Abstract
There is an intense interplay between HIV and the immune system, and the literature is replete with studies describing various immunological phenomena associated with HIV infection. By definition, HIV is the cause of the immunologic findings, for without HIV there is no AIDS. However, many of the phenomena that have been described seem too broad in scope to be attributable to either direct infection of cells by HIV, or the HIV-specific immune response. Recently, a more fundamental understanding of how HIV impacts upon various T cells and T cell compartments has emerged. This review will cover the role of immune activation in HIV immunopathogenesis, how that activation could be mediated directly by HIV replicating within and damaging the gut mucosal barrier, how HIV affects multiple T cell functions and phenotypes, and how chronic HIV replication induces immune modulatory pathways to negatively regulate certain functions in HIV-specific T cells.
Keywords: human immunodeficiency virus, T lymphocyte, Th17 cells, cytokines, PD-1
Introduction
The interplay between HIV and the immune system that ultimately leads to loss of immune control of multiple pathogens and cancers has been termed the immunopathogenesis of AIDS. While many basic concepts of how HIV is able to damage the immune system are unquestioned – HIV infects and destroys CD4 T cells, neutralizing antibodies have little effect on virus replication, cytotoxic T lymphoctes (CTL) limit, but do not stop HIV replication completely – other concepts are more controversial. Many have questioned how HIV can infect such an apparently small proportion of CD4 T cells (estimated at 1 in 100 to 1 in 1,000) and yet still overwhelm the T cell renewal capacity of the immune system. Others question what is driving the chronic immune activation in HIV infection; the virus, the virus-specific immune response, or another mechanism. Within this context, controversy remains over whether the virus drives immune activation, immune activation drives virus replication, or both. Finally, while it is known that CTL can help control virus replication, why HIV-specific CTL fail to do a better job than they do remains an intense area of investigation.
Recent discoveries allow some unifying hypotheses to emerge. What has become clear is that in the balance between viral replication and immune activation, chronic high level virus replication is fundamental to the process of HIV immunopathogenesis. If virus replication is blocked with antiretroviral therapy (ART), most immunologic defects will revert towards normal (some more rapidly than others). However, blocking immune activation has proven much less successful in restoring normalcy to the immune system. The literature on the immunopathogenesis of AIDS, 25 years into the epidemic, is too vast to allow a comprehensive review of this topic. Therefore we will touch on some of the newer findings which help address some of the outstanding questions of how HIV, by infecting certain CD4 T cells, is able to wreak such wide havoc upon the immune system, and how HIV is able to subvert the T cell response that is mobilized against it.
Immune activation in HIV infection
Chronic systemic immune activation is an almost pathognomonic feature of progressive HIV infection. Indeed, it is one of the strongest predictors of disease progression (1-4), is associated with impaired immune reconstitution with antiretroviral therapy (ART) (5) and is a critical factor that distinguishes pathogenic from non-pathogenic non-human primate SIV infection (6). Such immune activation is manifest in many ways including polyclonal B-cell activation (7), increased T-cell turnover (8), increased frequencies of T-cells with an activated phenotype (9), and increased serum levels of proinflammatory cytokines and chemokines (10).
While immune activation may have some beneficial consequences such as T cell proliferation and, by inference, the partial restoration of tissue memory CD4 T cells (11), there is general agreement that it is overwhelmingly detrimental to the HIV-infected person. High turnover of CD4 and CD8 T cells imposes a strain on their homeostatic mechanisms (12) resulting in a decrease in the overall half-life of T cells (13) and clonal exhaustion of T cells may ultimately result in drainage of memory T cell pools (14, 15). Inflammatory damage to lymphoid tissues may underlie thymic dysfunction (16, 17) and TGF-β-mediated fibrosis of lymph nodes (18, 19) which are, in turn, associated with abnormal retention of effector type T cells (20) and poor immune reconstitution with ART (21). Furthermore, and perhaps most importantly, immune activation results in the generation of activated T cell targets for the virus itself, further driving viral replication (22, 23). Hence HIV is a virus that, through the induction of immune activation, generates its own substrate for replication. Critically, it is the activation, infection and depletion of central memory CD4 T cells that are viewed, under normal circumstances, as a “self-renewing” source for tissue effector memory CD4 T cells which may correlate most closely with progression to AIDS (11).
Thus, constant damage to the cellular sources of, and anatomical niches that maintain, the CD4 T cell compartments caused by a quasi self-perpetuating relationship between the virus and immune activation further exacerbates the progressive net loss in CD4 T cell numbers and function and inevitably leads to AIDS. However, even though HIV has been shown to activate dendritic cells and NK cells of the innate immune system in vitro via TLR7/8 (24-26), its replication cannot alone account for the extent of systemic immune activation in HIV-infected individuals. For example, elite controllers who spontaneously control viral loads to very low or undetectable levels may have high immune activation, which also correlates with progressive CD4 T cell loss (3). Furthermore, individuals treated with ART who suppress virus but have incomplete restoration of CD4 T cells also have increased immune activation (5). Finally, in non-pathogenic SIV infection of natural non-human primate hosts such as sooty mangabeys and African green monkeys, while there is high systemic immune activation in the acute phase of the infection, this is rapidly attenuated in the chronic phase, even in the presence of persistently high viral loads (27-29). Thus, defining the factors that underlie systemic immune activation would appear to be critical to understanding the pathogenesis of progressive HIV infection. Recent studies have provided a direct link between immune activation in chronic HIV infection and catastrophic pathogenic events that occur at the mucosal surfaces during acute infection.
The gut mucosa and immune activation
The mucosal surface of the gastrointestinal (GI) tract forms a unique anatomical and physiological niche, serving as a structural and immunological barrier against the microorganisms of the outside world. In fact, the majority of the body's lymphocytes are contained in the GI tract (30, 31). It has long been known that HIV infection causes damage to this critical organ. In 1984, Kotler and colleagues observed that HIV-infected individuals had histological abnormalities of the GI mucosa, malabsorption, and lymphocyte depletion (32). More recently, a number of groups have examined this systematically and have shown that during the acute phase of HIV infection in humans or pathogenic SIV infection in rhesus macaques the majority of GI tract CD4 T cells are depleted, likely as a result of direct viral infection (20, 33-39). Moreover, this depletion continues throughout the entire disease course and represents a considerable assault to the immune system, neither the tempo nor extent of which is reflected in peripheral blood CD4 T cell counts. In addition to the loss of CD4 T cells, gene expression profiles of GI tract biopsies reveal that genes associated with cell cycle regulation, lipid metabolism, and epithelial cell barrier and digestive functions are down-regulated in HIV-infected individuals (40). The enteropathy, which can occur from the acute phase of the infection through to advanced disease, involves diarrhea, increased GI inflammation, increased intestinal permeability and malabsorption (41, 42).
Histologically, the enteropathy involves inflammatory infiltrates of lymphocytes and damage to the GI epithelial layer including villous atrophy, crypt hyperplasia, and villous blunting (43). Importantly these pathological changes occur in the absence of detectable bacterial, viral or fungal enteropathogens often associated with enteropathy (43). SIV-infected rhesus macaques also manifest enteropathy (44), the cause of which may be in part attributed to virus-mediated enterocyte apoptosis and occurs very early in the acute phase of infection (45). A number of investigators recently identified a preferential loss from the GI tract of a subset of T cells that are defined by their secretion of the cytokine IL-17 (46, 47). These Th17 cells are thought to be critical in the defense against bacteria and fungi, particularly at mucosal surfaces, and also contribute to the homeostasis of enterocytes. Importantly, although this loss is observed in HIV infection and pathogenic SIV infection of rhesus macaques (wherein it correlates with progression to AIDS (47)), it is not observed in non-pathogenic SIV infection of sooty mangabeys (46). Thus, it has become apparent that the GI mucosal barrier suffers a serious immunological and structural insult very early in HIV and pathogenic SIV infection and that this damage may adversely affect the barrier function of the gut in the defense against luminal microbes. In fact, it has long been known that damage to the barrier function of the GI tract, such as in inflammatory bowel disease or after chemo/radiotherapy for hematopoietic cell transplantation, results in the translocation of microbial products such as lipopolysaccharide (LPS) into the systemic circulation, in the absence of overt bacteremia, which correlates with systemic immune activation (48-50). Recent studies have shown that chronically HIV-infected individuals have significantly increased levels of plasma LPS compared to uninfected individuals (51). The increased levels of LPS are at a level commensurate with those capable of inducing an acute phase inflammatory response (52) and are associated with increased levels of soluble CD14 and lipopolysaccharide binding protein, and decreased levels of antibodies directed against LPS core antigen indicating bioactivity of LPS in vivo. Moreover, LPS levels are associated with both the frequency of activated memory CD8 T cells and plasma levels of the proinflammatory cytokine IFNα. Importantly, neither of these measures of activation could be directly attributed to LPS. These findings suggest that plasma LPS, in addition to its potent immunostimulatory activity through TLR-4 is also an indicator of the translocation of additional microbial products that stimulate the immune system through other receptors. Notably, in those elite controllers whose disease course nevertheless progresses, the degree of CD4 T cell depletion is closely associated with the level of T cell activation which is, in turn, associated with significantly raised levels of plasma LPS (3). These findings implicate microbial translocation as a cause of immune activation in chronically HIV-infected individuals, thus providing a direct link between the damage to the GI tract during the acute phase of infection and progression to immunodeficiency.
Depletion of GI tract CD4 T cells alone is clearly not sufficient to result in mucosal translocation and immune activation. While pathogenic SIV infection of rhesus macaques is also associated with microbial translocation and immune activation, recent studies have shown that African green monkeys (29) and sooty mangabeys (28), both natural hosts for SIV, lose a significant fraction of their GI tract CD4 T cells during acute SIV infection, yet typically have low levels of immune activation and plasma LPS even in the presence of high viral loads, and do not progress to AIDS (28, 29, 51). Importantly, both species manifest significant immune activation in the acute phase (27-29), with measurable microbial translocation in sooty mangabeys (28) but differ from pathogenic SIV/HIV infection in that the immune activation and microbial translocation is transient and is controlled as the infected animals enter the chronic phase. These observations suggest that natural host species may have evolved immunological mechanisms for control of mucosal pathogens that are less dependent upon CD4 T cells and may also be able to attenuate potentially harmful inflammatory responses in the face of ongoing viral replication.
Thus, in HIV and pathogenic SIV infection the GI tract is a site of massive CD4 T cell depletion, viral infection, enterocyte apoptosis and structural damage to the epithelial surface. New therapeutic directions might aim to prevent or reduce the propagation of HIV at mucosal surfaces and to restore the immunological and epithelial integrity of the mucosal barrier thereby circumventing the associated immune activation and disease progression (53). However, with such emphasis on immune activation as a cause of disease progression and its attenuation in chronic non-pathogenic SIV infection even in the presence of high viral loads, one should not be left with the impression that the virus plays a minor role in disease progression in pathogenic infection. Indeed, it plays a central role at all stages of disease; the relationship between immune activation and the virus may not always be a direct one, but clearly without the virus there is no immune activation. The efficacy of ART in reducing immune activation bears witness to this contention.
T cell function in HIV pathogenesis
Subsets of T cells can be defined by their specificity, surface phenotype, degree of maturation, location, or functions they express upon stimulation, and any and all of these parameters can be affected by HIV infection. Which of these changes are a cause, and which a consequence, of HIV infection has been the focus of intense study for many years. Gross changes in the representation of different T cell subsets have been described (54). Besides the loss of CD4 T cells, a destruction consequential to the infection of this subset by the virus, general patterns were seen that are true of all T cells, including CD8 and gamma-delta subsets. In general, untreated individuals show a progressive loss of resting subsets (with a preferential loss of resting naïve T cells during chronic disease), and elevated levels of activated T cells – for example, those expressing HLA-DR and CD38. Seminal studies by Giorgi and colleagues showed that these activated phenotypes of CD8 T cells were reasonably predictive of subsequent progression rates, in that high expression of CD38 was particularly poor prognostic, whereas HLA-DR expression in the absence of CD38 was favorable (4).
Further studies showed that ART reversed, at least to some extent, many (but not all) of the changes described in chronic HIV infection (55-59). Individuals in whom treatment was halted (STI trials) showed that these reversals were only temporary – suggesting that the remodeling of the T cell compartment accompanying HIV infection was largely a consequence of high viral loads, and that therapeutic control of virus would allow the immune system to partially heal. In general, the substantial phenotypic changes described for CD4 and CD8 T cells during HIV disease rarely correlate with disease progression and are probably more indicative of an immune system under duress.
With the advent of assays to identify antigen-specific T cells (intracellular cytokine staining, or ICS), attention shifted to characterizing those cells actively involved in controlling the virus, i.e., HIV-specific CD4 and CD8 T cells. ICS assays can enumerate the fraction of T cells (or absolute number) that respond by making one or more functional responses following stimulation with antigen; this is referred to as the magnitude of the response. In addition, by considering the types of different responses that are elicited by the stimulation, the quality of the response may be defined (60).
A number of studies demonstrate that the magnitude of the CD8 T cell response to HIV does not correlate with (nor predict) progression (61, 62). Indeed, the magnitude of this response is largely correlated to the viral load; successful control of viremia leads to a diminution of the T cell response (63-65). The failure to find a correlation between the CD8 T cell response to virus and pathogenesis came as a disappointment; there was every expectation that a vigorous T cell response to the virus would be associated with better control and clinical outcome.
Nonetheless, over the past few years it has become clear that the quality, if not the magnitude, of the T cell response may provide such a correlate. Initially, studies quantified the fractions of T cells that made IFNγ, IL2, or both on a cell-by-cell basis. While there was no difference between progressors and nonprogressors in terms of the magnitude of the IFNγ response, the fraction of the cells that made IL2 (alone or in combination with IFNγ) was elevated in nonprogressors (66-69). In other words, the quality of the response varied from an IFNγ-dominated response (progressors) to a more balanced, IL2-producing response (non-progressors) (Figure 2).
Figure 2. Functionally-defined T cell differentiation and HIV disease progression.
T cell stages can be defined either by phenotype (expression of cell surface markers such as CD45RA, CCR7, and CD28), or by function (expression of cytokines, chemokines, or other activities immediately following antigenic stimulation). Clinically-defined correlates of progression in HIV disease have been associated with T cell functions: better clinical outcome is associated with a prevalence of polyfunctional T cells. These cells are highly optimized for effector function by expressing multiple functions simultaneously as well as expressing high levels of cytokines on a per-cell basis. In contrast, there is little correlation between clinical outcome and the phenotype of antigen-specific cells, likely because of the broad overlaps between phenotypically-defined subsets and function.
A seminal paper by Betts et al. dramatically extended this paradigm (70). In this study, T cell quality was defined by the independent measurement of five different functions on a cell-by-cell basis; the concept of polyfunctional T cells (those cells capable of making a majority of measured functions) was born. By comparing clinically-defined progressors to nonprogressors, a substantial difference in the quality of the HIV-specific response was apparent: nonprogressors have a much larger fraction of their response comprised of polyfunctional T cells. Indeed, even within the progressor cohort, there was a correlation between the level of polyfunctionality and viral load – a correlation that had never before been seen with any functional measurement. This has been confirmed in a number of studies (67, 71-73).
Recent studies in HIV-2 infected adults provide additional support for the role of functionally, as opposed to phenotypically, defined T cell subsets in viral control (74, 75). HIV-2 is typically a far less pathogenic infection than HIV-1. As such, there are far more CD4 T cells preserved, and a detailed analysis of HIV-2-specific CD4 and CD8 T cells was possible. HIV-2 specific T cells were found to be more polyfunctional, akin to the HIV-1-specific T cells in nonprogressors. There was no correlation to be found with the phenotypes of these cells. Remarkably, the phenotypes of the antigen-specific T cells showed little correlation with function at all. Indeed, the only associations that were found were that more differentiated subsets produced less IL2 and more MIP1β; there was no association with IFNγ, TNF, or degranulation, and differentiation stage (defined phenotypically).
The potential role of polyfunctional T cells has been extended in a number of studies. Indeed, these cells appear to be relevant even in primary infection: patients with improved resolution of viral load had more polyfunctional responses (72, 76). Even within a single individual's response, there is heterogeneity that follows this pattern. First, responses to different HIV epitopes can be individually characterized; those epitopes that induced more polyfunctional responses also induced proliferative responses, in contrast to epitopes that induced only IFNγ production (68). And second, heterogeneity exists at the level of anatomical location: it appears that mucosal responses are more polyfunctional than those found in the blood. While rectal T cell responses were mildly more polyfunctional (77), T cell responses obtained from bronchoalveolar lavage (BAL) were far more polyfunctional (34). Interestingly, the BAL shows a far better preservation of CD4 T cells than the blood of these same individuals, indicating that pathogenesis is anatomically distinct (and correlates with the degree of polyfunctionality of the compartment).
Numerous studies now show that a higher level of T cells capable of IL2 production (or, more generally, that are polyfunctional) is associated with better outcome in HIV disease. However, to date these studies leave open the question of cause vs. effect. Polyfunctionality did not immediately increase in progressors undergoing ART (69, 70); however, long-term changes have not been well-defined. Nonetheless, there are good reasons to believe that polyfunctional T cell responses are superior and can directly lead to better control of virus. First, in an animal model, vaccine-elicited polyfunctional CD4 T cells were far better at providing protection against challenge with L. Major; indeed, the same number (magnitude) of monofunctional T cells provided essentially no protection (78). Second, each polyfunctional cell by definition elicts a wider repertoire of functions, both measured and unmeasured (for example, greater expression of CD40L, required for licensing dendritic cells) (67). Third, each polyfunctional T cells produces as much as 10-fold more of each cytokine than monofunctional T cells, bringing to bear a far larger effector response directly at the effector site (75, 78, 79). Finally, through the production of IL2, these cells are better equipped to proliferate and extend the response. In sum, these cells appear to be optimized effector cells that are also in the pivotal differentiation stage between central and effector memory T cells (60).
PD-1 and T cell dysfunction in HIV
Despite evidence that HIV- and SIV-specific CD8 T cells (CTL) are involved in the control of viral replication (80-82), there are intrinsic functional defects in these cells including decreased cytokine production, decreased proliferation, lack of polyfunctionality (described above), and lack of full effector differentiation which could at least partially explain their failure to clear the infection fully (83, 84). Similar defects in SIV-specific CTL also exist (85, 86). While chronic high-level antigen stimulation is associated with the generation of many of these defects, it is unclear if there is a central unifying mechanism that regulates all these functions, or whether the different defects, while all resulting from chronic antigen stimulation, are regulated through different pathways. Multiple surface molecules can transmit both positive and negative regulatory signals to T cells, any of which could be responsible for some or all of the observed defects in HIV-specific T cells (87).
Programmed death-1 (PD-1) has recently been identified as a crucial negative regulator of T cell function in HIV infection. PD-1 is a member of the CD28 family and was originally identified as a surface receptor involved in the apoptosis of cancer cells (88, 89). The role of PD-1 in regulating T cell function in chronic viral infection comes from studies of chronic LCMV infection in mice where the resultant CTL exhaustion mimics the defects seen in HIV infection of humans. In an elegant study, recovery from exhaustion of LCMV-specific CTL was accomplished in vivo by blocking the interaction between PD-1 and its ligand PD-L1 (90). This recovery of T cell function was accomplished even in CD4-depleted mice, making the findings relevant to HIV infection. This led to a flurry of activity to determine the role of PD-1 in T cell exhaustion in HIV infection.
Three near-simultaneous publications helped define the role of PD-1 in HIV infection (91-93). All three groups showed that PD-1 was highly expressed on HIV-specific CTL, and that CTL specific for less chronic or acute viruses had lower expression of PD-1. Trautmann et al and Day et al showed that there was an inverse relationship between viral load and PD-1 expression on HIV-specific CTL (91, 93), and a separate publication showed that HIV-specific CTL from long-term non-progressors have lower expression of PD-1 than progressors (94). All three groups showed that proliferation of virus-specific CTL could be improved by blocking the interaction of PD-1 with PD-L1 during antigen stimulation. However, there are some discrepancies in the literature over which CTL functions are directly controlled by PD-1. While expression of PD-1 on CTL has been linked by some to impaired production of cytokines (90, 91, 93) others have emphasized the predominant role of this receptor in regulating the survival of these cells (92, 95-98). Specifically, while blocking PD-1 increases the number of HIV-specific CTL that make cytokine in a multi-day assay, it is unclear if this is secondary to a direct effect of PD-1 on cytokine expression, or a reflection of the better proliferation/survival of HIV-specific CTL over the course of the assay. Petrovas et al were unable to demonstrate a direct effect of PD-1 on cytokine expression in HIV-specific CTL in short-term assays, suggesting a lack of direct linkage between PD-1 and cytokine production (92). Despite these minor differences, these findings collectively support the conclusion that PD-1 expression on HIV-specific CTL, and its engagement by PD-L1 on antigen presenting cells during chronic antigen stimulation, is responsible for at least some of the defects in CTL function.
What is the mechanism by which PD-1 engagement impairs CTL function? Petrovas et al investigated the association between PD-1 expression and apoptosis and concluded that PD-1 is a primary determinant of apoptosis sensitivity in CTL (92). Within any CTL population defined by any other set of surface markers, the PD-1+ population was always more sensitive to apoptosis than the PD-1− population. In addition, the level of PD-1 expression determined the sensitivity to apoptosis, and ligation of PD-1 was sufficient to induce apoptosis, indicating that PD-1 is not just a marker of, but a direct participant in, the apoptotic pathway. Therefore, PD-1 expression leads to a profound (but potentially reversible) survival defect.
There is evidence that chronic antigen stimulation drives the expression of PD-1. First, PD-1 expression decreases when viral replication is suppressed by antiretroviral therapy (91, 93). Second, within both HIV and SIV infections, PD-1 expression decreases on epitope-specific CTL once the epitope has escaped, while CTL specific for epitopes that have not escaped maintain high PD-1 expression (98, 99). Therefore, the PD-1-mediated impairment in CTL function is a direct consequence of high HIV-specific antigen stimulation and not general immune activation.
PD-1 is also expressed on CD4+ T cells. HIV-specific CD4+ T cells express high levels of PD-1 when compared to CMV-specific CD4+ T cells, and this expression correlates with viral load (91). As was seen with CD8+ CTL, PD-1 blockade significantly increased CD4+ T cell proliferation in vitro, suggesting a similar impact of chronic antigen stimulation, PD-1 expression, and functional impairment in both the CD4+ and CD8+ T cell arms of the immune response to HIV (91).
Collectively these findings define PD-1 as a potential therapeutic target for restoring the functional capacity of HIV-specific CTL (Figure 3). However, it should be appreciated that PD-1 expression attenuates potentially harmful T cell responses to many self antigens and other chronic pathogens. Given that many T cells express PD-1, it is likely that interventions which release all CTL from PD-1-mediated suppression will have untoward effects. That said, initial safety and tolerability studies of a PD-1 blocking antibody have been completed in monkeys, and clinical trials in cancer patients have begun. In addition, anti-PD-1 antibodies are being tested in SIV-infected monkeys. These initial studies will provide a foundation upon which decisions to move into human trials in HIV-infected subjects can be based. Use of anti-PD-1 interventions may be much easier in a vaccine setting, where one could target the intervention to T cells specific for a given antigen, thereby avoiding release of harmful responses from appropriate negative regulatory control. This could be accomplished by limiting the use of any anti-PD-1 intervention to either the time or location of the vaccination. While much needs to be done to better define the mechanisms and direct impact of PD-1 on T cell function, the recent discovery of a cell surface molecule that can be manipulated to reverse crucial T cell dysfunctions in HIV infection provides a very promising lead for further therapeutic development.
Figure 3. Model of PD-1 activity and therapeutic intervention.
Chronic stimulation of antigen presenting cells (APC) by HIV induces high expression of PD-L1 on the APC, and strong expression of PD-1 on responding CTL. Negative signaling through PD-1 in association with the peptide/MHC–TCR interaction leads to decreased proliferation and increased apoptosis of HIV-specific CTL. Anti-PD-1 therapy could block the negative regulatory signal through PD-1 thereby restoring proliferative potential in HIV-specific CTL despite the high expression of PD-1.
Summary
Because of the complexity of the interactions between HIV and the immune system, many differing and often competing hypotheses of how HIV causes AIDS have been put forth. In order to avoid emulating a group of blind men describing an elephant, specific findings need to be interpreted within the context of multiple other findings, and take into account not only what is observed in blood, but also at multiple other sites throughout the body. When such a view is taken, some unifying conclusions can be made. Among these are that HIV is the proximate cause of AIDS and that immune activation, while underlying the pathogenesis of ongoing viral replication, inhibition of T cell function, and impairment in immune reconstitution, only does this within the context and as a result of HIV infection itself. Recent data clearly demonstrate that the gut mucosal surface and the events that lead to its damage are crucial to the process by which generalized immune activation is established. This then drives further viral replication leading to more tissue destruction and a vicious cycle is established. Within this context, the major antiviral T cells which would normally control viral replication are negatively impacted upon first by the generalized immune activation and second by the high antigen loads and chronic T cell stimulation. This leads to T cell functional impairments that further impact upon the ability of the immune response to curtail HIV replication.
There are several bits of good news within this doomsday scenario. First, we know that potent anti-retroviral therapy, by shutting down HIV replication, leads to an often slow and incomplete but deliberate return of the immune system towards normality. Second, many of the processes described in this review are potential targets for new therapeutic or vaccine interventions. Finally, while there is still much to learn, our better understanding of how HIV causes AIDS will ultimately translate into better treatment options for HIV-infected people.
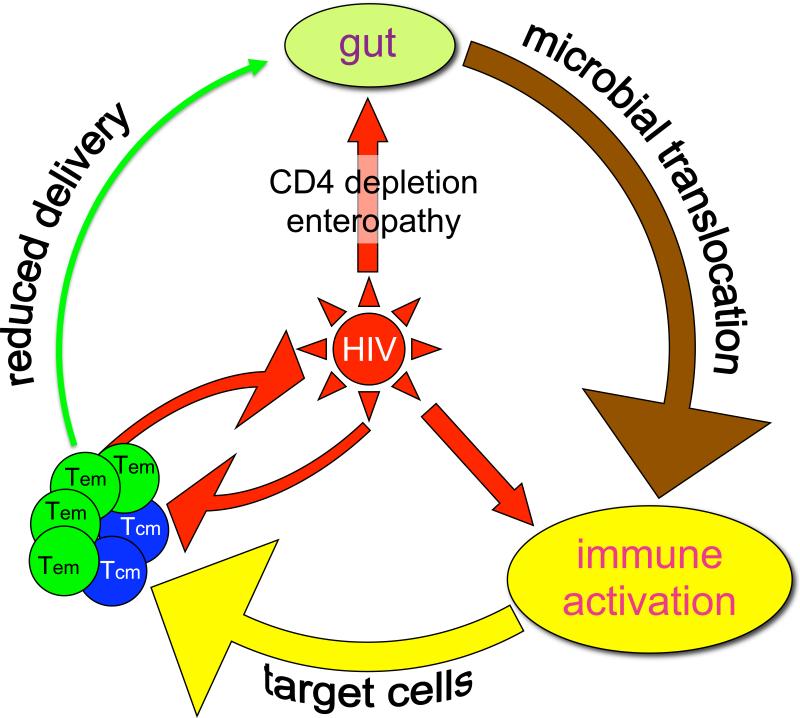
Figure 1. Central role of HIV in immunopathogenesis.
In acute infection CD4 T cell depletion and enteropathy results in microbial translocation which contributes to immune activation. The virus itself may also contribute to immune activation. Activation leads to the generation of more target cells for the virus thus perpetuating viral replication and mucosal damage. Infection and depletion of the critical central memory CD4 T cell pool results in reduced tissue delivery of effector memory CD4 T cells and further loss of immune control at mucosal and other tissue sites.
Acronyms
- HIV
Human immunodeficiency virus
- AIDS
Acquired immunodeficiency syndrome
- CTL
cytotoxic T lymphocyte
- ART
Antiretroviral therapy
- SIV
simian immunodeficiency virus
- LPS
lipopolysaccharide
- ICS
intracellular cytokine staining
- PD-1
programmed death 1
- PD-L1
ligand for programmed death 1
- LCMV
lymphocytic choriomeningitis virus
Footnotes
Terms/Definitions
Immunopathogenesis of AIDS: interaction between HIV and the immune system leading to AIDS
Microbial translocation: process by which gut microbial products gain access to the systemic circulation in the absence of overt bacteremia
Polyfunctional T cells: individual antigen-specific T cells that respond to cognate antigen by liberating more than one or two functions simultaneously: ie they simultaneously make IL2, IFNγ, and TNF
T cell exhaustion: functional characteristic of CTL often seen in chronic viral infections where CTL proliferate, kill, and liberate cytokines poorly in response to stimulation
Literature cited
- 1.Deeks S, Kitchen C, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 3.Hunt P, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Cumberland WG, Hultin LE, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hunt P, Martin J, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 6.Silvestri G, Paiardini M, Pandrea I, et al. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–54. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane HC, Masur H, Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Eng. J. Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 8.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 9.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood. 2000;95:249–55. [PubMed] [Google Scholar]
- 10.Valdez H, Lederman M. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997:187–228. [PubMed] [Google Scholar]
- 11.Okoye A, Meier-Schellersheim M, Brenchley J, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–85. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–41. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellerstein M, McCune J. T Cell Turnover in HIV-1 Disease. Immunity. 1997;7:583–9. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 14.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 15.Grossman Z, Meier-Schellersheim M, Sousa AE, et al. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–23. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 16.Dion M, Poulin J, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–68. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 18.Estes J, Wietgrefe S, Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–61. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 19.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacker T, Reilly C, Beilman G, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–71. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 22.Douek DC, Picker LJ, Koup RA. T Cell Dynamics in HIV-1 Infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 23.Grossman Z, Feinberg MB, Paul WE. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc Natl Acad Sci U S A. 1998;95:6314–9. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter G, Suscovich T, Teigen N, et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–66. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 25.Beignon A, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–75. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–91. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estes J, Gordon S, Zeng M, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon S, Klatt N, Bosinger S, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–34. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandrea I, Gautam R, Ribeiro R, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–46. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald T. The gut is still the biggest lymphoid organ in the body. Muc Immunol. 2008;1:246–7. [Google Scholar]
- 31.Pabst R, Russell M, Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 2008;29:206–8. doi: 10.1016/j.it.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Kotler D, Gaetz H, Lange M, et al. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–8. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 33.Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. Aids. 2000;14:1761–5. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 34.Brenchley J, Knox K, Asher A, et al. High Frequencies of Polyfunctional HIV-specific T cells are Associated with Preservation of Mucosal CD4 T cells in Bronchoalveolar Lavage. Muc Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Duan L, Estes J, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 37.Mattapallil J, Douek D, Hill B, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 38.Mehandru S, Poles M, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 40.Sankaran S, Guadalupe M, Reay E, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A. 2005;102:9860–5. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–6. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smale S, Tibble J, Bjarnason I. Small intestinal permeability. Curr Opin Gastroenterol. 2000;16:134–9. doi: 10.1097/00001574-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Batman P, Miller A, Forster S, et al. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol. 1989;42:275–81. doi: 10.1136/jcp.42.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kewenig S, Schneider T, Hohloch K, et al. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–23. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Estes J, Duan L, et al. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis. 2008;197:420–9. doi: 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- 46.Brenchley J, Paiardini M, Knox K, et al. Differential Th17 CD4 T cell depletion in pathogenic and non-pathogenic lentiviral infections. Blood. 2008 doi: 10.1182/blood-2008-05-159301. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cecchinato V, Trindade C, Laurence A, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Muc Immunol. 2008;1:279–88. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caradonna L, Amati L, Magrone T, et al. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–14. [PubMed] [Google Scholar]
- 49.Cooke K, Olkiewicz K, Erickson N, Ferrara J. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8:441–8. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- 50.Penalva J, Martinez J, Laveda R, et al. A study of intestinal permeability in relation to the inflammatory response and plasma endocab IgM levels in patients with acute pancreatitis. J Clin Gastroenterol. 2004;38:512–7. doi: 10.1097/01.mcg.0000129060.46654.e0. [DOI] [PubMed] [Google Scholar]
- 51.Brenchley J, Price D, Schacker T, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 52.Suffredini A, Hochstein H, McMahon F. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis. 1999;179:1278–82. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- 53.Veazey R, Klasse P, Schader S, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 54.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol Cell Biol. 2007;85:6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 55.Elrefaei M, McElroy MD, Preas CP, et al. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173:2184–9. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 56.Oxenius A, Price DA, Gunthard HF, et al. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci U S A. 2002;99:13747–52. doi: 10.1073/pnas.202372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oxenius A, Sewell AK, Dawson SJ, et al. Functional discrepancies in HIV-specific CD8+ T-lymphocyte populations are related to plasma virus load. J Clin Immunol. 2002;22:363–74. doi: 10.1023/a:1020656300027. [DOI] [PubMed] [Google Scholar]
- 58.Rehr M, Cahenzli J, Haas A, et al. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol. 2008;82:3391–404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tussey LG, Nair US, Bachinsky M, et al. Antigen burden is major determinant of human immunodeficiency virus-specific CD8+ T cell maturation state: potential implications for therapeutic immunization. J Infect Dis. 2003;187:364–74. doi: 10.1086/367707. [DOI] [PubMed] [Google Scholar]
- 60.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 61.Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–92. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Betts MR, Ambrozak DR, Douek DC, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–91. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–16. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray CM, Lawrence J, Schapiro JM, et al. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J Immunol. 1999;162:1780–8. [PubMed] [Google Scholar]
- 65.Kalams SA, Goulder PJ, Shea AK, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–8. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–72. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 67.Kannanganat S, Kapogiannis BG, Ibegbu C, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007;81:12071–6. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peretz Y, Ndongala ML, Boulet S, et al. Functional T cell subsets contribute differentially to HIV peptide-specific responses within infected individuals: correlation of these functional T cell subsets with markers of disease progression. Clin Immunol. 2007;124:57–68. doi: 10.1016/j.clim.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Potter SJ, Lacabaratz C, Lambotte O, et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;81:13904–15. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–85. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turk G, Gherardi MM, Laufer N, et al. Magnitude, breadth, and functional profile of T-cell responses during human immunodeficiency virus primary infection with B and BF viral variants. J Virol. 2008;82:2853–66. doi: 10.1128/JVI.02260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zimmerli SC, Harari A, Cellerai C, et al. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci U S A. 2005;102:7239–44. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duvall MG, Jaye A, Dong T, et al. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol. 2006;176:6973–81. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 75.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–63. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Northfield JW, Loo CP, Barbour JD, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J Virol. 2007;81:5759–65. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Critchfield JW, Lemongello D, Walker DH, et al. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–71. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 79.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 83.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 85.Vogel TU, Allen TM, Altman JD, Watkins DI. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J Virol. 2001;75:2458–61. doi: 10.1128/JVI.75.5.2458-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong Y, Luscher MA, Altman JD, et al. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J Virol. 2001;75:3028–33. doi: 10.1128/JVI.75.6.3028-3033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–38. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- 88.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 89.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 91.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 92.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 94.Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–8. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 95.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 96.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–35. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 97.Muhlbauer M, Fleck M, Schutz C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–8. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 98.Petrovas C, Price DA, Mattapallil J, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–36. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Streeck H, Brumme ZL, Anastario M, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]