Abstract
Introduction
Lung contusion (LC) from blunt thoracic trauma is a clinically-prevalent condition that can progress to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Patients with LC are at risk for gastric aspiration at the time of trauma, but the combined insults have not been well-studied in animal models. This study tests the hypothesis that concurrent gastric aspiration (combined acid and small gastric particles, CASP) at the time of trauma significantly increases permeability injury and inflammation compared with LC alone, and also modifies the inflammatory response to include distinct features compared with the aspiration component of injury.
Materials and Methods
Four groups of adult male Long-Evans rats were studied (LC, CASP, LC + CASP, uninjured controls). LC was induced in anesthetized rats at a fixed impact energy of 2.0J, and CASP (1.2mL/kg body weight, 40mg particles/mL, pH = 1.25) was instilled through an endotracheal tube. Lung injury and inflammation were assessed by arterial blood gases and levels of albumin, cells, and cytokines/chemokines in bronchoalveolar lavage (BAL) at 5 and 24hours.
Results
Rats with LC + CASP had lower mean PaO2/FiO2 ratios compared with LC alone at 24hours, and higher BAL albumin concentrations compared with either LC or CASP alone. Rats with LC + CASP versus LC had more severe inflammation based on higher levels of PMN in BAL at 5hours, increased whole lung myeloperoxidase (MPO) activity at 5 and 24hours, and increased levels of inflammatory mediators in BAL (TNFα, IL-1β, and MCP-1 at 5 and 24hours; IL-10, MIP-2, and CINC-1 at 5hours). Rats with LC + CASP also had distinct aspects of inflammation compared with CASP alone, i.e., significantly higher levels of IL-10 (5 and 24hours), IL-1β (24hours), CINC-1 (24hours), and MCP-1 (24hours), and significantly lower levels of MPO (5hours), MIP-2 (5hours), and CINC-1 (5hours).
Conclusions
Concurrent gastric aspiration can exacerbate permeability lung injury and inflammation associated with LC, and also generates a modified inflammatory response compared with aspiration alone. Unwitnessed gastric aspiration has the potential to contribute to more severe forms of LC injury associated with progression to ALI/ARDS and pneumonia in patients with thoracic trauma.
Keywords: lung contusion, gastric aspiration, acute lung injury, acute respiratory distress syndrome
Introduction
Lung contusion (LC) is an important and common problem in the care of trauma patients. Thoracic injury is involved in nearly one-third of all acute trauma admissions to the hospital [1–3]. LC is frequently associated with pneumonia, and is also an independent risk factor for the development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [1–3]. The clinical syndromes of ALI/ARDS reflect severe inflammatory lung injury, and have high mortality and morbidity despite sophisticated respiratory support and intensive care [4]. Aside from causing severe clinical consequences, the societal and economic impacts are significant when thoracic trauma-induced LC progresses to ALI/ARDS. The incremental hospital cost per trauma patient is much higher for those who develop ALI ($36,713) or ARDS ($59,633) compared with those who do not meet criteria for these clinical lung injury syndromes ($24,715) [5].
The pathophysiology of LC includes hypoxemia, hypercarbia, increased work of breathing, and decreased pulmonary volumes in association with ventilation/perfusion mismatching, edema, and segmental lung damage [1, 6, 7]. One of the vexing clinical aspects of blunt trauma-induced LC is that there is frequently not a clear correlation between the volume of affected lung and the severity and duration of hypoxemia [1]. Patients with small contusions (seen only on CT scan) may exhibit more severe hypoxemia than those with large contusions (visible on chest X-ray). This suggests that factors in addition to trauma-induced tissue damage may contribute to respiratory pathology in some patients with LC injury. One potential contributor of this kind is gastric aspiration, which is known to be a major cause of direct pulmonary ALI/ARDS [8, 9]. Patients are at increased risk for gastric aspiration at the time of trauma, particularly if they experience a brief loss of consciousness or have associated risk factors such as recent food or alcohol intake [8, 9]. Many cases of gastric aspiration are unwitnessed or unreported, and aspiration pneumonitis is often a diagnosis of exclusion in patients without other known causes of respiratory compromise [8, 9]. The scenario of concomitant pulmonary contusion plus occult gastric aspiration in chest trauma patients may be significantly underappreciated, since affected individuals have a readily apparent presumptive cause of lung injury from trauma alone.
The present study seeks to improve understanding about inflammatory mechanisms contributing to blunt trauma induced LC plus superimposed gastric aspiration. Experiments use a recently developed rat model of sublethal bilateral LC injury with minimal associated cardiac trauma [10, 11]. Gastric aspiration is simulated by an intratracheally-instilled combination of acid and small nonacidified gastric food particles (CASP), which has previously been shown to lung injury in rats in the absence of LC (e.g., [12–16]). Rats with LC + CASP, LC, or CASP are assessed for arterial oxygenation, pulmonary mechanics, and albumin in bronchoalveolar lavage (BAL) as measures of lung injury severity, and for levels of whole lung myeloperoxidase (MPO) and inflammatory cells and cytokines/chemokines in BAL as measures of innate pulmonary inflammation. A major hypothesis tested is that rats with the combination injury of LC + CASP will have more severe permeability lung injury and inflammation compared with LC alone. In addition, a secondary hypothesis is that the combined insults of LC + CASP will significantly modify the levels and time-dependent relationships of inflammatory mediators in BAL compared with CASP alone. Changes in specific inflammatory cytokines/chemokines in LC + CASP compared with the component injuries are important not only for mechanistic understanding, but also for the future development of diagnostic/prognostic paradigms and inflammation-based therapies to improve the management of patients with blunt chest trauma.
Materials and Methods
Animal Protocols
All animal studies were approved by the Institutional Animal Care and Use Committee at the University at Buffalo-SUNY, and complied with New York State, Federal, and National Institutes of Health regulations. Male 250–300 g Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN) were randomly assigned to one of four groups: (1) uninjured controls, (2) LC, (3) CASP, and (4) LC + CASP. Animals were sacrificed either at 5 hours or 24 hours post-insult to allow assessments of both early-onset injury and later (but still acute) progressing injury. A total of 8-12 animals per group were examined for lung injury and inflammatory variables at each time point.
Induction of Bilateral LC
Following induction of halothane anesthesia (2%), a 300 g cylindrical weight was dropped 68.0 cm (impact energy = 2.00 J) through a vertical guiding tube onto a Lexon plate with an attached precordial shield placed on the chest of a supine rat [10, 11]. The precordial shield directed the impact force bilaterally to the lungs so as to prevent cardiac trauma [10, 11]. The 2.00 J impact energy level was chosen to minimize animal mortality in the combined injury group where both contusion and gastric aspiration were present. Mortality in all animal groups was <15%.
Gastric Aspiration (CASP) Lung Injury
The CASP aspirate used contained 40 mg/mL of small gastric food particles adjusted to a pH of 1.25 with hydrochloric acid as defined in our prior work [12–16]. Small nonacidified gastric food particles were obtained from the stomach contents of healthy Long-Evans rats, followed by washing in normal saline and coarse-filtration through gauze to generate a mean particle diameter <10 μm [14, 15]. CASP was instilled to anesthetized rats through a 14 gauge endotracheal tube, with anatomical placement verified by a continuous end-tidal CO2 tracing obtained with a RASCAL II Raman light scattering spectrophotometer (Ohmeda, Salt Lake City, UT). The chest wall was compressed by hand and rapidly released as CASP was instilled (1.2 mL/kg body weight), followed by a 0.5 mL air-bolus chaser to facilitate pulmonary distribution. Animals in the LC + CASP group were allowed to recover in 100% O2 following contusion until regular, spontaneous breathing was obtained before the instillation of CASP (approximately 3 to 5 minutes post-LC). Following the induction of injury (LC + CASP, LC, or CASP), all animal groups were maintained in room air until being assessed for oxygenation and BAL levels of albumin, cells, and inflammatory mediators at 5 and 24 hours.
Blood Sampling and Arterial Blood Gas Measurements
At the designated post-injury time point, rats were re-anesthetized with halothane (2%) and arterial oxygenation was measured as the ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2). Prior to blood sampling, animals breathed 98% O2 for 5 min (FiO2 = 0.98) in order to allow the severity of pulmonary shunting to be assessed. Blood (approximately 0.5 mL) was collected from the descending aorta in a heparinized syringe and was analyzed with an ABL5 blood gas analyzer (Radiometer America, Westlake, OH).
BAL and Cell Counts
Following arterial blood sampling, animals were euthanized by transecting the vena cava. A midline incision was made through the sternum, and the lung vasculature was flushed by injecting 20 mL HBSS at 37 °C into the beating right ventricle. BAL was performed by injecting 5 × 10 mL of 37 °C normal saline through a tracheal cannula. Recovered BAL fluid was centrifuged at 1500 × g at 4 °C for 3 minutes to pellet cells, and the supernatant was frozen for albumin and cytokine/chemokine analyses. The cell pellet was resuspended in 4 mL of phosphate buffered normal saline (PBS) + 0.1% sodium azide, and the total numbers of BAL-recovered erythrocytes (RBCs) and leukocytes (WBCs) were determined with a Multisizer 3 Coulter Counter (Beckman Coulter, Fullerton, CA). Differential counts were performed on pelleted cells following cytocentrifugation (Cytospin 3; Shandon Southern Instruments, Sewickley, PA) and staining with Diff-Quik (Baxter, Detroit, MI).
Albumin Concentrations in BAL
Albumin concentrations (μg/mL) in cell-free BAL were measured as an assessment of permeability injury. Albumin levels were quantitated by ELISA with a polyclonal rabbit anti-mouse albumin antibody (generously provided by Dr. Daniel Remick, Boston University, Boston, MA) and a HRP-labeled goat anti-rabbit IgG (BD Biosciences Pharmingen, San Diego, CA) [17]. Rat albumin (Sigma, St. Louis, MO) was used as a standard.
Cytokines and Chemokines in BAL
Concentrations (pg/mL) of tumor necrosis factor-α (TNFα), interleukin (IL)-1β, IL-6, interferon-γ (IFNγ), and IL-10, plus the rodent CXC chemokines macrophage inflammatory protein-2 (MIP-2) and cytokine-induced neutrophil chemoattractant-1 (CINC-1), and the CC chemokine monocyte chemoattractant protein-1 (MCP-1), were measured in cell-free BAL by ELISA methodology using reagents from R and D Systems (Minneapolis, MN). In addition, biologically-active TNFα was quantitated by a cytotoxicity bioassay described previously by Davidson et al. [14].
Whole Lung Myeloperoxidase (MPO) Activity as a Marker for Granulocyte Activity and a Measure of Pulmonary Leukostasis [18]
After BAL, lungs were excised and ice-cold normal saline with 1× protease inhibitor “cocktail” (500 μM AEBSF HCl, 150 nM aprotinin, 1 μM E-64, 0.5 mM EDTA disodium, 1 μM leupeptin hemisulfate final concentrations; Calbiochem, LA Jolla, CA) was added to a total weight of 10 g (tissue plus saline). The lungs were then homogenized on ice with a Polytron TP-2000 tissue homogenizer (Brinkman Instruments, Westbury, NY). The tissue homogenate was centrifuged at 40,000 × g for 10 minutes at 4 °C, and MPO was extracted from the pellet by resuspension in 5 mL phosphate buffer (pH = 6.0) containing 0.5% hexadecyltrimethylammonium bromide and 5 mM EDTA followed by three freeze-thaw-sonication cycles (1 minute, 50% duty cycle Branson Sonifier with microtip probe; Branson Ultrasonics, Danbury, CT). Centrifugation was carried out as before, and the supernatant was combined with the supernatant from a second MPO extraction of the pellet. MPO activity was measured by combining 10 μL of extracted sample with 300 μL of assay buffer (pH = 6.0, containing 50 mM KH2PO4, 176 mM H202, and 52.5 mM o-dianisidine dihydrochloride) in a 96-well microplate (Sarstedt, Newton, NC). Absorbance was recorded at 460 nm for 90 seconds at 2 second intervals on a SPECTRAmax190 plate reader (Molecular Devices, Sunnyvale, CA). MPO activity was expressed in arbitrary units as the absorbance change per minute over the linear portion of the curve, and was normalized to the total volume of extracted sample from the whole lung [14].
Lung Pressure-Volume (P-V) Mechanics
Quasi-static lung P-V measurements were performed after sacrificing the animals. After re-induction of halothane anesthesia (2%) as above, animals breathed 100% O2 for 5 minutes to facilitate adsorption atelectasis. A 14-gauge steel cannula was inserted and secured by a suture in the trachea through a 2 cm ventral midline neck incision, followed by exsanguination via the abdominal inferior vena cava. Air was then injected into the lungs at a rate of 25 mL/min by a syringe pump connected to the tracheal cannula. Inflation pressure was monitored continuously by an in-line pressure transducer connected to an Apple PowerBook G4 (Apple Computer, Cupertino, CA) equipped with a National Instruments data acquisition board (Austin, TX) and custom software in Lab VIEW 6.0. At 40 cm H2O, the syringe pump was reversed and deflation pressures were monitored. Volumes were calculated based on the rate of injection or withdrawal, and were normalized to kg body weight [17].
Statistical Analyses
Graphic displays and descriptive statistics for each variable are expressed as mean ± standard error of the mean (SEM). Due to the non-normal character of much of the data, results were analyzed using the Kruskal-Wallis rank-sum test using the statistical software JMP I ver.5.1 (SAS Institute Inc., Cary, NC). If the null hypothesis (i.e., all group means being the same) was rejected (P < 0.05), then pair-wise comparisons between each group (six comparisons) were made using an analogue of the Bonferroni pair-wise comparison based on observation ranks. The two-sided family level of significance for all comparisons was set at α = 0.05 (to adjust for multiple comparisons, individual comparisons were considered significant if P < 0.0083).
Results
The Combination of LC + CASP Produces More Severe Lung Injury Compared with LC Alone
Arterial Oxygenation
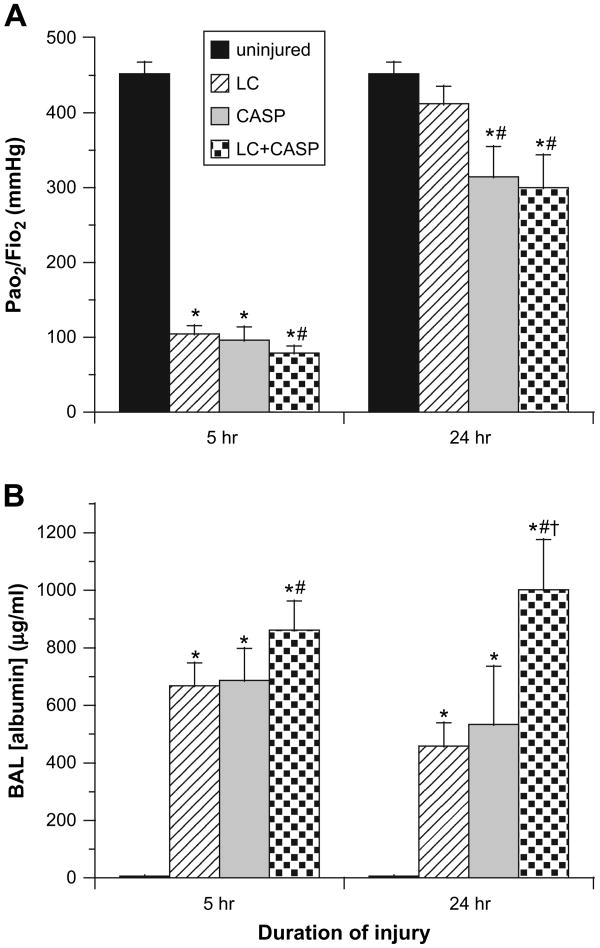
At 5 hours, all three injury groups (LC, CASP, and LC + CASP) had significant hypoxemia compared with uninjured control rats (P < 0.00001, Fig. 1A). PaO2/FiO2 ratios in all injury groups met criteria for the clinical definition of ARDS (i.e., ≤200 mm Hg [19]) at this early time point. However, PaO2/FiO2 ratios were lower in rats injured with LC + CASP (79 ± 9 mmHg) compared with LC alone (105 ± 11 mm Hg, P < 0.006, Fig. 1A). Arterial oxygenation improved in all injury groups by 24 hours, but PaO2/FiO2 levels in rats given LC + CASP (300 ± 44 mm Hg) or CASP (314 ± 40 mm Hg) were lower than for rats with LC alone (412 ± 24 mm Hg, P < 0.006, Fig. 1A). PaO2/FiO2 ratios at 24 hours for rats given LC alone were not significantly different from uninjured controls (452 ± 16 mm Hg) (Fig. 1A).
Fig. 1.
Assessment of lung function and injury. Rats breathed 98% oxygen (FiO2 = 0.98) for 5 minutes just prior to sacrifice at 5 or 24 hours post-injury with lung contusion (LC), aspiration of combined acid and small gastric particles (CASP), or both (LC + CASP). Arterial blood was drawn and analyzed for the partial pressure of oxygen (PaO2), and albumin concentration was measured in cell-free BAL supernatant by ELISA. (A) Arterial oxygenation expressed as PaO2/FiO2 (mm Hg, mean ± SEM, n = 6–12). (B) Albumin (μg/mL, mean ± SEM, n = 6–11). Kruskal-Wallis (rank sum) statistical analysis was performed on data at each time point, and inter-group comparisons were made with a Bonferroni correction for multiple comparisons such that P < 0.0083 was considered significant (family-wise α error = 0.05). *P < 0.00001 compared with uninjured controls; **P < 0.006 compared with LC alone; ***P < 0.00006 compared with CASP alone.
Alveolar-Capillary Integrity (BAL Albumin Concentrations)
Albumin levels in cell-free BAL were higher in all three injury groups compared with uninjured controls at both 5 and 24 hours post-injury (P < 0.00001, Fig. 1B). Rats given LC + CASP had higher concentrations of albumin in BAL at both 5 and 24 hours compared with rats given LC alone (861 ± 101 versus 669 ± 79 μg/mL at 5 hours and 1,002 ± 174 versus 458 ± 81 μg/mL at 24 hours, P < 0.006, Fig. 1B). In addition, albumin concentrations in BAL at 24 hours for rats given LC + CASP were significantly higher compared with rats given CASP (533 ± 202 μg/mL, P < 0.00006) (Fig. 1B).
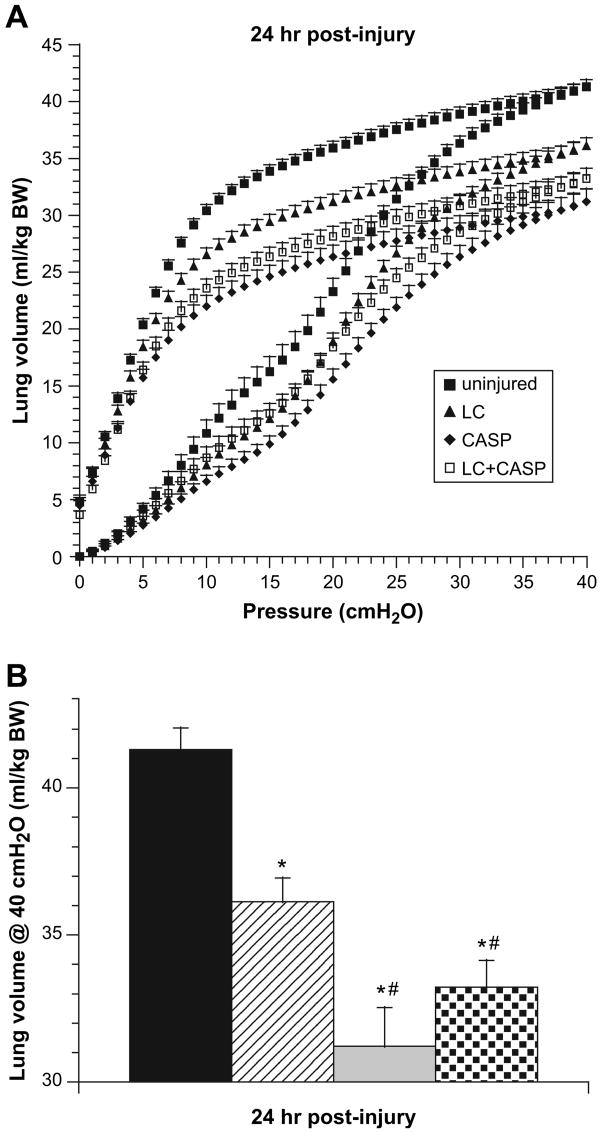
Lung P-V Curves
Quasi-static closed-chest P-V measurements at 24 hours post-insult indicated that pulmonary compliance and volumes were significantly reduced in all injury groups (LC + CASP, LC, and CASP) compared with uninjured controls (Fig. 2A and B). However, rats given LC + CASP or CASP had a greater impairment in overall P-V behavior at 24 hours compared with rats given LC alone (Fig. 2A). Lung vital capacity approximated as the inflation lung volume at 40 cm H2O was not significantly different in animals given LC + CASP and CASP (33.2 ± 0.9 and 31.2 ± 1.3 mL/kg bodyweight for LC + CASP and CASP, respectively, Fig. 2B). Lung vital capacity was larger for rats given LC alone (36.1 ± 0.8 mL/kg bodyweight, P < 0.004 compared with animals given LC + CASP or CASP, Fig. 2B). Vital capacity values in all three injury groups were reduced compared with uninjured controls (41.3 ± 0.7 mL/kg bodyweight, P < 0.0003, Fig. 2B).
Fig. 2.
Pressure-volume (P-V) mechanics as an assessment of lung injury. “Quasi-static” closed chest P-V behavior was measured at 24 hours post-injury in rats. (A) Complete inflation-deflation P-V hysteresis curves; (B) lung vital capacity approximated as the volume at 40 cmH2O (mean ± SEM, n = 6 animals per group). The same statistical analyses as in Fig. 1 were performed. *P < 0.0003 compared with uninjured controls; **P < 0.004 compared with LC alone.
Rats Given LC + CASP Differ From LC and CASP in Cell Numbers and Inflammatory Mediators in BAL
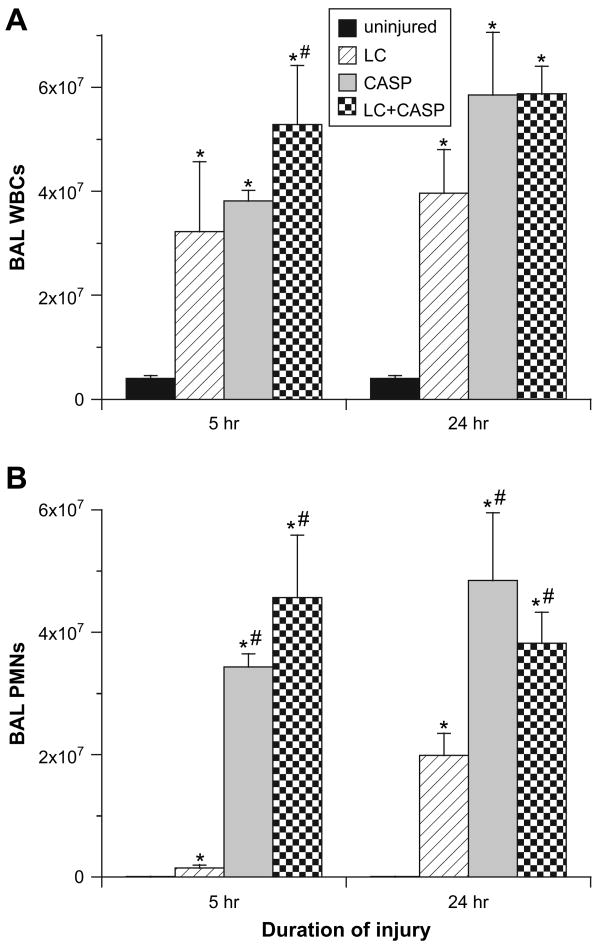
Cellular Characteristics of BAL
There were significant differences between animals given LC + CASP, LC, and CASP in cellular aspects of the inflammatory response. All injury groups had increased total numbers of leukocytes (WBCs) in BAL compared with uninjured controls at both 5 and 24 hours post-injury (P < 0.002, Fig. 3A). All injury groups also had significantly larger numbers of polymorphonuclear neutrophils (PMNs) in BAL compared with uninjured controls at 5 and 24 hours (P < 0.002, Fig. 3B). However, rats given either LC + CASP or CASP had significantly increased numbers of PMNs in BAL at 5 and 24 hours compared with rats given LC (P < 0.0083, Fig. 3B). There were no significant differences in the numbers of PMNs in BAL at either 5 or 24 hours for rats given LC + CASP compared with CASP. In additional studies (data not shown), rats given LC + CASP or CASP had lower numbers of macrophages in BAL at 5 hours post-injury compared with rats given LC alone. Also, rats with contusion (LC + CASP or LC) had increased numbers of RBCs in BAL, consistent with intrapulmonary hemorrhage associated with the initial blunt trauma insult (data not shown).
Fig. 3.
Numbers of inflammatory cells in recovered BAL fluid. Cells recovered by BAL were enumerated and a differential count performed as described in Methods. (A) Total leukocytes (WBC's) in BAL; (B) total polymorphonuclear neutrophils (PMN's) in BAL (mean ± SEM, n = 6–12 animals). The same statistical analyses as in Fig. 1 were performed. *P < 0.002 compared with uninjured controls; **P < 0.0083 compared with LC alone.
MPO Levels in Whole Lung Tissue
The activity of MPO in whole lung tissue was examined as a further marker of neutrophil pulmonary activation (Fig. 4). Whole lung MPO levels for all injury groups were increased compared with uninjured controls at 5 and 25 hours post-injury (P < 0.001, Fig. 4). However, rats with LC + CASP or CASP had significantly greater whole lung MPO activity at 5 and 24 hours compared with rats given LC alone (P < 0.0002, Fig. 4). The highest levels of whole lung MPO activity were present at 5 hours in rats given CASP (P < 0.004 compared with rats with LC + CASP at this time point, Fig. 4).
Fig. 4.
Myeloperoxidase (MPO) activity in whole lung homogenates. Lungs were harvested and homogenized following the BAL, and MPO was extracted and its activity measured as a reflection of pulmonary neutrophil infiltration in response to injury (arbitrary units, mean ± SEM, n = 6–12). The same statistical analyses as in Fig. 1 were performed. *P < 0.001 compared with uninjured controls; **P < 0.0002 compared with LC alone; ***P < 0.004 compared with CASP alone.
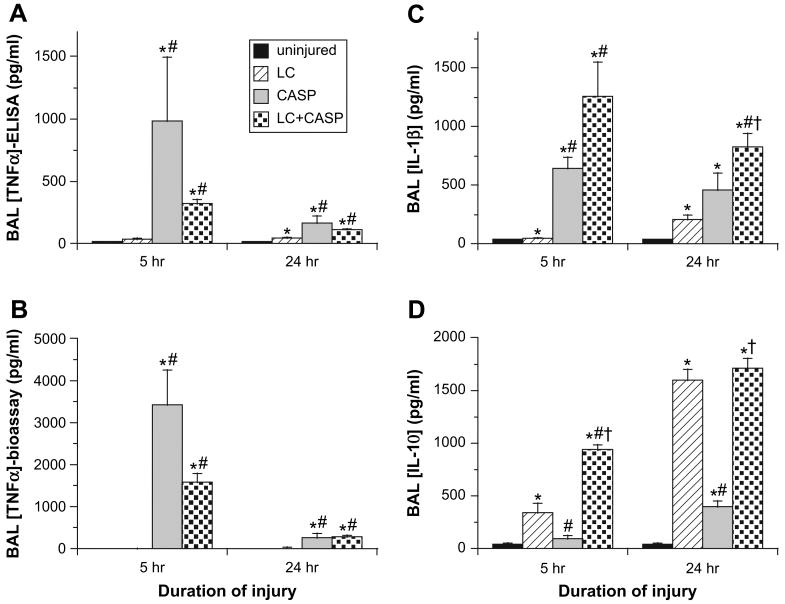
Cytokine Levels in Cell-Free BAL
The injury groups studied had significantly different patterns of cytokine responses in BAL (Fig. 5). Levels of the proinflammatory cytokines TNFα (measured either by enzyme-linked immunosorbent assay (ELISA) as total immunoreactive protein or by bioassay as the biologically-active unbound form) and IL-1β, () were greater for rats given LC + CASP compared with LC alone at 5 and 24 hours post-injury (Fig. 5A–C). Levels of TNFα in BAL were not statistically different between rats given LC + CASP and CASP, but levels of IL-Iβ were higher in rats given LC + CASP compared with CASP at 24 hours (P < 0.004, Fig. 5C). Levels of the down-modulatory cytokine IL-10 in BAL differed significantly between all injury groups. At both 5 and 24 hours post-injury, rats with LC-containing injury (LC + CASP or LC alone) had higher levels of IL-10 in BAL than rats given CASP or uninjured control rats (Fig. 5D). At 5 hours post-injury, IL-10 levels in BAL from rats given LC + CASP were significantly greater than for either CASP or LC alone (P < 0.003-0.004, Fig. 5D). Further analysis by two-way ANOVA indicated that LC + CASP had a synergistic interaction of the two component injuries for increasing IL-10 levels at this 5 hours post-injury time point (P < 0.0001). Measurements of two other cytokines (IFNγ and IL-6) in BAL indicated that these mediators were present only in relatively small amounts for all injury groups, and did not identify any significant intergroup differences (data not shown).
Fig. 5.
Cell-free BAL samples were analyzed by ELISA (A), (C), and (D) or by cytotoxicity bioassay (B) for the concentrations (pg/ml) of TNFα(A), (B), IL-1β (C), and IL-10 (D). Levels of TNFα, IL-1β, and IL-10 in uninjured control rats were at or below the lower limit of detection of the assays used. Data are mean ± SEM for n = 6–12 rats. The same statistical analyses as in Fig. 1 were performed. *P < 0.002 compared with uninjured controls; **P < 0.003 compared with LC alone; ***P < 0.004 compared with CASP alone.
chemokine levels in cell-free bal
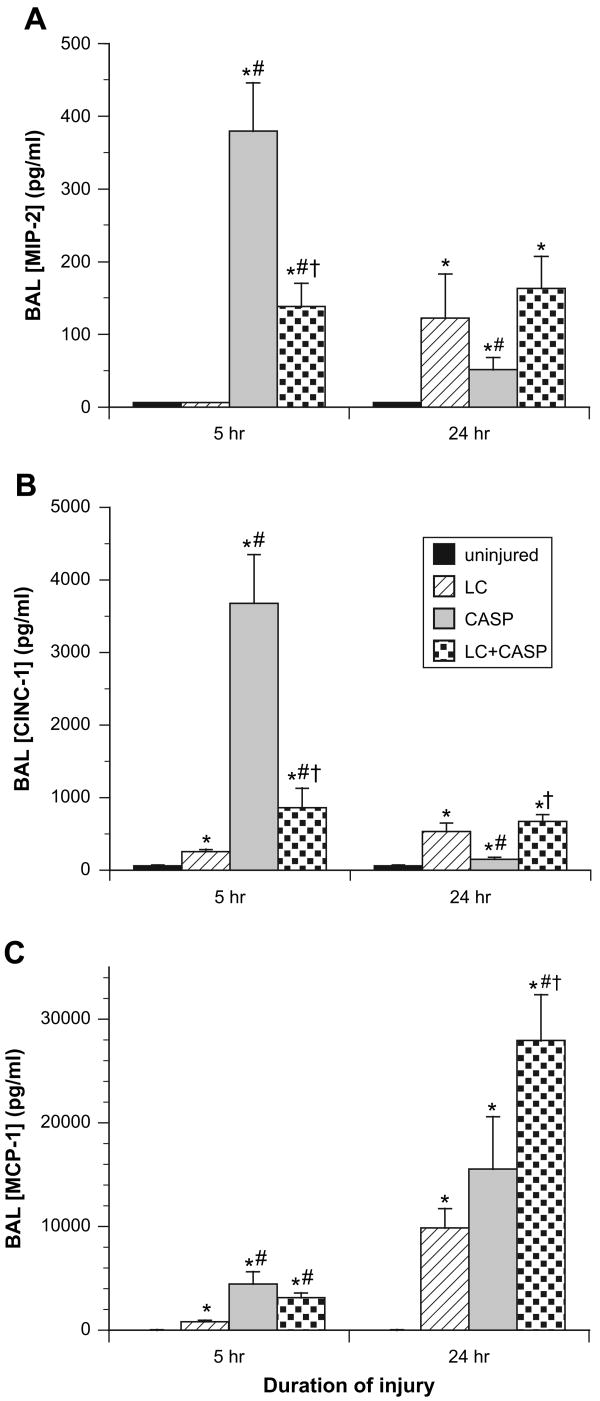
The three injury groups all had greater levels of the CXC chemokines (MIP-2, CINC-1) and the CC chemokine MCP-1 compared with uninjured controls (P < 0.002, Fig. 6). However, the quantitative levels of these chemokine mediators in BAL differed significantly among the injury groups themselves. At 5 hours post-injury, rats given LC + CASP or CASP had higher levels of both MIP-2 and CINC-1 in BAL compared with rats given LC alone (P < 0.007, Fig. 6A and B). At this early 5-hour time point, rats given CASP also had greater levels of these CXC chemokines than rats given LC + CASP (P < 0.002). However, at 24 hours post-injury, levels of MIP-2 and CINC-1 in BAL decreased for rats given CASP, and concentrations of CINC-1 were significantly higher for rats given LC + CASP versus CASP (Fig. 6B, P < 0.002). Levels of MCP-1 in BAL were greater at 5 and 24 hours for rats given LC + CASP compared with LC alone (Fig. 6C, P < 0.007). Rats given CASP also had higher levels of MCP-1 at 5 hours compared with rats given LC alone (P < 0.007, Fig. 6C). At 24 hours post-injury, rats given LC + CASP had the highest levels of MCP-1, significantly greater than rats given CASP (P < 0.002, Fig. 6C).
Fig. 6.
Levels of selected chemokines in BAL. Cell-free BAL samples were analyzed by ELISA for the concentrations (pg/mL) of MIP-2 (A), CINC-1 (B), and MCP-1 (C). Chemokine levels in uninjured controls were at or below the lower limit of detection of the assays used. Data are mean ± SEM for n = 6–12 rats. The same statistical analyses as in Fig. 1 were performed. *P < 0.002 compared with uninjured controls; **P < 0.007 compared with LC alone; ***P < 0.002 compared with CASP alone.
Discussion
This study has utilized a recently developed rat model to examine lung injury severity and innate pulmonary inflammation in rats with concurrent contusion and aspiration (LC + CASP) compared with the component injuries alone. The results of our experiments support the primary hypothesis that rats given LC + CASP had increased lung injury severity and inflammation compared with LC alone. In addition, while a significant amount of inflammatory response could be attributed to the CASP component, the results do support the secondary hypothesis that there were distinguishing features in the overall innate pulmonary inflammatory responses exhibited by the three insults studied (LC + CASP, LC, and CASP). The largest differences in inflammation were found in comparing LC alone with either LC + CASP or with CASP. Fewer differences in lung injury and inflammation were found between LC + CASP and CASP, but significant variations between these two insults were still present. Specific experimental findings are summarized and discussed in more detail below.
Increases in lung injury severity and inflammation in rats given LC + CASP compared with LC alone were documented by a variety of measurements. At 5 and 24 hours post-injury, rats given LC + CASP had more severe injury based on lower PaO2/FiO2 ratios, decreased lung volumes and compliance, and higher BAL albumin concentrations compared with rats given LC alone (Fig. 1 and Fig. 2). Rats with the LC + CASP combination insult also had a significant increase in pulmonary inflammation compared with rats given LC alone based on increased total WBCs in BAL at 5 hours post-injury (Fig. 3A), increased PMNs in BAL at 5 and 24 hours post-injury (Fig. 3B), increased whole lung MPO activity at 5 and 24 hours (Fig. 4), and increased levels of early response proinflammatory cytokines (TNFα and IL-1β) in BAL at 5 and 24 hours (Fig. 5A–C). BAL levels of chemokines (MIP-2 and CINC-1 at 5 hours and MCP-1 at 5 and 24 hours) were also increased for rats given LC + CASP compared with LC alone (Fig. 6), as were levels of the modulatory cytokine IL-10 at 5 hours (Fig. 5C). Increases in BAL levels of IL-10 at 5 hours in rats with the combination insult of LC + CASP were synergistic (interactive) compared with the component injuries of LC or CASP alone (Fig. 5D).
The increased lung injury severity and inflammation in rats with LC + CASP compared with LC alone was consistent with major contributions from the added CASP component of the combination insult. Rats given CASP alone were found to have significantly decreased oxygenation (Fig. 1A) and reduced pulmonary volumes and compliance (Fig. 2) at 24 hours post-insult compared with those given LC. Rats given CASP versus LC also had more severe inflammation based on increased PMN's in BAL (5 and 24 hours, Fig. 3B), increased whole lung MPO levels (5 and 24 hours, Fig. 4), and increased BAL concentrations of TNFα (5 and 24 hours, Fig. 5A and B), IL-1β (5 and 24 hours, Fig. 5C), MIP-2 (5 hours, Fig. 6A), CINC-1 (5 hours, Fig. 6B), and MCP-1 (5 hours, Fig. 6C). Mechanistically, abnormalities in lung compliance such as those found here in all three injury groups (Fig. 2) are frequently related to surfactant dysfunction [20, 21]. In agreement with this interpretation, we have previously shown that surfactant dysfunction based on reduced large aggregate content and impaired surface activity is present in rats injured with CASP [17]. We have also recently shown that surfactant dysfunction is present in rats with LC injury, and that the severity of surfactant abnormalities increases in the combination injury of LC + CASP [22].
We have previously shown that lung injury following isolated LC in rats induced at a chest impact energy level similar to that used here is relatively self-limiting, with a return to near normal levels of arterial oxygenation by 24 hours post-contusion and in BAL albumin concentrations by 48 hours post-contusion [10, 11]. Clinically, many patients diagnosed with pulmonary contusion following blunt chest trauma develop transient arterial hypoxemia that recovers in 24 to 48 hours, similar to these rodent findings. However, about 25% of patients with pulmonary contusion have a more severe form of disease that manifests as ALI/ARDS and/or subsequent ventilator-associated pneumonia (VAP), and requires prolonged respiratory support with substantial mortality and morbidity [1, 3]. Our findings that the combination insult of LC + CASP in rats leads to increased lung injury and inflammation relative to LC alone supports the possibility that gastric aspiration may be an important factor in at least a subgroup of patients with pulmonary contusion who progress to severe ALI/ARDS. In the present study, rats given LC + CASP had increased BAL albumin levels at 24 hours compared with rats given either LC or CASP alone. Mean levels of BAL albumin reflective of permeability injury were actually decreased in rats with LC alone or CASP alone at 24 hours compared with 5 hours, while mean levels of BAL albumin were still increasing in rats with LC + CASP at 24 hours, indicating progressive injury (Fig. 1B).
Although rats given LC + CASP and CASP had a number of similarities in their patterns of inflammation as noted earlier, measurable differences in cytokine/chemokine levels were still present for these conditions. In particular, rats given LC + CASP versus CASP had significantly increased levels of IL-10 (5 and 24 hours), IL-1β (24 hours), CINC-1 (24 hours), and MCP-1 (24 hours), and significantly decreased levels of MPO (5 hours), MIP-2 (5 hours), and CINC-1 (5 hours). Mechanisms underlying specific differences in inflammatory mediators for LC + CASP versus CASP were not examined in our study. However, one possibility for the lower levels of MPO, MIP-2, and CINC-1 found at 5 hours in rats given LC + CASP versus CASP (Fig. 5 and Fig. 6) is that contusion-induced blood in the lungs may have acted as a buffering solution to reduce some of the caustic effects of low pH aspiration during the initial phase of lung injury compared with the case of CASP aspiration alone.
Increases in early inflammation in animals given CASP are thought to be associated with its acid component, which generates an acute aspiration pneumonitis. We have previously shown that rats injured with CASP have a more rapid onset of inflammation compared with rats given nonacidified gastric particles alone [12–14, 16]. However, the particle component of CASP causes a prolonged inflammatory response apparent at 24 hours post-insult. It is thus likely that both acid-induced chemical pneumonitis and gastric particle-induced inflammation from CASP aspiration interacted with mechanical tissue trauma to exacerbate and prolong inflammation in rats with LC + CASP compared with LC alone (Fig. 5 and Fig. 6). In terms of cell-based inflammatory responses, previous work from our laboratory has shown that depleting neutrophils with Vinblastine can improve isolated LC injury in rats [11]. Results here were consistent with a similar prominent role for neutrophils in inflammatory injury in rats given LC + CASP, since animals receiving this combination insult had increased neutrophils in BAL (Fig. 3B) and increased whole lung MPO activity as a marker of granulocyte activity and leukostasis [18] compared with rats given LC alone (Fig. 4).
Further studies are needed to define the mechanistic significance of different mediators in the inflammatory response following pulmonary contusion and/or aspiration. One mediator of particular importance in rats given LC + CASP appeared to be IL-10, which underwent a synergistic increase at 5 hours in these combination injury animals compared with those given either of the component insults alone (Fig. 5D). Levels of IL-10 in BAL were also significantly elevated at 24 hours in rats given LC + CASP or LC compared with those given CASP or uninjured controls (Fig. 5D). The finding that IL-10 was increased at 24 hours in rats with LC alone compared with uninjured controls differs from a previous report from our laboratory that utilized a less sensitive assay for this cytokine [11]. IL-10 has been shown to be an important immuno-modulator in our prior work, predicting the severity of aspiration lung injury in rats [13]. Increases in MCP-1 found in rats with LC + CASP and CASP compared with LC (Fig. 6C) may also have mechanistic significance. The recruitment of monocytic cells that produce more MCP-1 than resident macrophages has been reported to be a sensitive biomarker for severe, sustained acute lung injury, and associated poor outcome in patients with ARDS [23]. MCP-1 has also been shown to be important in survival following CASP aspiration in mice [12].
One practical ramification of the distinct patterns of pulmonary inflammation found here for rats given LC + CASP, LC, and CASP concerns the potential for developing future diagnostic or prognostic paradigms based on cytokine/chemokine profiles in BAL in analogy with our prior work differentiating different kinds of gastric aspirates [24]. The present results support the potential for eventually developing related statistical predictive biomarker tests to allow more specific early diagnosis of LC + CASP, LC, and CASP in blunt chest trauma patients. For example, because the onset of inflammation in gastric aspiration is earlier and more pronounced than in pulmonary contusion, a biomarker profile showing increased rapid-onset inflammation in thoracic trauma patients diagnosed with contusion may be indicative of superimposed unwitnessed gastric aspiration. However, research elucidating specific mediators having particular predictive power for humans is needed before such approaches are feasible. Moreover, for clinical applications, biomarker profiles in blood have significant potential practical advantages compared with mediator profiles in BAL as assessed here.
In summary, the results of this study support the hypothesis that the combined insults of pulmonary contusion plus gastric aspiration (acid/particulates) produce more severe lung injury with increased inflammation at 5 and 24 hours compared with contusion alone. Given the significant potential for unwitnessed gastric aspiration in the setting of trauma, this concurrent insult may be an important contributor to clinical cases of blunt trauma-induced pulmonary contusion that are associated with severe or progressive impairments in respiration and the development of ALI/ARDS.
Acknowledgments
The authors gratefully acknowledge the support of the National Institutes of Health grants K08 GM-73826 (KR), DC-14685 (KR), HL-48889 (BAD, PRK), and the support of the Buswell foundation at the University at Buffalo (KR).
References
- 1.Cohn SM. Pulmonary contusion: Review of the clinical entity. J Trauma. 1997;42:973. doi: 10.1097/00005373-199705000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Lewis FR. Thoracic trauma. Surg Clin North Am. 1982;62:97. doi: 10.1016/s0039-6109(16)42636-8. [DOI] [PubMed] [Google Scholar]
- 3.Miller PR, Croce MA, Bee TK, et al. ARDS after pulmonary contusion: Accurate measurement of contusion volume identifies high-risk patients. J Trauma. 2001;51:223. doi: 10.1097/00005373-200108000-00003. discussion 229. [DOI] [PubMed] [Google Scholar]
- 4.Notter RH, Finkelstein JN, Holm BA, editors. Lung Injury: Mechanisms, pathophysiology and therapy. New York: Marcel Dekker; 2005. [Google Scholar]
- 5.Treggiari MM, Hudson LD, Martin DP, et al. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 2004;32:327. doi: 10.1097/01.CCM.0000108870.09693.42. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer L, Craven KD, Forkert L, et al. Pathophysiology of pulmonary contusion in dogs. J Appl Physiol. 1979;47:718. doi: 10.1152/jappl.1979.47.4.718. [DOI] [PubMed] [Google Scholar]
- 7.Van Eeden SF, Klopper JF, Alheit B, et al. Ventilation-perfusion imaging in evaluating regional lung function in nonpenetrating injury to the chest. Chest. 1989;95:632. doi: 10.1378/chest.95.3.632. [DOI] [PubMed] [Google Scholar]
- 8.Lockey DJ, Coats T, Parr MJ. Aspiration in severe trauma: A prospective study. Anesthesia. 1999;54:1097. doi: 10.1046/j.1365-2044.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 9.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 10.Raghavendran K, Davidson BA, Helinski JD, et al. A rat model for isolated bilateral lung contusion from blunt chest trauma. Anesthesia and Analgesia. 2005;101:1482. doi: 10.1213/01.ANE.0000180201.25746.1F. [DOI] [PubMed] [Google Scholar]
- 11.Raghavendran K, Davidson BA, Woytash JA, et al. The evolution of isolated, bilateral lung contusion from blunt chest trauma in rats: Cellular and cytokine responses. Shock. 2005;24:132. doi: 10.1097/01.shk.0000169725.80068.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghavendran K, Davidson BA, Mullan BA, et al. Acid and particulate induced aspiration injury in mice: Role of MCP-1. Am J Physiol: Lung Cell Mol Physiol. 2005;289:L134. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 13.Knight PR, Davidson BA, Nader ND, et al. Progressive, severe lung injury secondary to the interaction of insults in gastric aspiration. Exp Lung Res. 2004;30:535. doi: 10.1080/01902140490489162. [DOI] [PubMed] [Google Scholar]
- 14.Davidson BA, Knight PR, Helinski JD, et al. The role of tumor necrosis factor-alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology. 1999;91:486. doi: 10.1097/00000542-199908000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Knight PR, Rutter T, Tait AR, et al. Pathogenesis of gastric particulate lung injury: A comparison and interaction with acidic pneumonitis. Anesth Analg. 1993;77:754. doi: 10.1213/00000539-199310000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Shanley TP, Davidson BA, Nader ND, et al. Role of macrophage inflammatory protein-2 in aspiration-induced lung injury. Crit Care Med. 2000;28:2437. doi: 10.1097/00003246-200007000-00041. [DOI] [PubMed] [Google Scholar]
- 17.Davidson BA, Knight PR, Wang Z, et al. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol. 2005;288:L699. doi: 10.1152/ajplung.00229.2004. [DOI] [PubMed] [Google Scholar]
- 18.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985;59:1978. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Holm BA, Matalon S, et al. Surfactant activity and dysfunction in lung injury. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: Mechanisms, pathophysiology, and therapy. Boca Raton: Taylor Francis Group, Inc; 2005. pp. 297–352. [Google Scholar]
- 21.Notter RH. Lung surfactants: Basic science and clinical applications. New York: Marcel Dekker, Inc; 2000. [Google Scholar]
- 22.Raghavendran K, Knight PR, Wang Z, et al. Surfactant dysfunction in lung contusion with and without superimposed gastric aspiration in a rat model. Shock. doi: 10.1097/SHK.0b013e3181673fc5. (in press) (ePub date Feb 21, 2008, PMID: 18323743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosseau S, Hammerl P, Maus U, et al. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279:L25. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- 24.Hutson AD, Davidson BA, Raghavendran K, et al. Statistical prediction of the type of gastric aspiration lung injury based on early cytokine/chemokine profiles. Anesthesiology. 2006;104:73. doi: 10.1097/00000542-200601000-00013. [DOI] [PubMed] [Google Scholar]