Matthews G A, Turnstall J P, editors. Wallingford, UK: CABI; 1994. pp. 107–148. [Google Scholar]
- 9.Simmons A L, Dennehy T J, Tabashnik B E, Bartlett A, Gouge D, Staten R. Proc Beltwide Cotton Conf. 1998;2:1025–1030. [Google Scholar]
- 10.Patin A L, Dennehy T J, Sims M A, Tabashnik B E, Liu Y B, Gouge D, Henneberry T J, Staten R. Proc Beltwide Cotton Conf. 1999;2:991–996. [Google Scholar]
- 11.Tabashnik B E. J Econ Entomol. 1991;84:703–712. doi: 10.1093/jee/84.3.703. [DOI] [PubMed] [Google Scholar]
- 12.Tabashnik B E, Schwartz J M, Finson N, Johnson M W. J Econ Entomol. 1992;85:1046–1055. [Google Scholar]
- 13.Flint H M, Antilla L, Leggett J E, Parks N J. Southwestern Entomol. 1996;21:229–235. [Google Scholar]
- 14.Flint H M, Parks N J. Southwestern Entomol. 1999;24:13–20. [Google Scholar]
- 15.Liu Y B, Tabashnik B E, Dennehy T J, Patin A L, Bartlett A C. Nature (London) 1999;400:519. doi: 10.1038/22919. [DOI] [PubMed] [Google Scholar]
- 16.Alyokhin A V, Ferro D N. J Econ Entomol. 1999;92:510–515. [Google Scholar]
- 17.Liu, Y. B., Tabashnik, B. E., Meyer, S. K., Carrière, Y. & Bartlett, A. C. J. Econ. Entomol., in press. [DOI] [PubMed]