Abstract
The ectoparasitic mite, Sarcoptes scabiei, produces molecules that depress initiation of host inflammatory and immune responses. Some of these down-regulate expression of adhesion molecules or secretion of chemokines or cytokines on and by cultured dermal endothelial cells (HMVEC-D). This study was undertaken to determine if the response of HMVEC-D to scabies is altered in the presence of various proinflammatory cytokines (tumor necrosis factor α and interleukins 1α, 1β and 6), histamine, and lipid-derived mediators (prostaglandins D2 and E2, leukotriene B4, platelet activation factor) that likely occur in scabietic lesions in vivo. Scabies extract down-regulated the TNFα-induced expression of VCAM-1 by HMVEC-D and this down-regulation still occurred in the presence of the other proinflammatory cytokines, histamine or the lipid-derived mediators. Scabies inhibited the IL-1α and IL-1β-induced secretion of IL-6, while a combination of scabies and histamine or LTB4 reduced the TNFα-induced secretion of IL-6. Scabies extract inhibited secretion of IL-8. Histamine, PGD2, PGE2, LTB4, PAF, and IL-6 alone had no effect on this inhibition, but the scabies-induced inhibition of IL-8 secretion was reduced in a dose-dependent fashion in the presence of IL-1α and IL-1β.
Keywords: Human microvascular endothelial cells, Sarcoptes scabiei, histamine, prostaglandin, leukotriene
1. Introduction
The scabies mite, Sarcoptes scabiei, burrows in the epidermis of the skin of mammalian hosts. Living mites' physical activity and secretions, and products from disintegrating dead mite bodies can activate a complex cascade of interacting events that eventually result in local and systemic inflammatory and immune reactions in the host. In humans, the clinical manifestations of a primary infestation with S. scabiei frequently do not appear for 4-8 weeks [1-3]. Evidence suggests that the mites produce substances that depress the early initiation of the host inflammatory and immune responses [4-8]. Presumably, this delay allows the mite population time to become established in the host before a strong defense response is induced. However, in sensitized hosts the inflammatory and immune reactions appear more quickly.
Substances released by live mites into the host skin may include salivary secretions, excretory and fecal materials, and secreted substances associated with metamorphosis and the molting process (e.g. chitinase and protease molting enzymes). In addition, humans produce chitinase (e.g. acidic mammalian chitinase, AMCase) and chitinase-like proteins that lack enzymatic activity (e.g. human cartilage glycoprotein 39 or YKL-40) [9-12]. These glycosidases and other serum proteases may degrade the chitin and protein matrix of the mite body exoskeleton while it is still in the skin. Therefore, many substances are liberated from mite bodies after the mites die in the skin and their bodies disintegrate (e.g. molecules in the hemolymph such as hormones including ecdysones and juvenile hormones, fat body enzymes, molecules from muscle, and endosymbiont bacteria and their products). The surge in antibody titer and the continued pruritis that occurs after a host has been treated for scabies (while many dead mite bodies remain in the epidermis until they are shed with the stratum corneum) are evidence that the dead mite bodies are important in the host response [13].
Previous studies have shown that molecules in extracts made from mite bodies and from live mites that burrow into human skin equivalents have the ability to modulate the function of a number of blood and skin cell types that are involved in the host's inflammatory and immune responses to these mites [4-8]. The cell types shown to be modulated by scabies include circulating monocytes and the dendritic cells derived from them, circulating lymphocytes, and skin cells such as keratinocytes, fibroblasts and dermal microvascular endothelial cells [5-8]. It has been shown that molecules in an extract of S. scabiei bodies can up-regulate the secretion of interleukin (IL)-6, IL-8, granulocyte colony stimulating factor (G-CSF) and vascular endothelial growth factor (VEGF) by dermal fibroblasts [5]. S. scabiei extract induces human keratinocytes to reduce secretion of IL-1 receptor agonist (IL-1RA), and IL-8, and increase secretion of IL-6, G-CSF, and VEGF [5]. S. scabiei extract also induces up-regulated secretion of IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNFα) from peripheral blood mononuclear cells from human donors [6]. Dendritic cells derived from monocytes down-regulate secretion of IL-6 and IL-8 when stimulated with S. scabiei extract [6]. Previous studies have demonstrated that S. scabiei extract contains molecules that can down-regulate the expression of the adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, and the secretion of the chemokine IL-8 while stimulating increased secretion of IL-6 by human dermal microvascular endothelial cells [8].
While some molecules from scabies mites up-regulate the function of many cells as demonstrated, it also appears that scabies mites specifically produce and secrete some molecules for the specific purpose of down-regulating the host's inflammatory and immune responses so that the mites can successfully reproduce and establish a population on the host. Likewise, while some molecules from the mites modulate specific aspects of the inflammatory and immune responses, some molecules are antigenic and as the mite population increases the amount of these immunogenic molecules increases. It may be that the increase in antigenic load tips the balance and the host may eventually produce inflammatory and immune responses that may override the inhibitory effect of the down-regulating molecules.
As mites burrow in the skin, mite molecules may stimulate the activities of keratinocytes, fibroblasts, Langerhans cells, dendritic cells, macrophages and monocytes, mast cells, neurons, smooth muscle and venule endothelial cells of the microvasculature. Specific cells can release a selected set of chemical mediators. These chemical mediators may include histamine (from degranulation of mast cells) and arachidonic acid derivatives (from the metabolism of membrane phospholipids) and include prostaglandins, leukotrienes, thromboxanes, bradykinins, and platelet-activating factor from mast cells, macrophages, monocytes, and neutrophils in addition to proinflammatory cytokines and chemokines in the skin from a variety of cells as already reported.
Post-capillary venule endothelial cells control cell diapedesis during inflammation and immune responses. Because of their key role in the regulation of the migration of inflammatory and immune cells into the site of the mite infestation in the dermis, venule endothelial cells that line the microvasculature of the skin are potential targets for immune modulation by S. scabiei. Our previous research has demonstrated that scabies extract influences the function of individually cultured dermal microvascular endothelial cells [8]. In particular, it was able to down-regulate expression of some cytokines, chemokines and adhesion molecules leading to tissue recruitment of leukocytes from the circulation. This research, which demonstrates the modulatory properties of these mites on endothelial cells, was done using monocultures of cells that were stimulated with mite extract in the absence of other exogenous inflammatory mediators that undoubtedly occur in vivo. However, the functions of endothelial cells of the microvasculature in response to S. scabiei may be influenced by the milieu of cytokines, chemokines, histamine, and lipid-derived mediators secreted by surrounding cells in response to bioreactive substances from the mites. The release of these molecules can be influenced by scabies mites. How these chemical mediators orchestrate the response of endothelial cells to S. scabiei has not been elucidated but it is important to understanding the mechanism for delaying or stimulating inflammation in the scabietic lesion. The purpose of this study was to determine if the response of endothelial cells to S. scabiei is altered in the presence of inflammatory cytokines, histamine, and some common proinflammatory lipid-derived mediators that likely occur in scabietic lesions in vivo.
2. Materials and methods
2.1. Sarcoptes scabiei mite extract
Extract was prepared from S. scabiei variety canis mites as described previously [6]. Briefly, mites of all life stages were collected, killed by freezing, and stored at -80°C. An aqueous whole body homogenate extract of S. scabiei was prepared in glass-distilled water at 1/20 (wt/vol). Mites were ground in a TenBroeck homogenizer on ice until smooth (10 strokes) and allowed to extract 24 h at 4°C. The soluble extract was collected by centrifugation, and the pellet was subjected to a second 24-h extraction. Pooled supernatants were sterile filtered (0.22 μm) into a sterile vial. Total protein concentration in the extract was measured by the Bradford protein assay using bovine serum albumin as the standard [14].
2.2. Reagents
Recombinant human IL-8, IL-1α, IL-1β, TNFα, and IL-6 as well as biotinylated monoclonal antibodies directed against human intercellular adhesion molecule -1 (ICAM-1) and VCAM-1 were purchased from eBioscience, Inc. (San Diego, CA). Biotinylated monoclonal antibodies directed against human E-selectin were purchased from R&D Systems, Inc. (Minneapolis, MN). Histamine, prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), leukotriene B4 (LTB4) and platelet activating factor (PAF) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Horseradish peroxidase-conjugated strepavidin and goat anti-mouse Ig were purchased from Fisher Scientific (Pittsburgh, PA). Recombinant TNFα was purchased from Endogen (Rockford, IL). Preliminary experiments were performed to determine optimum antibody dilutions for each antibody (data not shown).
2.3. Endothelial cells
Cryopreserved adult dermal microvascular endothelial cells (HMVEC-D) and all media and growth factors were purchased from Cambrex Bio Science, Walkersville, Inc (Walkersville, MD). Cells were cultured in Clonetics Endothelial Cell Basal Medium-2 supplemented with EGM-2-MV growth factors (EGM-2) at 37°C in 5 - 7% CO2. Cells were plated and grown to confluency in Costar 96-well culture plates (Corning, Inc., Corning, NY) for subsequent cell ELISA and cytokine secretion studies. All studies were performed on cells between passages 6 and 10.
2.4. Stimulant Preparation
Stock solutions of IL-6, TNFα, IL-1α, IL-1β and histamine were made in EGM-2. Stock solutions of PGD2, PGE2, LTB4 and PAF were made in absolute ethanol. All further dilutions were made in EGM-2. Where appropriate, medium control wells contained ethanol in an amount equivalent to the highest concentration present in the stimulant to monitor for any effects of the ethanol on cell growth and function. Stimulants were tested in the following concentration ranges: IL-6 (150 - 15,000 pg/ml); TNFα (0.04 - 4 ng/ml); IL-1α (10 - 1,000 pg/ml); IL-1β (1 - 100 pg/ml); Histamine (0.1 - 1,000 uM); PAF (20 - 2,000 nM), PGD2 (1,000 - 10,000 nM); PGE2 (0.25 - 2500 nM); PAF (20 - 2,000 nM).
2.5. Cell-ELISA procedure
The expression of cellular adhesion molecules was determined using a cellular-ELISA procedure modified from Yokote et al. [15] and described previously [8]. All cell stimulations reported here were conducted for 6 h and S. scabiei extract was used at 100 μg/ml as determined previously [8].
2.6. Cytokine assays
Culture supernatants collected at the conclusion of the stimulation of endothelial cells were assayed for the presence of various cytokines. DuoSet ELISA kits for detection of IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1) were purchased from R&D Systems Inc., Minneapolis, MN. Absorbance was read using the BioTek EL800X microplate reader set to 450 nm.
2.7 Data analysis
All cell challenge experiments were performed several times. The data presented here are the average ± standard error of the mean (SEM) for triplicate wells from representative experiments.
3. Results
3.1 Scabies extract down-regulated expression of VCAM-1 in the presence of lipid-derived mediators, histamine, TNFα and other proinflammatory cytokines
Enhanced expression of cell adhesion molecules on the luminal surface of post-capillary venules facilitates recruitment of leukocytes into the dermis of the host during the inflammatory and immune responses to scabies mites. Experiments were conducted to determine if S. scabiei extract could down-regulate expression of these molecules by endothelial cells in the presence of a variety of proinflammatory cytokines and lipid-derived mediators that are likely produced by neighboring dermal cells in response to a burrowing scabies mite.
Endothelial cells constitutively expressed ICAM-1 but not VCAM-1 or E-selectin (data not shown). Stimulation with scabies mite extract alone induced increased expression of ICAM-1 above constitutive levels, and some secretion of E-selectin but not VCAM-1 (Figure 1). Alone, IL-6, PGD2, PGE2, histamine, LTB4 or PAF did not significantly influence the constitutive expression of ICAM-1 nor did they induce the expression of E-selectin or VCAM-1. Co-stimulation with S. scabiei extract and IL-6, PGD2, PGE2, histamine, LTB4 or PAF did not change the level of expression of ICAM-1, E-selectin or VCAM-1 that was seen with scabies alone or the mediators alone (data not shown).
Fig. 1.
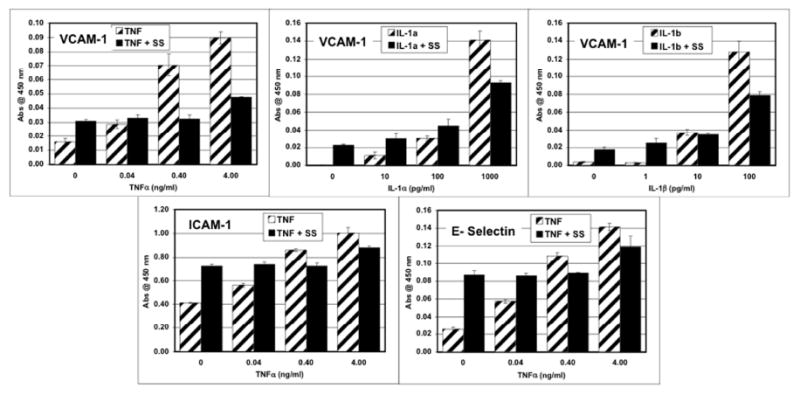
Dose dependent expression of adhesion molecules by human microvascular endothelial cells stimulated for 6 hours with varying doses of the proinflammatory cytokines TNFα, IL-1α or IL-1β in the absence or presence of S. scabiei (SS) extract at 100 μg/ml.
Stimulation with IL-1α, IL-1β, or TNFα alone induced a dose-dependent increased expression of ICAM-1, E-selectin and VCAM-1 by endothelial cells (Figure 1). Co-stimulation of endothelial cells with scabies extract in the presence of TNFα, IL-1α or IL-1β down-regulated this induced expression of VCAM-1 (Figure 1). When TNFα was used to induce VCAM-1 expression, scabies extract was still able to down-regulate this expression of VCAM-1 in the presence of IL-1α, IL-1β, IL-6, PGD2, PGE2, histamine, LTB4 or PAF (Figure 2).
Fig. 2.
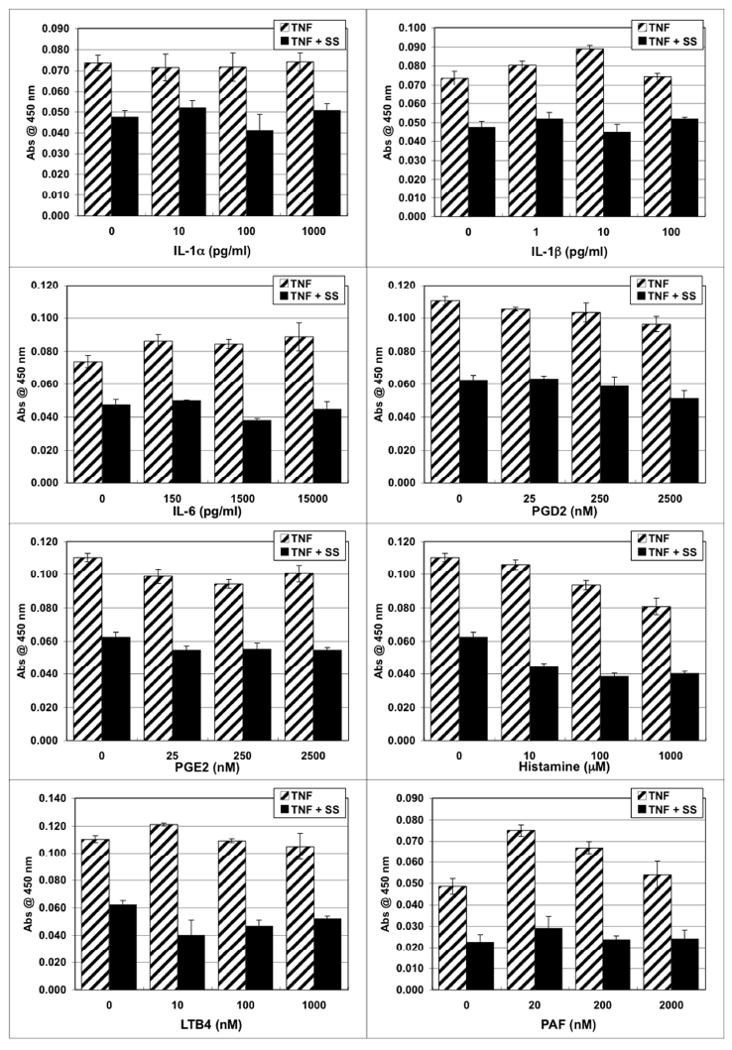
Scabies extract down-regulated expression of vascular cell adhesion molecule-1 (VCAM-1) in the presence of lipid-derived mediators, histamine, TNFα and other proinflammatory cytokines. Human microvascular endothelial cells were stimulated for 6 hours with varying doses of stimulant in the absence or presence of S. scabiei (SS) extract at 100 μg/ml and TNFα at 4 ng/ml.
3.2 Scabies extract down-regulated IL-6 secretion in the presence of TNFα along with histamine, LTB4, IL-1α or IL-1β
IL-6 is an important proinflammatory cytokine that is produced by keratinocytes, fibroblasts, and endothelial cells in the skin in response to scabies mites [5,8]. In this study, endothelial cells did not constitutively secrete IL-6, however, stimulation with scabies extract alone induced the secretion of very small amounts of IL-6 (Figure 3). IL-1α, IL-1β or TNFα alone stimulated secretion of IL-6 in a dose-dependent manner while PGD2, PGE2, histamine, LTB4 or PAF had no effect (Figure 3). The IL-1α and IL-1β-induced secretion of IL-6 was inhibited in the presence of scabies extract. Co-stimulation with scabies extract along with PGD2, PGE2, histamine, LTB4 or PAF had little effect on secretion of IL-6 above levels induced by scabies extract alone. TNFα plus IL-1α, IL-1β, PGD2, PGE2 or histamine induced greater secretion than TNFα alone. The TNFα-induced secretion of IL-6 was reduced when cells were stimulated with scabies in the presence of IL-1α, IL-1β, histamine or LTB4.
Fig. 3.
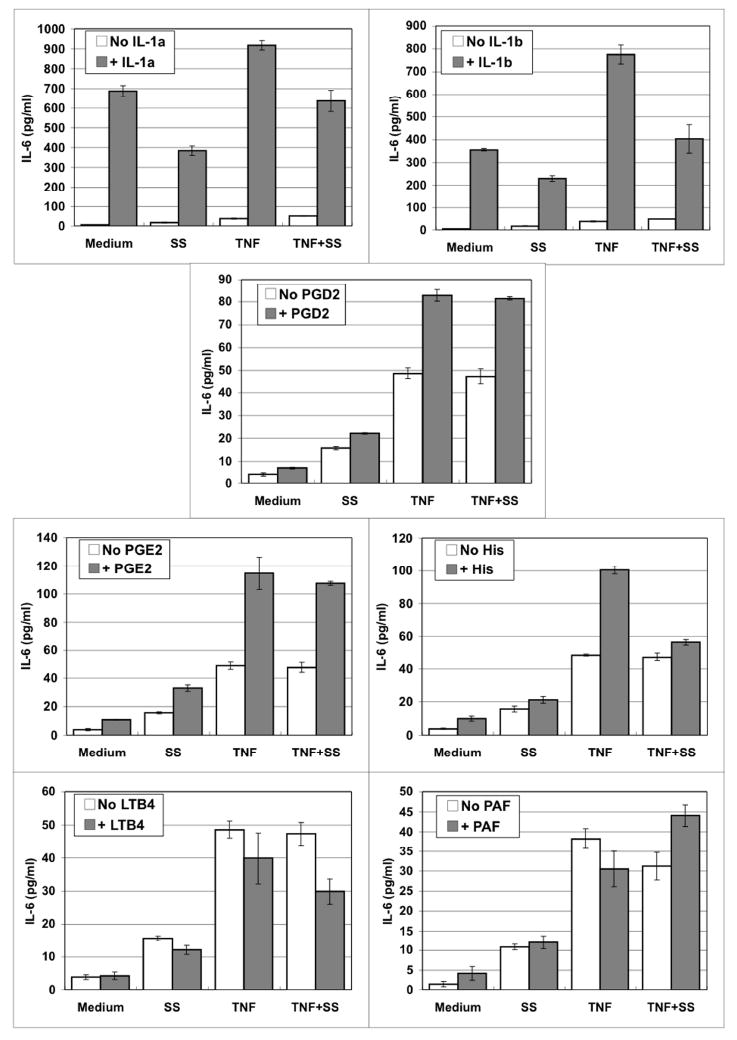
Scabies extract down-regulated IL-6 secretion in the presence of TNFα along with histamine, LTB4, IL-1α or IL-1β. Human microvascular endothelial cells were stimulated for 6 hours with varying doses of stimulant in the absence or presence of S. scabiei (SS) extract at 100 μg/ml and TNFα at 4 ng/ml.
3.3 Scabies extract down-regulated IL-8 secretion in the presence of lipid-derived mediators, histamine, TNFα and other proinflammatory cytokines
IL-8 is a chemoattractant for neutrophils that leads to recruitment of these cells into the tissues from the circulation. We found that endothelial cells constitutively secreted IL-8 and stimulation with TNFα, IL-1α or IL-1β more than doubled this secretion above the constitutive levels (Figure 4). Scabies extract depressed both the constitutive secretion of IL-8 and the TNFα, IL-1α and IL-1β induced secretion of IL-8. IL-6, PGD2, PGE2, histamine, LTB4 or PAF had no effect on the constitutive secretion of IL-8. The scabies-induced depression of constitutive secretion of IL-8 was not affected by the presence of IL-6, PGD2, PGE2, histamine, LTB4 or PAF. However, the scabies-induced depression of TNFα-induced IL-8 secretion was partly overcome by the presence of IL-1α or IL-1β in a dose-dependent fashion.
Fig. 4.
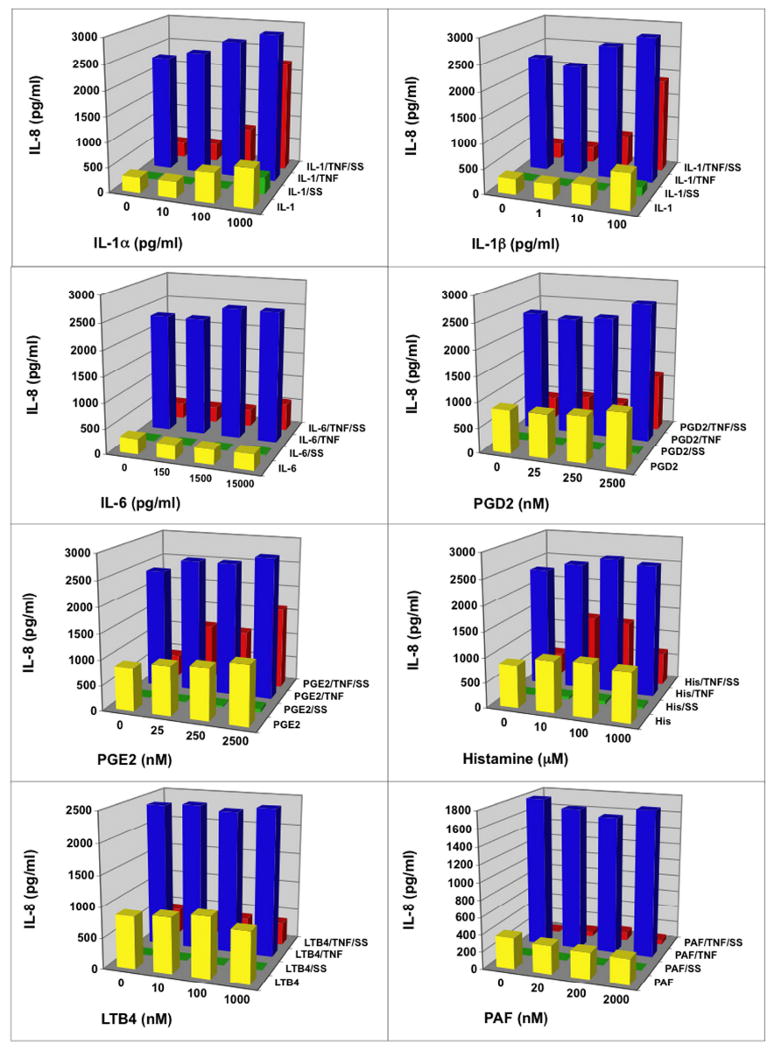
Scabies extract down-regulated IL-8 secretion in the presence of lipid-derived mediators, histamine, TNFα and other proinflammatory cytokines. Human microvascular endothelial cells were stimulated for 6 hours with varying doses of stimulant in the absence or presence of S. scabiei (SS) extract at 100 μg/ml and/or TNFα at 4 ng/ml.
3.4 Scabies extract down-regulated MCP-1 secretion in the presence of lipid-derived mediators, histamine, TNFα and other proinflammatory cytokines
MCP-1 (CCL2) is a cytokine produced by endothelial cells and keratinocytes that is a chemoattractant for monocytes and can activate them to become macrophages. This is important in the inflammatory and immune responses in the skin.
In these experiments, scabies extract stimulated secretion of MCP-1 above constitutive levels (Figure 5). IL-1α, IL-1β and TNFα alone induced secretion of MCP-1 in a dose dependent manner. IL-6, PGD2, PGE2, histamine, LTB4 or PAF did not stimulate secretion of MCP-1 and did not influence the secretion induced by scabies extract alone. Scabies extract down-regulated the TNFα-induced secretion of MCP-1 and only the addition of the highest dose of IL-6 overcame this suppression.
Fig. 5.
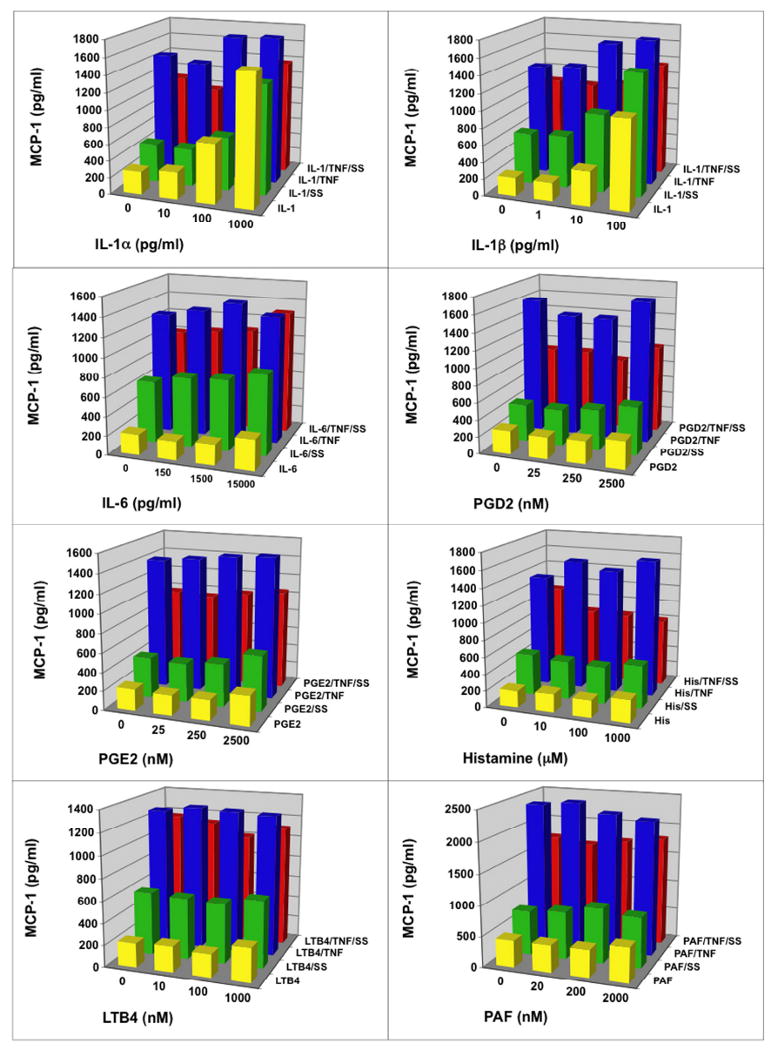
Scabies extract down-regulated monocyte chemotactic protein (MCP-1/CCL2) secretion in the presence of lipid-derived mediators, histamine, TNFα and other proinflammatory cytokines. Human microvascular endothelial cells were stimulated for 6 hours with varying doses of stimulant in the absence or presence of S. scabiei (SS) extract at 100 μg/ml and/or TNFα at 4 ng/ml.
4. Discussion
The skin around a burrowing scabies mite is composed of a limited number of resident and transient cell types that can play a role in an initial localized inflammatory response to the scabies mite and molecules from it. These cells include keratinocytes and Langerhans cells in the epidermis and fibroblasts, monocytes and macrophages derived from them, mast cells, and vascular endothelial cells in the dermis. Scabies mites reside among these cells or in close proximity to them. It is clear that cells in the epidermis and dermis respond to modulating molecules and antigens from scabies mites by changing the expression of cytokines, chemokines, and cellular adhesion molecules. The main cytokines, chemokines, and other mediators of inflammation released from these cells include IL-1α, IL-1β, TNFα, IL-6, IL-8, interferon-γ, histamine, prostaglandins, leukotrienes, platelet activating factor, and thromboxanes. Vascular endothelial cells of the skin regulate (control) the extravasation of inflammatory and immune cells from the post-capillary venule into the dermis by the way that they express adhesion molecules and chemokine receptors and secrete cytokines and chemokines.
We previously demonstrated that scabies extract (in the presence of TNFα) inhibited expression of VCAM-1, E-selectin and chemokine receptor-5 (CCR-5) and the secretion of IL-8 by cultured normal adult dermal microvascular endothelial cells, had no effect on the expression of ICAM-1 and secretion of MCP-1 but induced secretion of IL-6 and expression of chemokine receptors CXCR-1 and CXCR-2 [8]. However, the response of these cultured cells to other cytokines, chemokines and lipid mediators that might typically be produced by surrounding cells in response to a burrowing scabies mite (e.g. keratinocytes, fibroblasts, macrophages, mast cells, Langerhans cells, lymphocytes) was not studied since we did not include these in the culture medium. Therefore, in the present study we examined the response of endothelial cells to scabies extract in the presence of histamine, lipid-derived mediators, cytokines, and chemokines that are likely present in the vicinity of a scabietic lesion, some of which are secreted by cells in the skin in response to scabies mite products [5,6,8].
Our study found that IL-6, histamine, prostaglandins (PGE2 and PGD2), leukotriene B4 and platelet activating factor did not effect the response of vascular endothelial cell expression of the adhesion molecules ICAM-1, VCAM-1 and E-selectin to scabies mite extract. Particularly significant is that scabies down-regulated the TNFα-induced expression of VCAM-1, and this down-regulation was not reversed in the presence of histamine, the proinflammatory cytokines IL-1α, IL-1β, or IL-6, or the lipid-derived mediators PGE2, PGD2, PAF and LTB4. This down-regulation is significant because VCAM-1 plays a key role in the rolling and attachment of leukocytes to the endothelial cells of the postcapillary venules in preparation for migration out of the blood stream and into a scabietic lesion.
We were interested in the effects of histamine and the lipid-derived mediators because of their role in allergic (IgE-mediated) reactions in the skin. Patients with ordinary and crusted scabies often exhibit circulating IgE antibodies to the molecules in a S. scabiei extract [16]. IgE bound to the Fc receptor on the mast cell is the key mediator that initiates the signaling pathway that leads to degranulation of mast cells following binding of allergen to the IgE molecule on the mast cell surface. Histamine and prostaglandin D2 from mast cells cause increased vascular permeability (vascular dilation) by their effects on endothelial cells. Histamine is the primary product of the degranulation of mast cells, however, prostaglandin D2 and leukotrienes B4, C4, D4, and E4 are released from mast cells during degranulation and the breakdown of the plasma membrane phospholipids [17], and it is these molecules that are responsible for allergic symptoms [18]. Once mast cells degranulate, the symptoms associated with an allergic reaction such as vascular dilation (redness), edema (loose tight junctions that result in fluid escape from the capillary), and pruritus (itching) become evident. These are all symptoms of ordinary and chronic scabies. Lipid mediators can also influence the inflammatory response independent of the mast cell IgE-mediated allergic response [19]. Macrophages are a source of prostaglandins, leukotrienes, and PAF, and these may be released even in patients not predisposed to an IgE response to scabies. Our findings suggest that histamine and prostaglandins do not enhance the effects produced by scabies mites once the host is sensitized and the host response overcomes the down-modulation that prevents inflammation early in an infestation.
Scabies alone induced only a slight increase in expression of the proinflammatory cytokine IL-6 from dermal endothelial cells. Our data show that IL-1α, IL-1β, and TNFα stimulated increased production of IL-6 by these cells. In addition, we demonstrated that these endothelial cells are synergistically stimulated by histamine, PGD2, PGE2, PAF, IL-1α, and IL-1β in the presence of TNFα, as might be expected in a dermal inflammatory response. Importantly, the addition of a combination of scabies extract and IL-1α, IL-1β, histamine or LTB4 to TNF-induced cells resulted in down-regulation of IL-6 production. Since IL-6 is a critical proinflammatory cytokine in the inflammatory response, this down-regulation may be a factor in the delayed inflammatory response often seen in a primary scabies infestation. More importantly, the minimal secretion of IL-6 that was induced by scabies extract alone was increased in the presence of histamine and the prostaglandins. Therefore, one would expect that the effect of scabies would be amplified during inflammation in the skin in the absence of any other inhibitors from the mites. However, we also observed that the level of secretion of IL-6 that was induced by co-stimulation with both scabies extract and TNFα together was reduced in the presence of histamine or LTB4 (a potent chemoattractant of neutrophils). It is interesting that the presence or absence of TNFα determines whether histamine and prostaglandin, respectively, increase or decrease the effect induced by scabies. The mechanism and significance of these opposite effects are not clear.
IL-8 is chemotactic for neutrophils. Many neutrophils are present in scabietic lesions and they are the earliest cell type to appear in the infiltrate once inflammation is initiated during both primary and secondary infestations [20,21]. In this study, we confirmed that scabies inhibited constitutive and TNFα-induced secretion of IL-8 by endothelial cells. More importantly, we found that the presence of histamine, PGD2, PGE2, LTB4, PAF and IL-6 had no effect on the scabies-induced inhibition. However, in the presence of the proinflammatory cytokines IL-1α and IL-1β, the scabies-induced IL-8 inhibition still occurred but it was reduced in a dose-dependant fashion. Therefore, although scabies alone reduced constitutive secretion of IL-8, the amount of IL-8 produced when cells were co-stimulated with scabies extract and IL-1α or IL-1β was greater than when scabies was present alone.
Neutrophil extravasation is initiated by expression of E-selectin on the endothelial cell surface that initiates the loose tethering and rolling by low affinity for the mucin-like CAM on the neutrophil. IL-8 activates the neutrophil integrin receptor that allows it to tightly bind to the Ig-like CAM on the endothelial cell. It appears that scabies is able to down-regulate this IL-8-regulated step even in the presence of histamine, leukotriene B4, prostaglandins D2 and E2, and cytokines TNFα, IL-1α, and IL-1β. We previously found that scabies down-regulated the expression of E-selectin and this too may contribute to the lack of inflammatory cells in the early scabietic lesion. Thus, it appears that down-regulated expression of E-selectin and IL-8 together may contribute to inhibiting neutrophil extravasation early in a scabies infestation.
5. Conclusion
Scabies mite extract contains molecules that down-regulate expression of VCAM-1 and E-selectin on human dermal microvascular endothelial cells, secretion of IL-8, and the IL-1α and IL-1β-stimulated secretion of IL-6 by these cells. In vivo endothelial cells may be influenced by the milieu of histamine, lipid-derived mediators and proinflammatory cytokines secreted by surrounding cells in the skin. In this study, we demonstrated that histamine and the lipid-derived biologic mediators did not influence the observed inhibitory effects of S. scabiei extract on endothelial cells, and in one situation (the TNFα-induced secretion of IL-6) a combination of histamine or LTB4 with scabies extract was able to down-regulate this secretion. In contrast, the proinflammatory cytokines IL-1α or IL-1β were able to partially overcome the inhibitory effects of S. scabiei on IL-8 secretion in a dose-dependent fashion.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Disease (AI-017252) to LGA. We thank DiAnn L. Vyszenski-Moher for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orkin M, Maibach H, Parish LC, Schwartzman LM. Scabies and pediculosis. Philadelphia: Lippincott; 1977. [Google Scholar]
- 2.Kemp DJ, Walton SF, Harumal P, Currie BJ. The scourge of scabies. Biologist. 2002;49:19–24. [PubMed] [Google Scholar]
- 3.Elgart ML. Cost-benefit analysis of ivermectin, permethrin and benzyl benzoate in the management of infantile and childhood scabies. Expert Opin Pharmacother. 2003;4:1521–4. doi: 10.1517/14656566.4.9.1521. [DOI] [PubMed] [Google Scholar]
- 4.Arlian LG, Vyszenski-Moher DL, Rapp CM, Hull BE. Production of IL-1 alpha and IL-1 beta by human skin equivalents parasitized by Sarcoptes scabiei. J Parasitol. 1996;82:719–23. [PubMed] [Google Scholar]
- 5.Arlian LG, Morgan MS, Neal JS. Modulation of cytokine expression in human keratinocytes and fibroblasts by extracts of scabies mites. Am J Trop Med Hyg. 2003;69:652–6. [PubMed] [Google Scholar]
- 6.Arlian LG, Morgan MS, Neal JS. Extracts of scabies mites (Sarcoptidae: Sarcoptes scabiei) modulate cytokine expression by human peripheral blood mononuclear cells and dendritic cells. J Med Entomol. 2004;41:69–73. doi: 10.1603/0022-2585-41.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Arlian LG, Morgan MS, Paul CC. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J Med Entomol. 2006;43:283–7. doi: 10.1603/0022-2585(2006)043[0283:etsmas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Elder BL, Arlian LG, Morgan MS. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J Med Entomol. 2006;43:910–5. doi: 10.1603/0022-2585(2006)43[910:ssasme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–82. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 10.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–27. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 11.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10. [PubMed] [Google Scholar]
- 12.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–91. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arlian LG, Morgan MS. Serum antibody to Sarcoptes scabiei and house dust mite prior to and during infestation with S. scabiei. Vet Parasitol. 2000;90:315–26. doi: 10.1016/s0304-4017(00)00251-x. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Yokote K, Morisaki N, Zenibayashi M, Ueda S, Kanzaki T, Saito Y, et al. The phospholipase-A2 reaction leads to increased monocyte adhesion of endothelial cells via the expression of adhesion molecules. Eur J Biochem. 1993;217:723–9. doi: 10.1111/j.1432-1033.1993.tb18298.x. [DOI] [PubMed] [Google Scholar]
- 16.Arlian LG, Morgan MS, Estes SA, Walton SF, Kemp DJ, Currie BJ. Circulating IgE in patients with ordinary and crusted scabies. J Med Entomol. 2004;41:74–7. doi: 10.1603/0022-2585-41.1.74. [DOI] [PubMed] [Google Scholar]
- 17.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–84. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–86. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 20.Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. The development of protective immunity in canine scabies. Vet Parasitol. 1996;62:133–42. doi: 10.1016/0304-4017(95)00854-3. [DOI] [PubMed] [Google Scholar]
- 21.Arlian LG, Rapp CM, Vyszenski-Moher DL, Morgan MS. Sarcoptes scabiei: histopathological changes associated with acquisition and expression of host immunity to scabies. Exp Parasitol. 1994;78:51–63. doi: 10.1006/expr.1994.1005. [DOI] [PubMed] [Google Scholar]


