Abstract
Human bone marrow–derived mesenchymal stem cells (hMSCs) represent an appealing source of smooth muscle cells (SMCs) for engineering small-diameter vascular grafts due to the limited availability and replicative capacity of somatic SMCs. However, lack of standardization of hMSC culture conditions has limited some progress in hMSC research. Because, at the moment, a chemically defined, serum-free medium without growth factors is not capable of amplifying hMSCs in vitro, the usage of serum (either human serum or fetal bovine serum [FBS]) continues in hMSC research. The emergence of commercial hMSCs and hMSC media opened a series of questions regarding the compatibility of commercial and homemade hMSCs and hMSC media. In this study, two types of commonly used FBS-containing hMSC media—MSCGM (containing 10% FBS) and MesenPro (containing 2% FBS), along with our homemade medium (low-glucose Dulbecco's modified Eagle's medium plus 10% selected lot FBS)—were compared in their ability to support SMC differentiation from hMSCs. The effects of FBS level, medium supplements (ascorbic acid, copper, etc.), and growth factors (transforming growth factor β1) were also examined for their impact on SMC differentiation. It was discovered that MesenPro and transforming growth factor β1 are the strongest SMC inducers from hMSCs. In contrast, hMSCs grown in homemade (10% Dulbecco's modified Eagle's medium) and commercial MSCGM media remained undifferentiated. FBS concentration did not affect SMC differentiation when 10% FBS was compared with 2%. Finally, the mechanism underlying SMC differentiation from hMSCs grown in FBS-containing medium was explored by following the expression changes of serum response factor during the establishment of hMSC culture.
Introduction
Cell source considerations hamper progress in many areas of tissue engineering, including vascular regeneration. Recently, it has been shown that the replicative ability of smooth muscle cells (SMCs) from older donors, the major population of recipients of vascular grafts for bypass or other vascular surgeries, is limited.1,2 Alternative SMC sources are being actively sought after to replace somatic SMCs in engineering vascular grafts, especially small-diameter (inner diameter < 6 mm) blood vessels.3,4
Our lab recently reported the feasibility of using human bone marrow–derived mesenchymal stem cells (hMSCs) as SMC progenitors to engineer small-diameter human vessel wall with substantial histological and molecular similarity to native vessels after optimization of the culture conditions.4 Although the effect of culture medium on hMSCs has been extensively investigated,5–10 the influence of culture medium on hMSC differentiation—in particular, SMC differentiation—has not been addressed by any literature so far. Therefore, the focus of this study was to assess various culture media on their support of SMC differentiation from hMSCs. It has to be emphasized that in addition to culture medium, our previous work has indicated that extracellular matrix substrate, soluble factors, cyclic strain, and culture medium composition are all important determinants of SMC differentiation from hMSCs.4
Lack of standardization of hMSC culture conditions has become an obstacle for hMSC research, causing not only variations of results but also difficulty in comparing the results obtained from one lab to another. Recently, commercial sources of hMSC culture media became available, including MSCGM™ from Lonza (Lonza Walkersville, former Cambrex, Gaithersburg, MD), Mesenchymal Stem Cell Medium from ScienCell Research Laboratories (Carlsbad, CA), MesenPro RS™ Medium from Invitrogen (Carlsbad, CA), and MesenCult from StemCell Technologies (Vancouver, BC, Canada), among others. These commercial hMSC media might serve as promising standards to allow comparison of results on hMSCs grown under the same conditions in different laboratories. However, these commercial media contain varying amounts (2–10%) of fetal bovine serum (FBS). Due to its ill-defined nature, serum has been criticized as poorly suited for clinical applications.11 The adoption of serum-free media will eventually be important in the hMSC field for standardization and consistency of the culture conditions, although none of serum-free media being investigated so far could support the proliferation of hMSCs in the absence of growth factors.12
The focus of this study was to investigate the influence of basic culture medium, medium supplements, growth factors, and FBS on SMC differentiation from primary cultures of hMSCs, and the difference between primary isolates and commercial sources of hMSCs in their response to these parameters and on their SMC differentiation capabilities. The goal of the study design was to systematically evaluate the vascular smooth muscle differentiation potential of both commercial and primary hMSCs, in the presence of various types of culture media.
Materials and Methods
Establishment of hMSC culture
hMSC primary cells were isolated from fresh human bone marrow (Lonza) as described previously.4,13 Control hMSCs were purchased from a commercial source (Lonza). Both hMSCs were cultured in mesenchymal stem cell basal medium (MSCBM) supplemented with MSCGM SingleQuots Kit (Lonza medium) and maintained at 37°C in a humidified atmosphere containing 5% CO2. Medium was changed twice per week, and cells were passaged into 75 cm2 flasks for protein and RNA isolation, or into chamber slides for histochemical analysis.
hMSC phenotype was confirmed by fluorescence-activated cell sorting analysis with SH2 and SH3 antibodies14 (ATCC, Manassas, VA) as well as differentiation induction evaluated by alkaline phosphatase–von Kossa dual-staining and Nile red (Sigma, St. Louis, MO) staining for osteogensis and adipogenesis, respectively, as previously reported.4,13
Experimental design of the three experiments is summarized in Table 2.
Table 2.
Summary of Experimental Designs for the Three Experiments
| Experiment 1 | Experiment 2 | Experiment 3 | ||
|---|---|---|---|---|
| Purpose | To observe SMC differentiation from primary hMSCs under different culture conditions | To compare the SMC differentiation potential of primary and commercial hMSCs | To study the involvement of SRF in hMSC culture establishment and SMC differentiation | |
| Experimental design | Illustration | Table 1 | Figure 1 | |
| Cells | Primary hMSCs | Primary & commercial hMSCs | Primary hMSCs | |
| Medium | MesenPro | Establishment Lonza | Short term: Lonza, 10% DMEM | |
| Lonza | Subculture Lonza | Long term: Lonza | ||
| eDMEM | Lonza + 10% | |||
| eDMEM + TGFβ1 10% DMEM | 10% DMEM | |||
| 2% DMDM | MesenPro | |||
| Duration | 7 days | 14 days | Short term: 6 days | |
| Long term: ∼ 1 month (4th passage) | ||||
| Readout | 1. Western blot on SM22α, SMA, and calponin | 1. Western blot on SM22α, SMA, and calponin | Short term: Western blot and RT-PCR on SRF | |
| 2. Immunofluorescence staining for SMA and calponin | 2. ELISA on bFGF, TGFβ1, and PDGF-BB | Long term: Western blot on SRF | ||
| Results | Figures 2 and 3 | Figures 4 and 5 | Figure 6 | |
| Conclusions | 1. eDMEM + TGFβ1, eDMEM, and MesenPro provided the most SMC-inducing conditions, as judged by expression of calponin. | 1. Culture environment plays a more significant role in determining the SMC differentiation potential of hMSCs than donor variation. | During the establishment of hMSC culture, SRF isotype switch occurred concurrently with cell attachment and the expression of SMC markers, suggesting a possible involvement of SRF in SMC differentiation from hMSCs. | |
| 2. Lonza medium and 10% DMEM provided the least SMC induction. | 2. The levels of TGFβ1, bFGF co-related with the degree of SMC induction on hMSCs. | |||
| 3. FBS concentration in DMEM does not make a difference in SMC differentiation. |
Experiment 1: treatment of primary hMSCs with various culture media to induce SMC differentiation
The purpose of this experiment was to observe SMC differentiation from primary hMSCs under different culture conditions. Confluent primary hMSC cultures at passage 3 were detached with trypsin and seeded at 2 × 103 cells/cm2 in 75 cm2 flasks (BD Biosciences, San Jose, CA) or chamber slides (Nunc, Naperville, IL) in the presence of six different types of culture media: MesenPro, Lonza, enhanced DMEM (eDMEM), eDMEM + transforming growth factor β1 (TGFβ1), 10% DMEM, and 2% DMEM (see Table 1 for details). After 7 days of culture in various media, the cells were harvested for protein isolation and lysed after treatment with RIPA lysis buffer (Boston BioProducts, Boston, MA). The protein lysates were centrifuged at 14,000 rpm for 30 min at 4°C, and stored at − 70°C for Western blot analysis. The cultures in chamber slides (Nunc) were fixed in cold 4% paraformaldehyde (Boston BioProducts) for immunofluorescence staining.
Table 1.
Summary of the Various Culture Conditions Used in This Paper
| Media | Components | Sources |
|---|---|---|
| MesenPro | Reduced serum (2%) MSC medium | Invitrogen |
| Lonza | MSCBM, MSCGM SingleQuots (10% FBS, pen-strep-L-glutamine) | Lonza |
| Enhanced DMEM (eDMEM) | Low-glucose DMEM, 10% selected lot of premium FBS, 100 U/mL penicillin G, 5 mmol/L HEPES, 3 ng/mL copper sulfate, 50 mg/mL proline, glycine, and ascorbic acid, and 20 mg/mL alanine | DMEM: Life Technologies FBS: Hyclone (lot#APH21746) Others: Sigma |
| eDMEM + TGFβ1 | Enhanced DMEM medium (above), 1 ng/mL TGFβ1 | TGFβ1 from R&D Systems |
| 10% DMEM | Low-glucose DMEM, 10% selected lot of FBS, pen-strep-L-glutamine | FBS: same lot as eDMEM |
| 2% DMEM | Low-glucose DMEM, 2% selected lot of FBS, pen-strep-L-glutamine | FBS: same lot as eDMEM and 10% DMEM |
Experiment 2: comparison of SMC differentiation potential in commercial and primary hMSCs
The goal of this experiment was to compare the SMC differentiation potential of primary and commercial hMSCs. The experimental design for this set of experiments is summarized in Figure 1. To explore the influence of postthaw culture medium, cell source, and expansion medium on SMC differentiation from hMSCs, cells were divided into three groups. Primary hMSCs established initially in Lonza medium were thawed in either Lonza (group A) or in 10% DMEM medium (group B), and then maintained in the same conditions for 7 days. A third group was commercial hMSCs (Lonza) that were thawed and maintained in Lonza medium only (group C).
FIG. 1.
A schematic (A) and summary (B) of the experimental design for Experiment 2.
The cells in each of the three groups were detached and subcultured in 25 cm2 flasks (BD Biosciences) at a seeding density of 2.4 × 103/cm2 in one of the following four types of media: Lonza medium, Lonza MSCBM (containing standard Lonza SingleQuots) plus 10% FBS (Hyclone, Logan, UT; lot # APH21746, same lot as in 10% DMEM) (Lonza + 10%), 10% DMEM, and MesenPro. One milliliter of each fresh medium of each type was saved for enzyme-linked immunosorbent assays (ELISA) for baseline growth factor levels. The cells were cultured in each of the four medium conditions for 14 days until the proteins from various cultures were isolated with RIPA lysis buffer, centrifuged at 14,000 rpm for 30 min at 4°C, and stored at − 70°C for Western blot analysis.
Experiment 3: involvement of serum response factor (SRF) in primary hMSC culture establishment and SMC differentiation
Proteins were harvested along the establishment of primary hMSC culture from fresh bone marrow. To obtain primary hMSCs, 25 mL of fresh human bone marrow was diluted 1:1 with sterile phosphate-buffered saline (PBS), mixed well, slowly loaded onto Ficoll-Paque Plus density media (StemCell Technologies), and fractionated at room temperature for 30 min at 1200 g with no brake. The mononuclear cell layer at the interface was removed and washed once with Dulbecco's PBS (D-PBS, Life Technologies, Gaithersburg, MD). Ten million fresh bone marrow mononuclear cells (BMMNCs) were saved for protein and RNA isolation before the rest of the BMMNCs were plated in 75 cm2 flasks in either Lonza or 10% DMEM medium. Protein and RNA was also isolated from the BMMNC culture every day afterward until day 6. Culture media were changed on day 7 when some cells started to attach to the flask. The culture media were then changed twice per week until the cultures became confluent.
The primary hMSC culture established in Lonza medium was then passaged and expanded in the same medium until passage 4. Proteins were harvested at time of initial isolation, 1 week when hMSCs started to attach, primary culture, and every passage afterward, quantified, and stored at −80°C for Western blotting.
Western blot analysis on SMC-specific markers and SRF
Protein lysates containing 25 μg of protein were separated on 10% SDS-PAGE gel and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated at 4°C overnight in blocking buffer (5% nonfat dry milk in TBST buffer) and then incubated for 1 h at room temperature with primary antibodies, 1:400 and 1:200 dilution for mouse anti-human SMA and calponin (Dako, Carpinteria, CA), respectively, and 1:6000 dilution for rabbit anti-human SM22α (Abcam, Cambridge, MA) in 1% nonfat dry milk (Bio-Rad, Hercules, CA) in TBST buffer. Then the blots were probed with secondary antibodies, horseradish peroxidase (HRP)–conjugated goat anti-mouse (1:2000) or goat anti-rabbit (1:4000) IgG (Santa Cruz Biotechnology, Santa Cruz, CA), for 30 min at room temperature. For SRF, the primary antibody was mouse monoclonal SRF IgM (A-11) (1:100, Santa Cruz Biotechnology). The secondary antibody was polyclonal goat anti-mouse IgM-HRP (1:2000; Abcam). After washing, the blots were developed using the SuperSignal West Pico Chemiluminescent detection system (Pierce, Rockford, IL). After visualization, membranes were then stripped in Restore™ Western Blot Stripping Buffer (Pierce) at room temperature for 5–15 min and reprobed for β-actin (1:5000 dilution; Sigma), which was used as equal loading control. Protein lysate was also obtained from coronary artery SMCs (CASMCs) as positive control. Quantification of Western blots was performed using Image J (National Institutes of Health, Bethesda, MD). The results were presented as relative density after correction with β-actin.
Quantitative PCR for SRF
Total RNA was harvested using RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer's protocols. cDNAs were generated from 1 μg of total RNA using SuperScript II RNase H− reverse transcriptase with random primers (Invitrogen). Gene expression level of SRF was determined using TaqMan human SRF gene expression assay (Hs00182371_m1; Applied Biosystems, Foster City, CA) and detected by iCycler iQ (Bio-Rad). Human GAPDH (Hs99999905_m1; Applied Biosystems) was used as internal control for standardization. Standard curves were plotted for each PCR. Relative expression levels were calculated using GAPDH expression levels for comparison.
Immunofluorescence staining
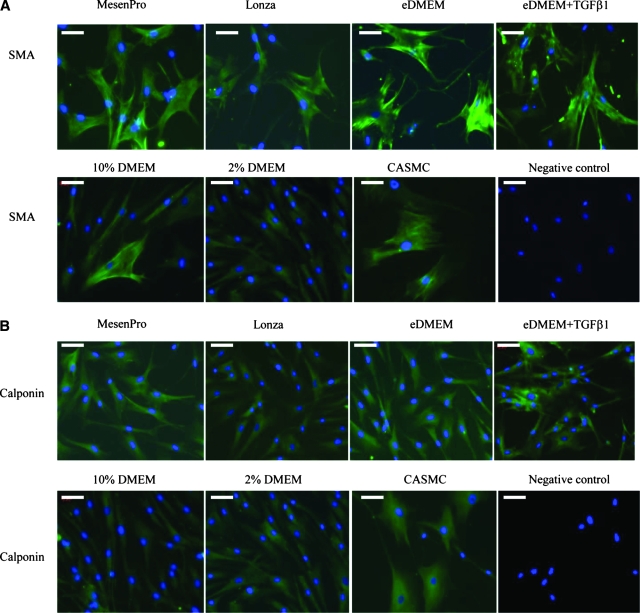
Immunofluorescence staining for SMC markers was performed as previously described.4 After fixation with cold 4% paraformaldehyde, the chamber slides were incubated with monoclonal primary antibodies, mouse anti-human SMA and calponin (Dako), both diluted 1:200 in blocking buffer (5% FBS in PBS) for 1 h at room temperature. After three washes with sterile water, the secondary antibody, a fluorescein isothiocyanate–conjugated goat anti-mouse IgG (Santa Cruz Biotechnology), 1:2000 diluted in blocking buffer, was added and incubated for 30 min at room temperature. After three washes, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Images were visualized with a Zeiss Axiovert 200M inverted fluorescence microscope (Carl Zeiss MicroImaging, Jena, Germany) and analyzed with AxioVision software (Carl Zeiss MicroImaging).
Human CASMCs (Lonza) were cultured in the smooth muscle growth medium (SmBM) supplemented with SmGM-2 SingleQuots (Lonza) and stained for SMA and calponin as positive controls. Primary deletes from staining of CASMCs served as negative control for both stainings.
Enzyme-linked immunosorbent assays
The plasma concentrations of basic fibroblast growth factor (bFGF) and TGFβ1 were measured using a Quantikine Human bFGF and TGFβ1 ELISA kit (R&D Systems, Minneapolis, MN), respectively, according to the manufacturer's instruction. The minimum detectable dose of bFGF and TGFβ1 is 3 and 1.7–15.4 pg/mL (mean 4.61), respectively. The absorbance of ELISA samples was then measured at 450 nm with an Umax kinetic microplate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis
All quantitative results from Western blots were obtained from triplicate samples for Western blot analyses. For ELISA assays, the results were average of triplicate for bFGF and quadruplicate for TGFβ1. Data were expressed as mean ± standard deviation values. Statistical analysis was carried out using ANOVA, followed by Bonferroni post hoc test. A value of p < 0.05 was considered to be statistically significant.
Results
Experiment 1 was designed to observe the SMC differentiation potential from primary hMSCs using six different media. Experiment 2 was designed to compare SMC differentiation capacity of primary and commercial hMSCs in response to four different culture media. To better understand the differential SMC-induction capacities of media in Experiment 2, we also assayed levels of several growth factors that we have shown previously to impact SMC differentiation (TGFβ1, PDGF-BB, and bFGF). Experiment 3 was designed to determine the role of SRF in hMSC culture establishment and SMC differentiation, using primary hMSCs derived from fresh bone marrow.
Experiment 1: effect of various culture conditions on primary hMSC differentiation into SMCs
Of these six media, MesenPro and Lonza were selected for evaluation as commonly utilized commercial hMSC media. It is interesting to note that MesenPro medium contains 2% FBS by volume, while Lonza contains 10% FBS. Obviously, the lots of FBS in these media are selected and prescreened by the respective manufacturers. To provide direct comparison of serum concentration with these two commercial media, we also evaluated simple DMEM containing one lot of FBS that we have selected in our laboratory, at concentrations of either 2% or 10%. In addition, we also evaluated the impact of an eDMEM, which is a medium developed by our laboratory and used by several groups for vascular tissue engineering applications, on SMC differentiation from hMSCs.15,16 Lastly, we also evaluated the impact of eDMEM that was supplemented with TGFβ1 (eDMEM + TGFβ1), because our previous studies showed a strong dependence of hMSC differentiation on this growth factor.4
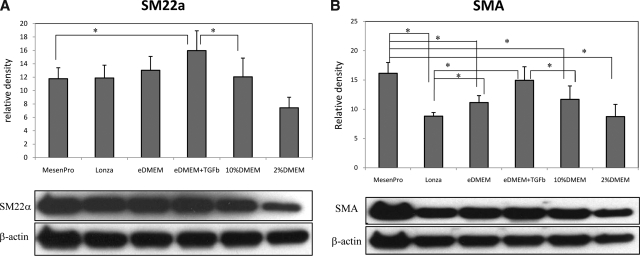
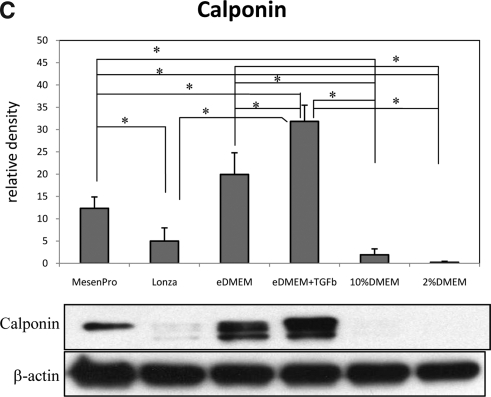
Based upon the observations of primary hMSCs cultured with six different media, MesenPro, eDMEM, and eDMEM + TGFβ1 seemed to provide the most SMC-inducing conditions, as judged by expression of the later marker calponin. On the other hand, Lonza medium and 10% DMEM provided the least SMC induction (Fig. 2A–C). These immunoblotting quantification results were consistent with immunofluorescence staining for SMA and calponin in all six different cultures (Fig. 3A, B). In other words, when primary hMSCs were grown in MesenPro, eDMEM, and eDMEM + TGFβ1 media, they were skewed to an SMC lineage. In contrast, other commonly used hMSC culture media, both Lonza medium and 10% DMEM, maintained primary hMSCs in a less differentiated state.
FIG. 2.
Effect of various culture media on SMC differentiation from hMSCs. The expression of SMC-specific markers, SM22α (A), SMA (B), and calponin (C), are compared among six different culture media. The results were expressed as relative density compared to β-actin as equal loading control (*p < 0.05). Representative Western blot film of three Western blots on the protein lysate from triplicates in each treatment group is shown below the bar graph of each protein.
FIG. 3.
Immunofluorescence staining of SMA (A) and calponin (B) on hMSCs grown in six different culture media. CASMCs served as positive control. Primary deletes from staining of CASMCs served as negative control for both staining. Scale bars shown in each figure represent 100 μm.
Lonza medium versus 10% DMEM
Lonza medium (MSCGM), a widely used mesenchymal stem cell medium, consists of basal medium (MSCBM) and MSCGM SingleQuots, the latter of which includes 10% FBS and pen-strep-L-glutamate. It was developed for growing large numbers of hMSCs without inducing differentiation. In contrast, vascular smooth muscle cells are typically cultured in media consisting of DMEM plus some amount of added serum, often 10% by volume. Since at the moment, a serum-free medium is not available for amplifying hMSCs in vitro, the type and lot of serum (fetal calf/bovine serum or human serum) that is used determines the consistency of results from lab to lab, and even from batch to batch in the same lab. Therefore, we compared our selected FBS-containing 10% DMEM medium with Lonza medium and observed that the two media seem to be the least SMC-inductive among the six media. This implies that both Lonza and 10% DMEM may be good initial culture media for hMSC expansion before induction of differentiation. On the other hand, MesenPro, eDMEM, and eDMEM + TGFβ1 should be avoided during the expansion of hMSC culture, because these media appear to strongly skew primary hMSCs to an SMC lineage.
2% versus 10% FBS
To determine whether FBS level affects SMC differentiation from hMSCs, MesenPro, a reduced (2%) serum medium specifically formulated to support the growth of hMSCs in culture, and 2% DMEM were compared to 10% DMEM medium. Among the three, MesenPro stood out as the most SMC-inducing medium, by significantly promoting SMA and calponin as compared to 2% and 10% DMEM (Fig. 2B, C). This finding is rather striking, given that MesenPro is developed for the expansion of hMSCs, rather than for driving a particular lineage commitment. However, there was no difference in the expression level of all three SMC markers between 10% and 2% serum in DMEM, suggesting that FBS concentration in DMEM does not make a difference in SMC differentiation.
One goal of directing hMSCs toward an SMC fate was to utilize these cells for vascular regeneration. To look into the effect-specific medium that has been developed to support vascular regeneration in vitro, we evaluated eDMEM, an enhanced medium developed15,16 and optimized by our lab to support engineered vessel culture by supporting extracellular matrix synthesis by SMCs.17 Compared to 10% DMEM, eDMEM medium significantly increased calponin expression (Fig. 2C) although the expression of the two earlier SMC markers, SMA and SM22α, was not significantly different between the two DMEM-based media (Fig. 2B).
Role of TGFβ1 in SMC differentiation
TGFβ1 has long been known as a potent SMC inducer.18,19 Consistent with our previous observations,4 supplementation of TGFβ1 in eDMEM significantly increased calponin expression level in hMSCs (Fig. 2C). In fact, among the six culture conditions included in this study, eDMEM + TGFβ1 medium provided the strongest SMC induction as evaluated by calponin, which is more SMC-specific than SM22α and SMA, which are earlier SMC markers that are also expressed by other cells.20–24 Although the eDMEM + TGFβ1 gave the best SMC induction, it is not included in the subsequent experiments because it is our lab's homemade recipe and not widely used in other labs.
Experiment 2: comparison of SMC differentiation from commercial and primary hMSCs
Because of the concern that hMSCs isolated from fresh human bone marrow in the lab are different from those from a commercial source, we compared SMC differentiation between hMSCs purchased from Lonza and primary hMSCs that were isolated from fresh human bone marrow. This allowed us to study the impact of various growth media on hMSCs that had not been subjected to extensive in vitro expansion before differentiation. To eliminate the complexity of different growth media on the establishment of hMSCs (discussed below), primary hMSC cultures were established and grown in Lonza medium and then frozen down, just as commercial hMSCs were. The scheme of the experimental design is summarized in Figure 1.
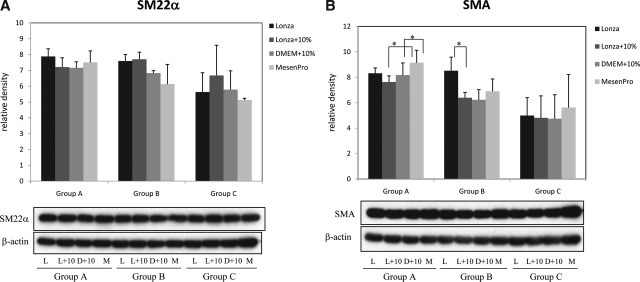
Regardless of the four culture conditions, SMA expression by immunoblotting appeared higher in primary hMSCs (groups A and B) than in commercial hMSCs (group C) (Fig. 4B). However, because differences in SMA and SM22α were mostly nonsignificant, the pattern of change in calponin levels in various culture conditions was compared among the three groups. In general, group A resembled group C more than group B (Fig. 4C). The fact that hMSCs in groups A and C, although obtained from different donors, responded similarly to different culture media (Fig. 4C) suggested that culture environment plays a significant role in determining the differentiation potential of hMSCs. For calponin expression, the similarity in pattern in all groups was striking. Lonza medium with 10% FBS added and MesenPro both generally induced the greatest amount of calponin expression.
FIG. 4.
Experiment 2: Effect of postthaw culture conditions on SMC differentiation from hMSCs. Expression of SM22α, SMA, and calponin are compared among different conditions within the same group by Western blots and shown in (A–C), respectively. β-Actin served as equal loading control. The results were expressed as relative density compared to β-actin (*p < 0.05). Representative Western blot film of three Western blots repeated on the same protein lysate from each treatment condition in each group was shown below the bar graphs of each protein. L, Lonza; L + 10, Lonza + 10% FBS; D + 10, DMEM + 10%; M, MesenPro.
In particular, it was noted that the difference between Lonza + 10% and 10% DMEM was consistent in all three groups (Fig. 4C), emphasizing the important role of basic medium on directing SMC differentiation. In other words, because the lot of FBS was the same in Lonza + 10% and 10% DMEM medium, the consistent enhanced SMC differentiation in the Lonza + 10% medium means that basal medium composition is an important driver of differentiation. Using the same lot of FBS, Lonza's MSCBM basal medium exerted a more potent SMC-inductive effect than low-glucose DMEM. (While the makeup of Lonza's MSCBM is proprietary, knowledge of the exact components in MSCBM would help to further dissect the elements that promote SMC differentiation.) Also in groups B and C, Lonza + 10% seemed to significantly increased calponin expression compared to Lonza medium. The difference should be attributed to FBS, which was the only difference between the two media. Obviously, our selected lot FBS supported SMC differentiation better than the FBS in the MSCGM SingleQuots Kit. Further, the combination of MSCBM and our selected lot of FBS seemed to have a comparable SMC-induction effect to MesenPro medium (Fig. 4C), which was one of the three strongest SMC-promoting media from Experiment 1.
A variance is noted in the level of calponin expression between Figures 2C and 4C. This variation can be explained by different donor source of hMSCs and different culture duration (1 week for Fig. 2C, and 3 weeks for Fig. 4C) in each experiment.
Comparison of bFGF and TGFβ1 levels in culture media
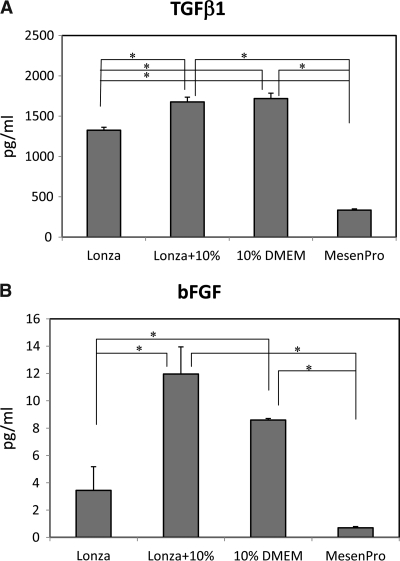
In previous studies, others and we have shown that TGFβ1 and bFGF have opposing effects on SMC differentiation from hMSCs.4 The levels of TGFβ1, a potent SMC differentiation inducer, and bFGF, a potent SMC differentiation inhibitor, were measured in four different culture media used in the previous experiment (Fig. 5A, B). The observation that Lonza + 10% contained a higher level of TGFβ1 compared to Lonza medium suggested that our selected lots of FBS has a higher level of TGFβ1 than Lonza's FBS, which is consistent with the results from Figure 4C, where our selected lot of FBS was a stronger SMC inducer than Lonza's FBS. Although MesenPro medium did not contain as much TGFβ1 as these two 10% FBS media, its bFGF level was less than one-tenth of the two 10% FBS media. This implies that a low level of an SMC differentiation inhibitor, bFGF, in MesenPro medium was a major contributor to MesenPro's strong SMC-induction effect.
FIG. 5.
ELISA analysis on the levels of TGFβ1 (A) and bFGF (B) in the four culture conditions in Experiment 2. The results were expressed in pg/mL as mean ± SD (n = 4 for TGFβ1; n = 3 for bFGF; *p < 0.05). (C) Summary of the ELISA results.
Experiment 3: involvement of SRF in hMSC culture establishment and SMC differentiation
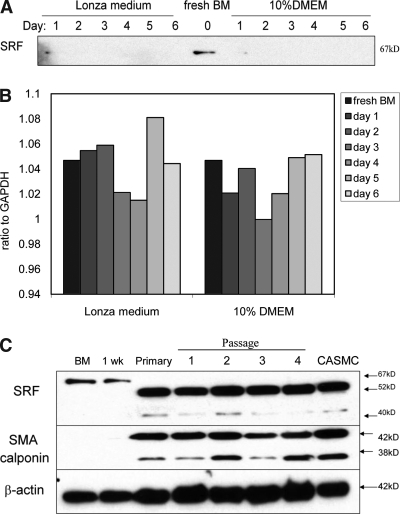
SRF has been shown to be involved in differentiation to an SMC fate during development.25,26 Hence, we sought to understand the expression patterns of SRF protein in newly isolated hMSC cultures and in differentiating cells. Levels of SRF protein expression were followed in the short term (from isolation to day 6) and in the long term (from isolation to over a month at four passages). During isolation and early culture, the patterns of SRF protein expression were similar in primary isolates of hMSCs that were established in either Lonza or 10% DMEM medium (Fig. 6A). Interestingly, SRF protein was expressed at a high level in BMMNCs freshly isolated from the bone marrow. But SRF level dropped significantly after 24 h and remained undetectable by immunoblotting after day 2 (Fig. 6A). However, the mRNA level of SRF decreased between days 2 and 3, but increased to a similar level as the early cultures after day 5 under both culture conditions (Fig. 6B), suggesting that posttranscriptional or posttranslational modification of SRF might be involved within the first week of hMSC culture establishment.
FIG. 6.
Experiment 3: Involvement of SRF in the establishment of hMSC culture and SMC differentiation from hMSCs. The expression of SRF was studied by Western blot (A) and RT-PCR (B) after the establishment of hMSC culture in Lonza or 10% DMEM medium in the short term from fresh human bone marrow up to 6 days. In (C), SMA and calponin expression mirrored SRF changes in hMSCs grown in Lonza medium by Western blot in the long term up to fourth passage.
To investigate the changes of SRF and SMC marker expression during the establishment and passage of hMSC culture, hMSC culture established in Lonza medium was followed from isolation to the fourth passage (Fig. 6C). Before the hMSC culture became established, a 67-kDa SRF isotype was detected in BMMNCs during the first week of culture. Once the hMSCs became attached and expanded to establish the primary culture, a lower molecular weight isotype, 52-kDa SRF, was detected in hMSC culture. We should point out that this unique isotype switch of SRF was only observed using a specific clone of SRF antibody from Santa Cruz Technologies (A-11), which, according to the manufacturer, will react with four different SRF isotypes, 67, 57, 52, and 40 kDa. Similarly, SMA and calponin proteins were not detected by Western blot until after the primary hMSCs became established. The finding that the SRF isotype switch and expression of SMC markers (SMA and calponin) coincided with the attachment and clonal expansion of hMSCs suggests that SRF might be associated with hMSC culture establishment and SMC differentiation.
Discussion
In this report, we systematically studied the impact of several culture media on the propensity of human mesenchymal stem cells to differentiate along a vascular smooth muscle lineage. Results of these studies may help to expand the utility of mesenchymal stem cells for various cardiovascular engineering applications, and to improve our understanding of the potential of MSC to take on vascular phenotypes.
We observed that serum is important for hMSC culture initiation and establishment, but that once the culture became established, FBS concentration does not appear to make a difference in SMC differentiation, because no difference was observed in the protein levels of all three SMC markers in hMSCs between 10% and 2% FBS DMEM (Fig. 2). Between the two commercial hMSC media that were studied, MesenPro appears to be a stronger SMC inducer than Lonza medium. However, there was no significant difference between Lonza medium and 10% FBS DMEM medium in supporting SMC differentiation. The similarity between Lonza and 10% DMEM media, coupled with the significant difference observed between Lonza and MesenPro, emphasizes the necessity to screen even commercial media for a particular culture requirement.
To investigate whether primary hMSCs are comparable to commercial hMSCs in their SMC differentiation ability in response to different culture conditions, primary hMSCs isolated and grown on Lonza medium but thawed and passaged in either Lonza or 10% FBS DMEM medium were compared to hMSCs purchased from Lonza (Fig. 4). Primary hMSCs grown exclusively in Lonza medium showed a similar pattern in the change of SMA and calponin levels to commercial hMSCs under four different culture conditions, but different from the same batch of primary hMSCs grown in 10% DMEM. This discrepancy between the primary hMSCs cultured in 10% FBS DMEM and Lonza medium suggested that the culture and expansion medium is another important factor that causes variations among cultures. Although we have shown that Lonza and 10% DMEM medium are not different with respect to hMSC differentiation, there might be a difference in their influence on hMSC proliferation and culture expansion, especially the response of hMSCs to SMC inducers contained in the culture medium.
One limitation of this study is that all the primary hMSCs used in this study were from the bone marrow of a single donor, while the commercial hMSCs are from another single donor. In other words, n equals 1 when we compared the hMSCs from two different sources. A large variability in terms of proliferative capacity and MSC life span was noted between donors.27 Despite the lack of literature on the variation of SMC differentiation potential in hMSCs from different donors, the variation is conceivable and should be considered in exploration of the conclusions from this study to hMSCs from different donors and from different sources other than bone marrow.
To further understand the mechanism underlying the different degrees of SMC induction by various culture media, the levels of two important growth factors were measured and compared among four different culture conditions. TGFβ1 is one of the primary differentiation factors for SMCs that not only upregulates several SMC differentiation markers such as SMA, calponin SM22α, and SM-MHC in vitro, but also induces expression of SMC markers in multipotent embryonic fibroblast (10T1/2 cells),28 adult hMSCs,4 and neural crest cells.29 bFGF is one of the most potent mitogenic growth factors for SMCs.4,30 However, bFGF-induced mitogen-activated protein kinase (MEK)/extracellular signal–regulated kinase signaling was shown to play an antagonistic role in TGFβ1-induced SMC gene expression through suppression of SRF function.31 The opposing effects of bFGF and TGFβ1 on SMC gene expression are postulated to control the phenotypic plasticity of SMCs.31 It is possible that the net effect from the opposing action of TGFβ1 and bFGF on SMC differentiation determined whether a culture medium promoted SMC differentiation. To further confirm the association between the effects of each type of culture medium on SMC differentiation and their bFGF/TGFβ1 content, additional studies are necessary with the uses of bFGF supplementation and TGFβ1-neutralizing antibody.
SRF regulates SMC-specific transcription by binding to CArG (CC(A/T)6GG) cis elements that are found in the promoters of nearly all of the SMC marker genes.32–35 TGFβ1 induction of smooth muscle cell phenotype requires transcriptional and posttranscriptional control of SRF.36 The finding that SRF isotype switch occurred concurrently with hMSC cell attachment and the expression of smooth muscle markers should be further explored to reveal the underlying mechanism. In a preliminary experiment, we introduced siRNA to SRF to the MNCs from fresh human bone marrow, but we were not able to establish hMSC culture because hMSCs did not attach to the culture flasks, suggesting that SRF may play a role in hMSC cell attachment.
In summary, culture medium plays a very important role in SMC differentiation from hMSCs. When carefully selected and screened, homemade medium can be comparable to commercial medium. Primary hMSCs, when grown in the same culture medium, respond similarly to SMC inducers as the commercial hMSCs. Although serum is currently indispensible for hMSC culture establishment and expansion, the trend and future in the hMSC field is to develop a chemically defined, serum-free medium. When using commercial FBS-containing hMSC medium, medium screening is necessary to maintain undifferentiated state of hMSCs due to the possibility that certain medium skewed the hMSCs down to certain lineage due to its unknown nature. Finally, in addition to the involvement in SMC differentiation from hMSCs, SRF might also play a critical role in cell adhesion and, hence, the establishment of hMSC culture.
Acknowledgments
This work was funded by National Institutes of Health RO1HL083895 and HL063766 (both L.E.N.), the Connecticut Stem Cell Research Fund, and the Center of Excellence in Molecular Hematology NIH P30 DK072442 (D.S.K.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Poh M. Boyer M. Solan A. Dahl S.L.M. Dawn P. Banik S.S.R. McKee J.A. Klinger R.Y. Counter C.M. Niklason L.E. Blood vessels engineered from human cells. Lancet. 2005;365:2122. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 2.McKee J.A. Banik S.S.R. Boyer M.J. Hamad N.M. Lawson J.H. Niklason L.E. Counter C.M. Human arteries engineered in vitro. EMBO Rep. 2003;4:633. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J.Y. Swartz D.D. Peng H.F. Gugino S.F. Russell J.A. Andreadis S.T. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Gong Z. Niklason L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore H. The medium is the message. Nat Biotechnol. 2006;24:160. doi: 10.1038/nbt0206-160. [DOI] [PubMed] [Google Scholar]
- 6.Sekiya I. Larson B.L. Smith J.R. Pochampally R. Cui J. Prockop D.J. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;6:530. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 7.Sotiropoulou P.A. Perez S.A. Salagianni M. Baxevanis C.N. Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 8.Dimarakis I. Levicar N. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells. 2006;24:1407. doi: 10.1634/stemcells.2005-0577. [DOI] [PubMed] [Google Scholar]
- 9.Berger M.G. Veyrat-Masson R. Rapatel C. Descamps S. Chassagne J. Boiret-Dupre N. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells. 2006;24:2888. doi: 10.1634/stemcells.2006-0387. [DOI] [PubMed] [Google Scholar]
- 10.Mannello F. Tonti G.A. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25:1603. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 11.Liu C.H. Wu M.L. Hwang S.M. Optimization of serum free medium for cord blood mesenchymal stem cells. Biochem Eng J. 2007;33:1. [Google Scholar]
- 12.Shahdadfar A. Fronsdal K. Haug T. Reinholt F.P. Brinchamann J.E. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 13.Gong Z. Wezeman F.H. Inhibitory effect of alcohol on osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Alcohol Clin Exp Res. 2004;28:468. doi: 10.1097/01.alc.0000118315.58404.c1. [DOI] [PubMed] [Google Scholar]
- 14.Haynesworth S.E. Baber M.A. Caplan A.I. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 15.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 16.Niklason L.E. Abbott W. Gao J. Klagges B. Hirschi K.K. Ulubayram K. Conroy N. Jones R. Vasanawala A. Sanzgiri S. Langer R.L. Morphologic and mechanical characteristics of bovine engineered arteries. J Vasc Surg. 2001;33:628. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 17.Dahl S.L. Rucker R.B. Niklason L.E. Effect of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005;14:861. [PubMed] [Google Scholar]
- 18.Bjorkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb. 1991;11:892. [PubMed] [Google Scholar]
- 19.Hautmann M.B. Madsen C.S. Owens G.K. Transforming growth factor β (TGFβ) control element drives TGFβ-induced stimulation of smooth muscle α-actin gene expression in concert with two CArG-elements. J Biol Chem. 1997;272:10948. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa A. Sakatsume M. Wang X. Sakamaki Y. Tsubata Y. Alchi B. Kuroda T. Kawachi H. Narita I. Shimizu F. Gejyo F. SM22alpha: the novel phenotype marker of injured glomerular epithelial cells in anti-glomerular basement membrane nephritis. Nephron Exp Nephrol. 2007;106:e77. doi: 10.1159/000103020. [DOI] [PubMed] [Google Scholar]
- 21.Ruzicka D.L. Schwartz R.J. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988;107:2575. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawtell N.M. Lessard J.L. Cellular distribution of smooth muscle actins during mammalian embryogenesis: expression of the alpha-vascular but not the gamma-enteric isoform in differentiating striated myocytes. J Cell Biol. 1989;109:2929. doi: 10.1083/jcb.109.6.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darby I. Skalli O. Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21. [PubMed] [Google Scholar]
- 24.Cintorino M. Belllizzi De Marco E. Leoncini P. Tripodi S.A. Xu L.J. Sappino A.P. Schmitt Graff A. Gabbiani G. Expression of alpha-smooth-muscle actin in stromal cells of the uterine cervix during epithelial neoplastic changes. Int J Cancer. 1991;47:843. doi: 10.1002/ijc.2910470609. [DOI] [PubMed] [Google Scholar]
- 25.Li S. Wang D.Z. Wang Z. Richardson J.A. Olson E.N. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA. 2003;100:9366. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schratt G. Philippar U. Berger J. Schwarz H. Heidenreich O. Nordheim A. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J Cell Biol. 2002;156:737. doi: 10.1083/jcb.200106008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardo M.E. Zaffaroni N. Novara F. Cometa A.M.M. Avanzini M.A. Moretta A. Montagna D. Maccario R. Villa R. Daidone M.G. Zuffardi O. Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 28.Hirschi K.K. Rohovsky S.A. D'Amore P.A. PDGF-BB, TGFβ, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah N.M. Groves A.K. Anderson D.J. Alternative neural crest cell fates are instructively promoted by TGFβ superfamily members. Cell. 1996;85:331. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 30.Owen G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 31.Kawai-Kowase K. Sato H. Oyama Y. Kanai H. Sato M. Doi H. Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor-β1-induced smooth muscle gene expression through extracellular signal regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24:1384. doi: 10.1161/01.ATV.0000136548.17816.07. [DOI] [PubMed] [Google Scholar]
- 32.Miano J.M. Carlson M.J. Spencer J.A. Misra R.P. Serum response factor-dependent regulation of smooth muscle calponin gene. J Biol Chem. 2000;275:9814. doi: 10.1074/jbc.275.13.9814. [DOI] [PubMed] [Google Scholar]
- 33.Madsen C.S. Hershey J.C. Hautmann M.B. White S.L. Owens G.K. Expression of the smooth muscle myosin heavy chain gene is regulated by a negative-acting GC-rich element located between two positive-acting serum response factor-binding elements. J Biol Chem. 1997;272:6332. doi: 10.1074/jbc.272.10.6332. [DOI] [PubMed] [Google Scholar]
- 34.Li L. Liu Z. Mercer B. Overbeek P. Olson E.N. Evidence for serum response factor-mediated regulatory networks governing SM22alpha transcription in smooth, skeletal, and cardiac muscle cells. Dev Biol. 1997;187:311. doi: 10.1006/dbio.1997.8621. [DOI] [PubMed] [Google Scholar]
- 35.Herring B.P. Smith A.F. Telokin expression in A10 smooth muscle cells requires serum response factor. Am J Physiol. 1997;272:C1394. doi: 10.1152/ajpcell.1997.272.4.C1394. [DOI] [PubMed] [Google Scholar]
- 36.Hirschi K.K. Lai L. Belaguli N.S. Dean D.A. Schwartz R.J. Zimmer W.E. Transforming growth factor-beta induction of smooth muscle cell phenotype requires transcriptional post-transcriptional control of serum response factor. J Biol Chem. 2002;277:6287. doi: 10.1074/jbc.M106649200. [DOI] [PMC free article] [PubMed] [Google Scholar]