Abstract
The development of a lentiviral system to deliver genes to specific cell types could improve the safety and the efficacy of gene delivery. Previously, we have developed an efficient method to target lentivectors to specific cells via an antibody-antigen interaction in vitro and in vivo. We report herein a targeted lentivector that harnesses the natural ligand-receptor recognition mechanism for targeted modification of c-KIT receptor-expressing cells. For targeting, we incorporate membrane-bound human stem cell factor (hSCF), and for fusion, a Sindbis virus-derived fusogenic molecule (FM) onto the lentiviral surface. These engineered vectors can recognize cells expressing surface CD117, resulting in efficient targeted transduction of cells in a SCF-receptor dependent manner in vitro, and in vivo in xenografted mouse models. This study expands the ability of targeting lentivectors beyond antibody targets to include cell-specific surface receptors. Development of a high titer lentivector to receptor-specific cells is an attractive approach to restrict gene expression and could potentially ensure therapeutic effects in the desired cells while limiting side effects caused by gene expression in non-target cells.
Introduction
The development of gene delivery has facilitated the utilization of nucleic acids as practical tools to understand and treat diseases (Verma and Weitzman 2005). One of the obstacles for gene delivery is accomplishing targeted gene expression in a specific subset of cells, which due to the great diversity of cell types continues to be a key challenge (Jang et al. 2007; Waehler et al. 2007). Furthermore, gene delivery to a particular type of cells would limit side effects caused by gene expression in non-targeted cells and ensure therapeutic effects in only the desired cells (Waehler et al. 2007). Efficient targeted transduction into specific cell types still represents a major barrier to gene therapy. Previously, we have reported a method to target lentivectors to specific cells via antibody-antigen mediated targeting (Yang et al. 2006). It remains unknown whether a natural ligand-receptor interaction can be similarly utilized to engineer lentivectors for selective modification of the receptor-expressing cells.
c-KIT is a proto-oncogene encoding CD117, a type III cell surface transmembrane tyrosine kinase receptor, that naturally binds stem cell factor (SCF) (Hamel and Westphal 1997). CD117 is expressed in many tissues including mast cells, gastrointestinal stromal tumors (GISTs), melanocytes in the skin, glial tumors, interstitial cells of Cajal in the digestive tract and is a precise marker in the bone marrow for hematopoietic progenitor cells (Edling and Hallberg 2007; Miettinen and Lasota 2005). Surface CD117 expression can serve as a unique molecular determinant to differentiate between cell types that can be targeted for gene therapy. Due to the prevalence of c-KIT receptor in associated malignancies, gene delivery to CD117 specific cells is an interesting target to demonstrate the potential of engineering targeted lentivectors utilizing cell surface receptor-ligand interactions.
The development of gene delivery vehicles that are targeted to CD117 has been the goal of many investigators working in the field of gene therapy. Several groups have targeted c-KIT using plasmid DNA complexes as well as modified adenoviruses (Chapel et al. 2004; Chapel et al. 1999; Itoh et al. 2003; Schwarzenberger et al. 1996; Zhong et al. 2001). However, these methods do not lead to long-term stable gene expression. Others have manipulated the gamma-retrovirus – an enveloped double stranded RNA virus that is capable of stable integration in the host genome. The investigations redirecting gamma-retroviral vectors to deliver genes to CD117 cells have focused on using membrane-bound SCF with ecotropic or amphotropic envelope glycoproteins of murine leukemia virus (Chandrashekran et al. 2004; Fielding et al. 1998). The challenge to this approach is that the native envelope glycoprotein’s fusion ability remains intimately linked with receptor binding. The unknown and delicate coupling mechanisms of binding and fusion make it extremely difficult to reconstitute fusion function once the binding determinant of the vector has been altered, which has resulted in inconsistent targeting and low viral titers (Kasahara et al. 1994; Sandrin et al. 2003; Zhao et al. 1999). To circumvent the need for specific targeting, current strategies depend upon direct injection to a localized site with cell specific promoters/enhancers (Logan et al. 2002; Lutzko et al. 2003; Moreau et al. 2004) or, ex vivo isolation, purification and transduction (Akporiaye and Hersh 1999; Cavazzana-Calvo et al. 2000).
One limitation to the utility of current viral vectors remains producing a high titer, long-term expressing, cell specific, gene delivery strategy. In this paper, we engineer lentivectors capable of specifically transducing receptor-specific cells using lentivectors incorporating a cognate native ligand and fusogenic molecule. Previously, we have reported a method to target lentivectors to specific cells via antibody-antigen mediated targeting (Yang et al. 2006). We report herein a novel approach to harness the natural ligand-receptor mechanism for targeted modification of c-KIT receptor-expressing cells. For targeting, we incorporate membrane-bound human stem cell factor (hSCF), and for fusion, a Sindbis virus-derived fusogenic molecule (FM) onto the lentiviral surface. Targeting lentivectors displaying these two proteins are effective for selective transduction of a c-KIT receptor model target cell line in vitro and in vivo. Development of an efficient lentivector for receptor-specific gene transfer could potentially limit the side effects caused by gene integration in non-target cells and ensure therapeutic effects in the desired cells.
Materials and Methods
Plasmid Construction
The fusion molecules have been previously described (Yang et al. 2008). Briefly, the original binding-deficient Sindbis virus-derived fusogenic molecule (Yang et al. 2006), designated FM1, was used as a template for PCR mutagenesis to introduce mutations at the E1 226 domain to replace amino acids AKN in FM1 with AGM, or SGM, or SGN to generate new mutant forms of fusogen designated FM2, FM3, and FM4, respectively (Figure 1). To generate phSCF, the genomic DNA was extracted from a cell line SI/SI4 hSCF220 (ATCC CRL-2453; Manassas, VA) (Toksoz et al. 1992). PCR was performed to amplify the cDNA of the membrane-bound human SCF (hSCF), which was then sub-cloned downstream of the CMV promoter in the mammalian expression plasmid pcDNA3 (Invitrogen, Carlsbad, CA) (Figure 1). To generate the expression plasmid for CD117-blind ligand CD20, human CD20 cDNA was sub-cloned downstream of the CMV promoter in the pcDNA3. The integrity of the DNA sequences was confirmed by DNA sequencing. The lentiviral backbone plasmid FUGW has been previously described (Figure 1) (Yang et al. 2006). The lentiviral backbone plasmid FUGL was constructed by removing the eGFP gene from FUGW and inserting the PCR assembly of the cDNA of firefly luciferase linked to self-cleaving P2A (Szymczak and Vignali 2005) followed by eGFP cDNA (Figure 1).
Figure 1.
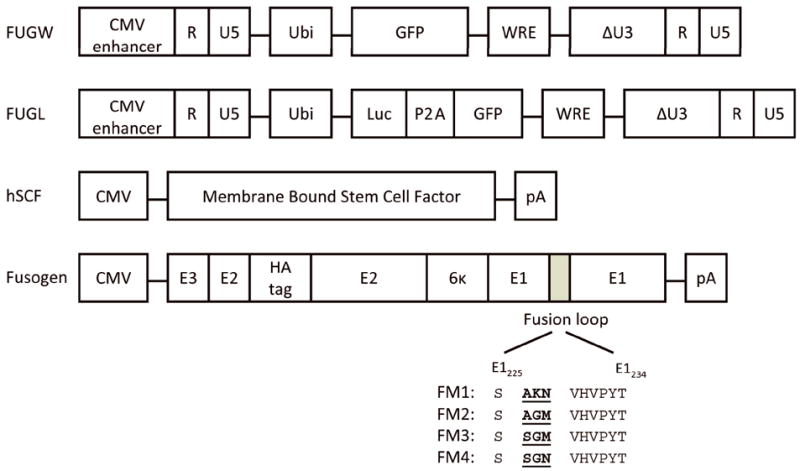
Virus-producing constructs used to make the recombinant targeting lentiviral vectors. Schematic diagrams of constructs encoding transfer lentiviral vector FUGW, lentivector expressing luciferase reporter FUGL, membrane-bound human stem cell factor (hSCF), and fusogenic molecules derived from Sindbis virus glycoprotein. CMV enhancer: the enhancer element derived from human cytomegalovirus; Ubi: the human ubiquitin-C promoter; GFP: enhanced green fluorescence protein; WRE: woodchuck responsive element; ΔU3: deleted U3 region that results in the transcriptional activation of the integrated viral LTR promoter; Luc: firefly luciferase gene; P2A: self-cleaving linker; CMV: human cytomegalovirus immediate-early gene promoter; pA: polyadenylation signal; E1, E2, 6κ, E3: glycoproteins of the Sindbis virus (E1 for fusion, E2 for receptor binding, 6κ a linker, and E3 is a signal sequence); HA tag: 10-residue epitope HA tag sequence (MYPYDVPDYA). Fusogen molecules were constructed by making several alterations (Morizono et al. 2005; Yang et al. 2006), including deletion of amino acids to disrupt the binding to heparin sulfate glycosaminoglycan, resulting in a binding deficient but fusion-active fusogenic protein FM1. Mutations were then made in the fusion loop region to create various engineered fusogens (FM2, FM3, FM4) amino acid sequences start at 226 of wild-type Sindbis E1.
Generation of Cell Lines
To create a stable cell line expressing human c-KIT receptor for the testing of targeted gene delivery, the cDNA of human c-KIT was sub-cloned downstream of the ubiquitin-C promoter in the lentiviral backbone plasmid FUW. This construct was termed FUW-hcKIT. Subsequently, VSVG-pseudotyped FUW-hcKIT was prepared using calcium-phosphate precipitation and the viral supernatant was used to transduce the parental Jurkat cell line (ATCC TIB-152) by spin infection. After several passages, the resulting cells were stained with PE-conjugated anti-human CD117 antibody and subjected to cell sorting to obtain a uniform population of c-KIT+ cells designated Jurkat.hcKIT.
Flow Cytometry Analysis
Antibodies and other staining reagents used for this study include: Biotin-conjugated anti-HA (Miltenyi Biotech, Bergisch Gladbach, Germany), PECy5-conjugated streptavidin (BD Biosciences, San Jose, CA), anti-hcKIT (Biolegend, San Diego, CA), anti-hSCF (R&D Systems, Minneapolis, MN). Flow cytometry was acquired using a FACScan (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Effects of Blocking SCF and NH4Cl on Viral Transduction
Jurkat.hcKIT cells (0.1 × 106) and 500 μL of viral supernatant (FUGW/hSCF+FM3) were co-incubated in the presence of soluble human SCF (R&D Systems, Minneapolis, MN), soluble IL-3 as a control (R&D Systems), or NH4Cl (Fisher Scientific, Waltham, MA). The culture media were then replaced 8 hours later with fresh media and incubated for another 4 days at 37°C, 5% CO2. Experiments were preformed in triplicate and eGFP expression was analyzed using flow cytometry.
Targeted Transduction in vitro
In a 24-well flat bottom plate, Jurkat.hcKIT or TF1α cells (0.2 × 106) were plated and mixed with 2 mL of fresh viral supernatant and 10 μg/mL polybrene (Chemicon International, Inc., Temecula, CA). Cells were spin-infected for 90 minutes at 2,500 rpm at 30°C in Jouan CR 412 centrifuge (Jouan S.A., Cedex, France). Subsequently the supernatant was removed and replaced with fresh culture medium and incubated for a further 3 days at 37°C with 5% CO2. Experiments were preformed in triplicate and one representative result is shown. Viral titers were preformed in duplicate in a 96-well flat bottom plate, Jurkat.hcKIT or Jurkat cells (0.02 × 106) were plated spin infected with 100μL dilutions of fresh viral supernatant. The transduction titer was measured in dilution ranges that exhibited a linear response of eGFP expression with viral serial dilution concentration.
Virus-cell Binding Assay
Jurkat or Jurkat.hcKIT cells (0.1 × 106) were incubated with 500 μL of viral supernatant at 4°C for half an hour and washed with 4 mL of cold PBS. After extensive washing using cold PBS, the cells were mixed with an anti-HA tag antibody (Roche Applied Science, Mannheim, Germany) to stain the fusogen molecule. After staining, cells were analyzed by flow cytometry. Experiment was conducted in duplicate and a representative result shown.
Mice
RAG2-/-γc-/- mice (Jackson Laboratory, Bar Harbor, ME) were maintained in the university’s animal facility and cared for in accordance to the NIH guideline and institute regulations. Treated mice were maintained on mixed antibiotics (sulfamethoxazole and trimethoprim oral suspension; Hi-Tech Pharmacal, Amityville, NY).
Targeting Jurkat.hcKIT in vivo
Mice (8-12 weeks old) were anesthetized with 2.5% isoflurane and injected either subcutaneously or intrafemorally with Jurkat.hcKIT cells suspended in PBS. For intrafemoral or intra-bone marrow (i.b.m.) injection, the right knee was shaved and sterilized. A 27-gauge needle (BD Bioscience) was inserted into the femur through the flexed knee to complete injection. Intrafemorally injected mice were given 2 mg/kg ketoprofen (Fort Dodge, Fort Doge, IA) for pain management. Lentivector was concentrated using ultracentrifugation (Optima L-80 K preparative ultracentrifuge, Beckman Coulter) for 90min at 50,000 × g. Viral particles were then resuspended in an appropriate volume of cold PBS. Either 8 hours later or 1 week later mice were injected with concentrated lentivector expressing firefly luciferase and eGFP through subcutaneous (s.c.) or i.b.m. administration. To analyze targeting efficiency, mice were anesthetized with 2.5% isoflurane (Abbott Animal Health, Abbott Park, IL) and 1.5 mg D-Luciferin (Xenogen, Hopkinton, MA) in PBS was injected intraperitoneally. After 5 minutes, the mice were imaged using a Xenogen IVIS 200 (Xenogen). The subcutaneous experiment was repeated three times and the i.b.m. experiment was repeated twice. Images were analyzed using Living Image 2.50.1 software and a representative of each group is presented.
Results
Targeting of Human c-KIT-expressing Cell Lines In Vitro
The natural budding mechanism of the lentivirus and the plasticity of envelope membrane to be altered allow pseudotyping and insertion of ligands, peptides, cytokines and single-chain antibodies that can direct the vectors to specific cell types (Aires da Silva et al. 2005; Gollan and Green 2002; Kueng et al. 2007; Morizono et al. 2005). Here we report engineering lentivectors capable of specifically transducing receptor-specific cells using a cognate natural receptor ligand and fusogenic molecule. We report herein a study to evaluate using this approach to harness the natural ligand-receptor binding mechanism for targeted modification of c-KIT receptor-expressing cells. By co-transfection of plasmids, a fusion molecule described previously (termed FM1) (Yang et al. 2006) as well as membrane-bound ligand (hSCF) may be incorporated into the cell membrane, which will be transferred to the viral envelope as it buds from the producing cell (Morita and Sundquist 2004). Besides the FM1 as the fusogen, we developed additional fusion molecules (FM2-4) (Figure 1) by performing mutations in a cholesterol fusion dependent domain (E1 226) of the Sindbis virus envelope glycoprotein (Lu et al. 1999). To generate lentivectors enveloped with both targeting ligands and fusogenic molecules, 293T cells were transiently transfected by a standard calcium phosphate-mediated precipitation method with lentiviral vector FUGW, which carries the enhanced green florescent protein (eGFP) reporter gene under control of the human ubiquitin C promoter, packaging plasmids (encoding viral gag, pol, and rev genes) and one of the fusion molecules (FM1-FM4) plus the targeting ligand hSCF or a CD117-blind targeting molecule CD20 to produce FUGW/hSCF+FM or FUGW/CD20+FM.
A stably expressing CD117 cell line was generated for the study of receptor-targeted transduction. To assess the expression level of CD117, the relative expression of the Jurkat cells expressing CD117 (Jurkat.hcKIT) was compared to that of the parental Jurkat cell line by antibody staining and quantified by flow cytometry (Figure 2C). The Jurkat.hcKIT cell line was found to express human c-KIT receptor stably and uniformly at significant levels above the parental Jurkat cell line. Vectors incorporating one of the fusogens and either hSCF or CD20 were spin-infected with Jurkat.hcKIT cells. The transduction efficiency was measured by the difference in mean fluorescence intensity (MFI) or eGFP fluorescence activity where non-infected cells were used for setting the fluorescence gate. FUGW/hSCF+FM vector specifically transduced Jurkat.hcKIT cells with greater than 80% efficiency and MFI 13 times higher than non-targeted vector (Figure 2A). In contrast, the FUGW/CD20+FM vector was unable to significantly transduce Jurkat.hcKIT cells (low MFI), indicating that hSCF confers transduction efficiency of the vector with Jurkat.hcKIT cells (Figure 2A). To extend these observations, we analyzed the relative ability of the eight vector preparations to transduce TF-1α cells, which have been previously been confirmed by flow cytometry to naturally express high levels of c-KIT (Chandrashekran et al, 2004). Using SCF or CD20-targeted vectors and TF-1α cells, the FUGW/hSCF+FM vector gave transductions of up to 70% and conferred a similar increase in MFI beyond that of non-targeted vector. The CD117-blind FUGW/CD20+FM vector was unable to significantly transduce these cells (Figure 2B). All FUGW/hSCF+FM vectors displayed targeting ability with FM3-bearing vectors resulting in the highest transduction whereas CD20-bearing vectors resulting in low background transduction.
Figure 2.
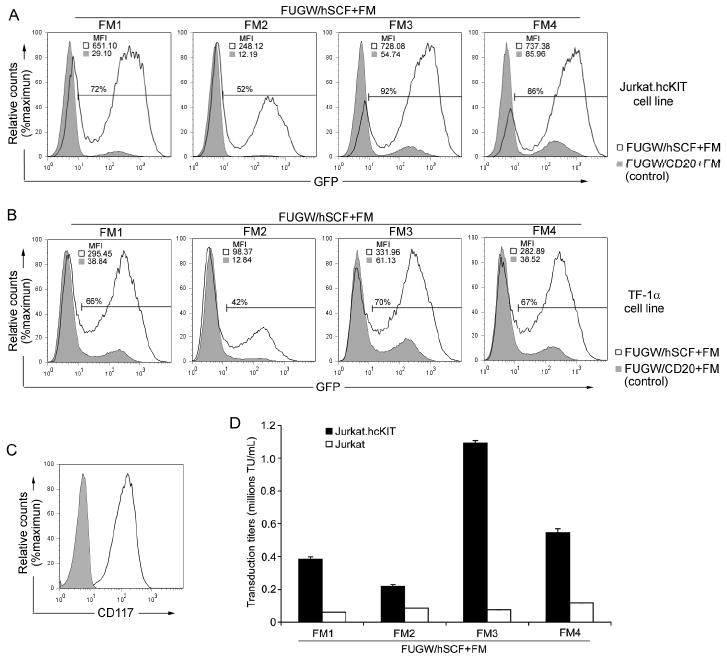
Engineered lentiviral vector transduction of targeted cells in vitro. (A&B) Fresh unconcentrated recombinant lentiviral vectors (2 mL) either bearing: hSCF and the indicated fusogen (FUGW/hSCF+FM) (solid line) or CD20 (control ligand, no specificity to CD117) and the indicated fusogen (FUGW/CD20+FM) (shaded area), were added to CD117-expressing Jurkat.hcKIT cells (A) or TF-1α (B). Three days post-transduction the resulting eGFP expression was taken as an indication of transduction efficiency and mean fluorescence intensity was analyzed by flow cytometry with cell counts normalized and a representative result of fluorescence shown with gating of FUGW/hSCF+FM transduced cells set using non-infected cells. (C) Flow cytometry analysis of the Jurkat.hcKIT cell line by surface staining using anti-CD117 antibody. Solid line: CD117 expression on Jurkat.hcKIT cell line; Shaded area: CD117 expression on Jurkat cell line (as a control). (D) Specific viral titers for various engineered lentiviral vectors. Fresh unconcentrated lentiviral vectors incorporating hSCF and a fusogen were used to transduce (2 × 104) Jurkat.hcKIT or Jurkat cells in a 96 well plate. The viral titer was conducted in duplicate and measured in dilution ranges that exhibited a linear response of eGFP expression with viral dilution.
The titer of FUGW/hSCF+FM3 (fresh viral supernatant) was estimated on Jurkat.hcKIT to be ~1 × 106 transduction units (TU)/mL (Figure 2D); the titer was determined in the dilution ranges that showed a linear response of eGFP expression with viral serial dilution. In contrast, the FUGW/hSCF+FM3 resulted in a small background infection level with a titer of ~7.6 × 104 TU/mL on Jurkat cells lacking the expression of CD117 (Figure 2D). Each fusion molecule had a slightly different effect on the ability of the vector to selectively transduce CD117-expressing Jurkat cells. FUGW/hSCF+FM4 resulted in the highest non-targeted titer ~1 × 105 TU/mL in Jurkat cells. The titers were conducted in duplicate experiments with a deviation of ±0.01 × 106 in the results. Furthermore, when comparing the infectivity of the same viral vector on the targeted Jurkat.hcKIT cell line, the transduction units of FUGW/hSCF+FM4 only increased 4.6 folds compared to a 14-fold increase with FUGW/hSCF+FM3 (Figure 2D). Thus, the mutations which generate each different fusion molecule were found to effect targeted viral transduction and FUGW/hSCF+FM3 resulted in the highest infectivity in target Jurkat.hcKIT and the lowest background infectivity in the parental Jurkat cell line. Because of its improved targeted transduction efficiency, the FUGW/hSCF+FM3 vector was chosen for the further investigations.
The targeted viral vectors must perform two critical functions for efficient, precise transduction. First, they must specifically bind with the target cell. To confirm that the recognition is a consequence of interaction between hSCF and CD117, the binding of the viral particles to the c-KIT receptor was examined. Jurkat.hcKIT or parental Jurkat cells were mixed at 4°C to prevent internalization of the viral particles, with lentivector incorporating one type of FM and hSCF. Cells were then stained for the presence of viral particles on the cell surface using an anti-FM antibody and quantified by MFI using flow cytometry (Figure 3A). Flow cytometry analysis revealed that lentivectors bearing hSCF and FM were able to bind to CD117-expressing Jurkat cells (Figure 3A). The control parental Jurkat cells with no CD117 expression displayed no detectable FM, indicating that the viral particles binding to cells are due to a specific interaction between the cell surface c-KIT receptor and the viral surface hSCF molecules. In another control, the vector bearing FM and CD20 antigen exhibited no ability to bind either cell line, indicating that the FM lacked the capacity for cell binding (data not shown). The mean fluorescent intensity revealed that the difference in FM does not appear to affect the binding of the targeted virus to CD117, which indicates that the observed differences in transduction are not a result of the differences in binding moieties.
Figure 3.
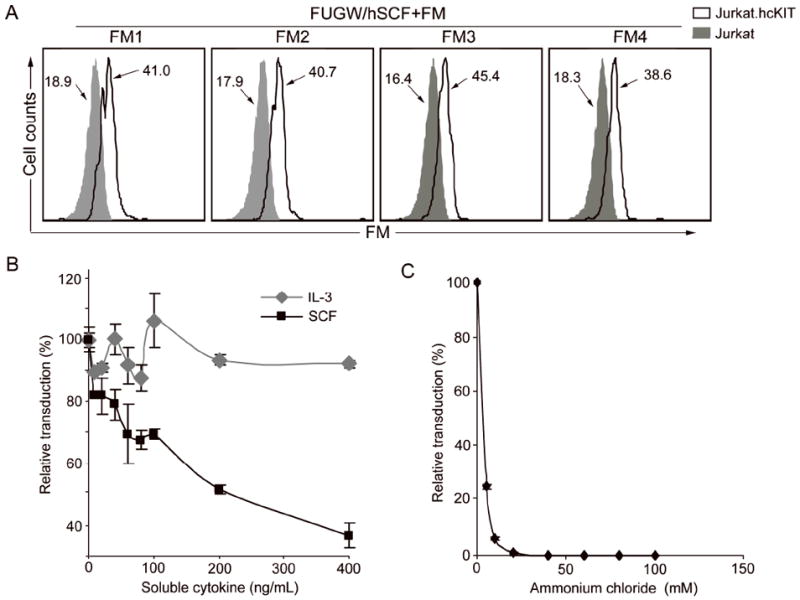
Study of the properties of in vitro targeted transduction. Fresh unconcentrated lentiviral vectors bearing hSCF and a fusogen (FUGW/hSCF + FM) were produced. (A) FACS analysis of the targeted virus cell binding by surface staining using anti-HA tag antibody against fusogen was used to detect the change in MFI due to viral vectors bound to the cells. Lentivector (FUGW/hSCF+FM) was mixed at 4°C to prevent internalization with Jurkat.hcKIT (solid line) or parental Jurkat (shaded area) as a control. (B) Effect of addition of soluble hSCF on targeted transduction. Either soluble hSCF (black square) or a control cytokine IL-3 (gray diamond) was added to the wells during transduction of Jurkat.hcKIT with FUGW/hSCF+SGM. Eight hours later, the media was replaced with fresh media and the cells placed in the incubator for four days before FACS analysis of eGFP expression. (C) Various concentrations of NH4Cl (dissolved in PBS, pH=7.4) were added into viral supernatant (FUGW/hSCF+SGM) for 8h, after which the medium was replaced with fresh medium and cells were cultured for 3 days before FACS analysis of eGFP-positive cells. The data is presented as the percentage of reduced transduction as compared to the result of transduction without treatment of either soluble cytokine (B) or NH4Cl (C).
Efficient transduction of Jurkat cells was dependent upon the expression of CD117. To further examine how the specific interaction between targeting ligand on the lentiviral surface and c-KIT receptor on the surface of the target cells mediated the targeted transduction by FUGW/hSCF+FM3, soluble human SCF cytokine was added at increasing levels to a virus-cell mixture. As measured by eGFP expression, transduction of FUGW/hSCF+FM3 was inhibited by >60% during incubation with soluble hSCF but not the negative control interleukin (IL)-3 (Figure 3B). Furthermore, soluble SCF-mediated inhibition of viral transduction with Jurkat.hcKIT cells was dose-dependent (Figure 3B). These data confirmed that FUGW/hSCF+FM3 transduction is mediated by binding of the hSCF ligand to the human c-KIT receptor.
The second critical function targeted viral vectors must perform for efficient, precise transduction is viral endosomal fusion. Upon binding, the vector should then induce endocytosis, bringing the lentiviral particle into an endosome where the fusogenic molecule will respond to the low pH environment and trigger membrane fusion, allowing the viral core to enter the cytosol. To examine whether c-KIT receptor mediated endocytosis triggers the pH dependent fusion, we incubated FUGW/hSCF+FM3 with Jurkat.hcKIT cells in the increased presence of ammonium chloride (NH4Cl), which can raise the pH of the endosomal compartments (Mellman et al. 1986). Addition of NH4Cl to cells directly abolished transduction in a dose dependent manner (Figure 3C). These results are consistent with the observed low pH requirement of the Sindbis derived glycoprotein to trigger membrane fusion and mediate infection (Lu et al. 1999).
Targeting Subcutaneous Tumor Cells In Vivo
A subcutaneous tumor model was developed to determine whether the engineered vectors could specifically modify CD117-expressing cells in vivo. To assess the transduction efficiency and specificity, lentiviral vector FUGL, which carries genes for the firefly luciferase linked to eGFP through a self cleaving 2A peptide (Szymczak and Vignali 2005) under control of the human ubiquitin C promoter to facilitate co-expression of both factors (Figure 1), was used to transduce cells. Jurkat.hcKIT cells (10 × 106 per mouse) were transferred into irradiated immunodeficient RAG2-/-γc-/- mice on the right flank subcutaneously (s.c.). Eight hours post-cell injection, 10 × 106 TU of engineered lentivector bearing either membrane-bound hSCF and FM3 or vectors pseudotyped with VSVG (a viral envelope derived from vesicular stomatitis virus that has a broad tropism) were injected subcutaneously into both flanks. Figure 4A illustrates the experimental design. Fourteen days post-inoculation, the animals were anesthetized with isoflurane and the reporter substrate D-luciferin was injected intraperitoneally and luciferase activity was imaged using a Xenogen IVIS 200 system. The experiment was repeated three times and one representative result was shown (Figure 4B). Mice transduced with FUGL/hSCF+FM3 show an increased tumor luciferase activity (~12× 104 p/s) in the right flank as compared with the left flank, suggesting that only tumor cells were transduced to express the reporter gene (Figure 4B). In contrast, the non-specific FUGL/VSVG elicited a much lower luciferase activity in the right flank of approximately 1000 p/s (Figure 4B). Finally, mice were euthanized; the tumors were collected and the cells were analyzed for surface antigen and eGFP expression. The xenograft tumor cells receiving the targeted vector were 10% positive for eGFP whereas the xenograft tumor cells receiving VSVG-pseudotyped vector were 2% positive for eGFP (Figure 4C). This verifies that the targeted FUGL/hSCF+FM3 vector is more efficient than the VSVG-pseudotyped vector in a xenografted subcutaneous Jurkat.hcKIT tumor model in vivo.
Figure 4.
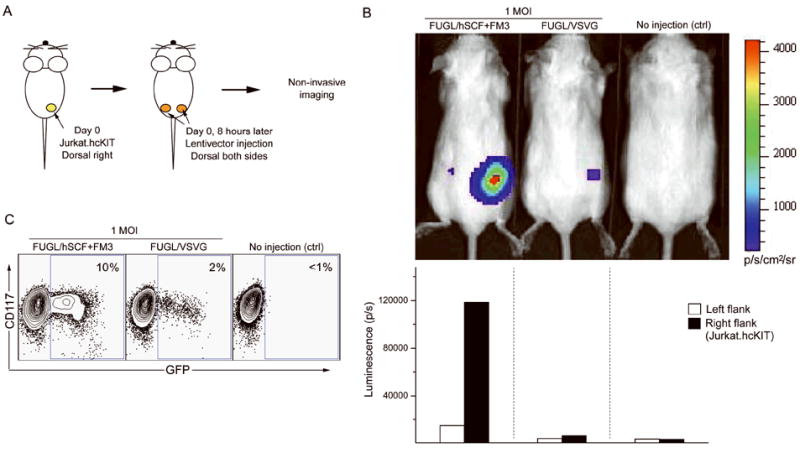
In vivo targeted transduction of Jurkat.hcKIT cells xenografted on immunodeficient mice by engineered lentiviral vectors. (A) Schematic diagram of the procedure used to target cells in vivo. Jurkat.hcKIT cells (10 × 106) were injected subcutaneously on the right flank of an immunodeficient RAG2-/-γc-/- mouse. After eight hours, concentrated lentiviral vectors (10 × 106 TU) expressing both firefly luciferase and eGFP either targeted (FUGL/hSCF+FM3) or non-targeted (FUGL/VSVG) were injected subcutaneously on both the right and left flank of the mouse. (B) Two weeks later mice were injected with the substrate D-luciferin and analyzed using bioluminescence imaging. The experiment was repeated three times and one representative result is shown with the indicated luminescence quantification. A mouse receiving no lentivector was used as a negative control. (C) When tumor cells were palpable the mice were culled and the tumor cells were collected and cultured. FACS analysis was used to determine CD117 and eGFP expression.
Intra-Bone Marrow Targeted Delivery
Having confirmed that Jurkat.hcKIT cells could be infected subcutaneously with targeting lentivector, a bone marrow model was established to determine whether cells expressing human c-KIT receptor can be specifically transduced after intra-bone marrow administration. Three mice were injected with 1 × 106 Jurkat.hcKIT cells in the right femur. Seven days later, 1 × 106 TU of either targeted lentivector (FUGL/hSCF+FM3) or a negative control vector with membrane-bound CD20 (FUGL/CD20+FM3) was injected into mice with or without Jurkat.hcKIT (Figure 5A). After another seven days, mice were injected with the reporter substrate D-luciferin and imaged. The experiment was repeated twice and one representative result was shown (Figure 5B). The mice inoculated with Jurkat.hcKIT and targeted lentivector resulted in the highest luminescence (24000 p/s), implying transduction of the target CD117 expressing cells in the bone marrow. The mice that received targeted vector but no Jurkat.hcKIT exhibited the least luminescence due to the low non-specific transduction of the vector. In contrast, mice receiving the negative control vector, FUGL/CD20+FM3, displayed similar levels of luminescence regardless of Jurkat.hcKIT injection (Figure 5B). To confirm targeted transduction, the Jurkat.hcKIT cells were collected from the bone marrow and the cells were analyzed for CD117 and eGFP expression (Figure 5C). The eGFP of mouse tumor cells expressing CD117 receiving the targeted vector was approximately 3.6 times higher than the mouse receiving the non-targeted vector.
Figure 5.
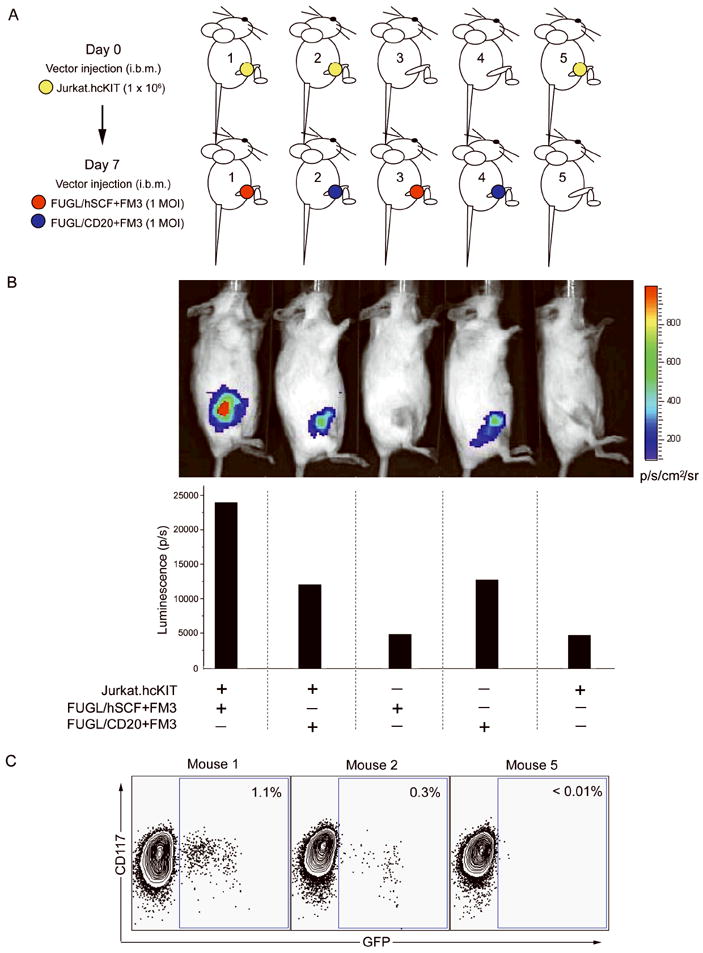
In vivo targeted transduction of Jurkat.hcKIT cells xenografted by intra-bone marrow injection with engineered lentiviral vectors. (A) Schematic diagram of the procedure used to target cell in the bone marrow. Jurkat.hcKIT cells (1 × 106) were injected into the right femur of the mice. One week later lentivector (1 × 106 TU) expressing both firefly luciferase and eGFP either targeted (FUGL/hSCF+FM3) or control vector (FUGL/CD20+SGM) was injected into the right femur of the mouse. (B) One week after the injection of lentivector, mice were injected with the substrate D-luciferin and analyzed using bioluminescence imaging. The experiment was repeated twice and one representative result is shown with luminescence quantification. A mouse receiving Jurkat.hcKIT with no lentivector was used as a negative control. (C) Mice were culled and the cells from the right femur were collected and cultured. FACS analysis was used to determine CD117 and eGFP expression.
Discussion
The focus of this study is to test the targeting of lentiviral vectors to specific cell receptors in vivo mediated by membrane-bound natural receptor ligands. Our results demonstrate that lentivectors bearing both membrane-bound human stem cell factor and a Sindbis virus derived fusogen molecule can target cells expressing the c-KIT receptor in vitro and in vivo. To generate targeting lentivectors, the vector-producing cells were transfected with plasmids to express both membrane-bound human stem cell factor and fusogen molecules. The natural budding mechanism of the lentivirus produces particles with an envelope bearing both a fusion molecule and a targeting hSCF ligand.
The specific binding of the vectors bearing hSCF to the c-KIT receptor on the target cell surface membrane initiates the mechanism for viral entry. Using flow cytometry, engineered lentivectors were confirmed to selectively bind to cells expressing CD117. Furthermore, vectors that bound to CD117 cells were stained with anti-fusogen antibody indicating that CD117 targeting lentivectors particles have both binding and fusogen capabilities. Engineered vectors were further confirmed to target cells in a CD117-dependent mechanism by a SCF inhibition assay. Addition of soluble SCF to the cells inoculated with targeted vector was found to inhibit transduction in a dose dependent manner, confirming the binding requirement for the observed targeting. Because transduction is dependent on binding of the engineered lentivector to the c-KIT receptor, we expect that the level of c-KIT receptor on the cell surface may affect the transduction efficiency. The targeted transduction of lentivector requires both specific binding by membrane-bound SCF and that the binding should induce endocytosis of lentivectors initiating viral entry.
Another key step of entry is fusogen-mediated endosomal fusion releasing the viral core. The viral fusogen molecules derived from Sindbis virus require a low pH in the early endosome to induce membrane fusion to deliver the viral payload (Joo and Wang 2008; Lu et al. 1999). The four Sindbis virus-derived fusogen mutants were adapted to efficiently envelope lentiviral vectors in a binding-deficient and fusion-competent manner with mutations in the E1 glycoprotein domain that alter fusion activity in a cholesterol dependent domain (Lu et al. 1999; Yang et al. 2008). Binding of the targeted lentivector was similar regardless of which fusion molecules were co-incorporated on the lentivector surface. Furthermore, the functional effect of the different fusion mutants resulted in a varied efficiency of transduction on target as well as on non-target cells in vitro. In vitro titer data for each targeted vector with the respective fusogen molecules resulted in the highest transduction in target Jurkat.hcKIT and the lowest background transduction by FUGW/hSCF+FM3. The mechanism for the enhanced viral transduction was revealed to be dependent on the endosomal pH. When ammonium chloride was added to neutralize the acidic endosomal compartments, targeted transduction was reduced. The sharp decrease in transduction with the addition of NH4Cl is indicative of the pH-dependent nature of the fusogenic molecules incorporated on the lentivector.
Targeted transduction in vitro shows that the targeting lentivector can preferentially transduce cells expressing c-KIT receptor. The potential of the targeted lentivector for in vivo targeted gene delivery was first evaluated using a subcutaneous xenografted Jurkat.hcKIT tumor model. The experiment was repeated only three times and as such the variances might not be fully captured and therefore shown is only one representative, which illustrates the observed results but not the variance. The VSVG-pseudotyped vector was compared to the engineered lentivector FUGL/hSCF+FM3 by measuring bioluminescence in live animals. VSVG is one of the most common pseudotypes used in lentivector studies due to its ability to efficiently transduce the majority of human and mouse cells in a non-specific manner. Although in vitro experiments revealed that VSVG-pseudotyped vectors can efficiently transduce Jurkat.hcKIT, in vivo the targeted lentivector is more effective at transducing the target tumor cells. Perhaps the reason VSVG is very efficient in vitro but not in vivo is due to non-specific binding of its pseudotyped vectors to multiple cell types which results in less viral vector reaching the intended cells. The difference in tumor transduction may be further exacerbated by the difference in growth rate of non-transduced versus transduced progeny. Overall, the FUGL/hSCF+FM3 vector is able to more efficiently transduce c-KIT expressing cells than a VSVG-pseeudotyped vector in a subcutaneous xenograft tumor.
Although direct injection of targeted gene delivery vehicles may be a reasonable approach for treatment of diseases that have well-defined anatomical barriers, the treatment of many more evasive diseases requires widespread delivery. Current protocols for delivery of viral vector-mediated gene delivery involve the localized injection of the vector to the tissue of interest, resulting only in localized gene expression. Restricted viral trophism would be simplest safe and effective method of achieving cell type specific distribution. Development of a gene delivery vehicle to receptor-specific cells is an attractive approach to restrict gene expression and to ensure therapeutic effects in the desired cells while limiting side effects caused by gene expression in non-target cells. We report herein a novel approach to harness the natural ligand-receptor mechanism for targeted modification of specific receptor-expressing cells. This new approach for targeted gene delivery into c-KIT expressing cells may be a useful tool for investigations focused on transducing specific cell types within a heterogeneous tissue.
Specific gene delivery to c-KIT-expressing cells in the bone marrow is particularly interesting because of the important role of this receptor in hematopoiesis. c-KIT expression is known to be a specific marker in the bone marrow for hematopoietic progenitor cells. To evaluate the targeting potential of the engineered lentivector in the bone marrow compartment, a Jurkat.hcKIT tumor was established intra-femorally in a mouse model. When the FUGL/hSCF+FM3 targeted vector was administrated via intra-bone marrow injection, it efficiently transduced c-KIT-expressing tumor cells, whereas when no tumor cells were present, the target vector had a no significant activity. In contrast, the viral vector carrying CD117-blind CD20 molecules and FM3 resulted in non-targeted transduction in mice with and without tumor cells. Our data shows that the targeting vectors can efficiently transduce c-KIT-expressing tumor cells when injected i.b.m. in a xenograft Jurkat.hcKIT intra-femoral tumor mouse model. c-KIT has been a popular target for gene therapy due to its association with hematopoietic stem cells (HSCs). However, clinically gene therapy to HSCs as a personal medicine remains time consuming and expensive. Our results demonstrate the potential for targeted stable gene transfer into c-KIT receptor expressing cells in the bone marrow. Future studies in other models are warranted to investigate more real-world physiological situations. Utilizing this CD117-specific lentiviral gene delivery system in other tissues may further broaden the prospects of c-KIT receptor targeted gene delivery.
Gene delivery to a particular type of cells would limit side effects caused by gene expression in non-target cells and ensure therapeutic effects in only the desired cells. Efficient targeted gene delivery to a specific subset of cells continues to be a key challenge for gene therapy. Previously, we have reported a method to target lentivectors to specific cells via antibody-antigen mediated targeting (Yang et al. 2006). Our findings suggest that another way to target specific cells is to engineer lentivectors bearing cognate natural ligands and fusogenic molecules. These vectors are able to confer selective transduction of receptor expressing cell type in vitro and in vivo. We demonstrate our targeting methodology using c-KIT receptor as a model target. Further implementation of this natural ligand-receptor targeting strategy may be adapted to other receptor targets thereby targeting various other cell types.
Acknowledgments
This work was supported by a National Institute of Health grant A1068978.
References
- Aires da Silva F, Costa MJ, Corte-Real S, Goncalves J. Cell type-specific targeting with sindbis pseudotyped lentiviral vectors displaying anti-CCR5 single-chain antibodies. Hum Gene Ther. 2005;16(2):223–34. doi: 10.1089/hum.2005.16.223. [DOI] [PubMed] [Google Scholar]
- Akporiaye ET, Hersh E. Clinical aspects of intratumoral gene therapy. Curr Opin Mol Ther. 1999;1(4):443–53. [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cetin N, Dienel G, Gokden M. CD117 expression in glial tumors. J Neurooncol. 2005;75(2):195–202. doi: 10.1007/s11060-005-2318-1. [DOI] [PubMed] [Google Scholar]
- Chandrashekran A, Gordon MY, Casimir C. Targeted retroviral transduction of c-kit+ hematopoietic cells using novel ligand display technology. Blood. 2004;104(9):2697–703. doi: 10.1182/blood-2003-10-3717. [DOI] [PubMed] [Google Scholar]
- Chapel A, Deas O, Bensidhoum M, Francois S, Mouiseddine M, Poncet P, Durrbach A, Aigueperse J, Gourmelon P, Gorin NC, et al. In vivo gene targeting of IL-3 into immature hematopoietic cells through CD117 receptor mediated antibody gene delivery. Genet Vaccines Ther. 2004;2(1):16. doi: 10.1186/1479-0556-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel A, Poncet P, Neildez-Nguyen TM, Vetillard J, Brouard N, Goupy C, Chavanel G, Hirsch F, Thierry D. Targeted transfection of the IL-3 gene into primary human hematopoietic progenitor cells through the c-kit receptor. Exp Hematol. 1999;27(2):250–8. doi: 10.1016/s0301-472x(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Duensing A, Heinrich MC, Fletcher CD, Fletcher JA. Biology of gastrointestinal stromal tumors: KIT mutations and beyond. Cancer Invest. 2004;22(1):106–16. doi: 10.1081/cnv-120027585. [DOI] [PubMed] [Google Scholar]
- Edling CE, Hallberg B. c-Kit--a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39(11):1995–8. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Fielding AK, Maurice M, Morling FJ, Cosset FL, Russell SJ. Inverse targeting of retroviral vectors: selective gene transfer in a mixed population of hematopoietic and nonhematopoietic cells. Blood. 1998;91(5):1802–9. [PubMed] [Google Scholar]
- Gollan TJ, Green MR. Selective targeting and inducible destruction of human cancer cells by retroviruses with envelope proteins bearing short peptide ligands. J Virol. 2002;76(7):3564–9. doi: 10.1128/JVI.76.7.3564-3569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel W, Westphal M. The road less travelled: c-kit and stem cell factor. J Neurooncol. 1997;35(3):327–33. doi: 10.1023/a:1005828921273. [DOI] [PubMed] [Google Scholar]
- Itoh A, Okada T, Mizuguchi H, Hayakawa T, Mizukami H, Kume A, Takatoku M, Komatsu N, Hanazono Y, Ozawa K. A soluble CAR-SCF fusion protein improves adenoviral vector-mediated gene transfer to c-Kit-positive hematopoietic cells. J Gene Med. 2003;5(11):929–40. doi: 10.1002/jgm.430. [DOI] [PubMed] [Google Scholar]
- Joo KI, Wang P. Visualization of targeted transduction by engineered lentiviral vectors. Gene Ther. 2008;15(20):1384–96. doi: 10.1038/gt.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara N, Dozy AM, Kan YW. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266(5189):1373–6. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- Kueng HJ, Leb VM, Haiderer D, Raposo G, Thery C, Derdak SV, Schmetterer KG, Neunkirchner A, Sillaber C, Seed B, et al. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J Virol. 2007;81(16):8666–76. doi: 10.1128/JVI.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan AC, Lutzko C, Kohn DB. Advances in lentiviral vector design for gene-modification of hematopoietic stem cells. Curr Opin Biotechnol. 2002;13(5):429–36. doi: 10.1016/s0958-1669(02)00346-4. [DOI] [PubMed] [Google Scholar]
- Lu YE, Cassese T, Kielian M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol. 1999;73(5):4272–8. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzko C, Senadheera D, Skelton D, Petersen D, Kohn DB. Lentivirus vectors incorporating the immunoglobulin heavy chain enhancer and matrix attachment regions provide position-independent expression in B lymphocytes. J Virol. 2003;77(13):7341–51. doi: 10.1128/JVI.77.13.7341-7351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13(3):205–20. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- Moreau T, Bardin F, Imbert J, Chabannon C, Tonnelle C. Restriction of transgene expression to the B-lymphoid progeny of human lentivirally transduced CD34+ cells. Mol Ther. 2004;10(1):45–56. doi: 10.1016/j.ymthe.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11(3):346–52. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Sandrin V, Russell SJ, Cosset FL. Targeting retroviral and lentiviral vectors. Curr Top Microbiol Immunol. 2003;281:137–78. doi: 10.1007/978-3-642-19012-4_4. [DOI] [PubMed] [Google Scholar]
- Schwarzenberger P, Spence SE, Gooya JM, Michiel D, Curiel DT, Ruscetti FW, Keller JR. Targeted gene transfer to human hematopoietic progenitor cell lines through the c-kit receptor. Blood. 1996;87(2):472–8. [PubMed] [Google Scholar]
- Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5(5):627–38. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, Martin FH, Williams DA. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992;89(16):7350–4. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ziegler L, Joo KI, Cho T, Lei Y, Wang P. Gamma-retroviral vectors enveloped with an antibody and an engineered fusogenic protein achieved antigen-specific targeting. Biotechnol Bioeng. 2008;101(2):357–68. doi: 10.1002/bit.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci U S A. 2006;103(31):11479–84. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhu L, Lee S, Li L, Chang E, Soong NW, Douer D, Anderson WF. Identification of the block in targeted retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1999;96(7):4005–10. doi: 10.1073/pnas.96.7.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Oliver P, Huang W, Good D, La Russa V, Zhang Z, Cork JR, Veith RW, Theodossiou C, Kolls JK, et al. Efficient c-kit receptor-targeted gene transfer to primary human CD34-selected hematopoietic stem cells. J Virol. 2001;75(21):10393–400. doi: 10.1128/JVI.75.21.10393-10400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


