Abstract
Background
Response to neoadjuvant chemotherapy is one of the most powerful prognostic factors for extremity osteosarcoma (OS). [F-18]-fluorodeoxy-D-glucose (FDG) positron emission tomography (PET) is a non-invasive imaging modality to predict histopathologic response. To determine the prognostic value of FDG PET response for progression-free survival (PFS) in OS, we reviewed the University of Washington Medical Center experience.
Methods
Forty patients with extremity OS were evaluated by FDG PET. All patients received neoadjuvant and adjuvant chemotherapy. FDG PET standard uptake values before (SUV1) and after (SUV2) neoadjuvant chemotherapy were analyzed and correlated with histopathologic response.
Results
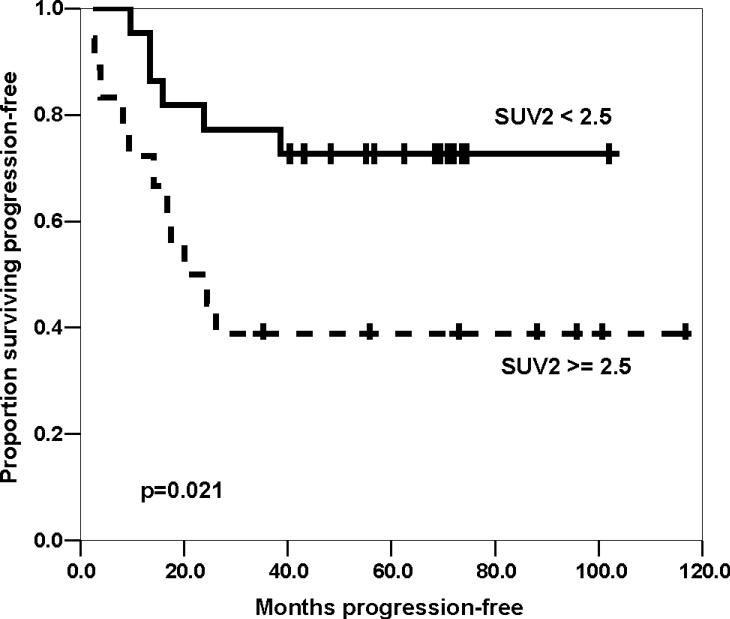
The median SUV1, SUV2, and ratio of SUV2 to SUV1 (SUV2:1) were 6.8 (range 3.0−24.1), 2.3 (1.2−12.8), and 0.36 (0.12−1.10), respectively. Good FDG PET response was defined as SUV2 < 2.5 or SUV2:1 ≤ 0.5. FDG PET response by SUV2 or SUV2:1 were concordant with histologic response in 58% and 68% of patients, respectively. SUV2 was associated with outcome (four-year PFS 73% for SUV2 < 2.5 versus 39% for SUV2 ≥ 2.5, p=0.021. Initial disease stage and histologic response were both associated with outcome.
Conclusion
FDG PET imaging of extremity OS correlated only partially with histologic response to neoadjuvant chemotherapy. SUV2 < 2.5 was associated with improved PFS. Future prospective studies to determine whether FDG PET imaging is a predictor of outcome independent of initial disease stage are warranted.
Keywords: osteosarcoma, fluorodeoxyglucose positron emission tomography, outcome
INTRODUCTION
Osteosaroma (OS) is the most common malignant bone tumors in children and young adults, with an incidence of 600 cases among children, adolescents, and young adults each year in the United States [1-3]. Multi-agent chemotherapy and surgery have dramatically improved the prognosis for OS, resulting in 60−70% progression-free survival (PFS) for children with localized disease [2-7]. In addition to facilitating limb-sparing resection, neoadjuvant chemotherapy allows radiographic and histologic assessment of chemotherapy efficacy on the tumor. Histologic response, measured by the percentage of viable tumor cells remaining after neoadjuvant chemotherapy [8,9], has prognostic value in predicting PFS [9,10]. If a favorable response to therapy is anticipated, limb-sparing surgical resection may be more feasible [5,11]. However, OS usually does not significantly change in size in response to chemotherapy, making computed tomography (CT) or magnetic resonance imaging (MRI) insensitive methods to determine chemotherapy-responsiveness [12]. Instead, a non-invasive surrogate marker of histologic response would be useful to assess the efficacy of pre-operative chemotherapy.
[F-18]-fluorodeoxy-D-glucose (FDG) positron emission tomography (PET) is functional imaging method with the potential to assess tumor response to chemotherapy. Malignant cells avidly take up and retain FDG, a glucose analog. We have previously reported the correlation between FDG PET changes and histologic response in pediatric Ewing sarcoma family of tumors (ESFT) and OS [13]. We have also reported an association between the reduction in SUV and outcome following neoajduvant doxorubicin-containing chemotherapy in extremity soft tissue sarcomas [14] and ESFT [15]. To determine the value of FDG PET response for predicting outcome in extremity OS, we reviewed the experience at our center with both pediatric and young adult patients.
PATIENTS AND METHODS
Patient population
This is an analysis of patients presenting to the Children's Hospital and Regional Medical Center (CHRMC) or University of Washington Medical Center (UWMC) with extremity OS who were enrolled prospectively in a study of FDG PET in sarcomas. All patients (or parents for minors) provided written informed consent for participation in the PET study and medical record review as approved by the UWMC Institutional Review Boards of Human Subjects and Radiation Safety in accordance with institutional and federal guidelines. All eligible patients with extremity OS who received treatment at CHRMC or UWMC between July 1, 1995 and August 1, 2004 underwent evaluation by FDG PET imaging. This series included 38 patients who received both chemotherapy and surgery at CHRMC or UWMC and two patients referred to CHRMC for surgical resection after neoadjuvant chemotherapy at other institutions. Patients who did not have FDG PET both before and after neoadjuvant chemotherapy were excluded. Patients underwent PET imaging no more than one week prior to the initiation of chemotherapy. All patients received neoadjuvant chemotherapy including cisplatin and doxorubicin, in most cases with the addition of high-dose methotexate [4]. Other treatment regimens included cisplatin, doxorubin, and ifosfamide [16], and cisplatin, doxorubicin, ifosfamide, and high-dose methotrexate [4]. One course of therapy was defined as a treatment cycle of doxorubicin-containing chemotherapy with or without high-dose methotrexate. PET imaging was repeated after the induction course of chemotherapy prior to surgical resection. Although the timing of the second FDG PET was not standardized, 30 (75%) patients had repeat imaging after two courses of chemotherapy. Histologic response to neoadjuvant chemotherapy was evaluated based upon the grading system by Salzer-Kuntschik M, et al for osteosarcoma [9]. For each patient, the percentage of viable tumor (calculated from multiple samples from the resected bone and surrounding soft tissue specimen) was used to determine the percent viable tumor cells using standard histopathologic analysis. Favorable response to chemotherapy was defined as ≤ 10% viable tumor cells and unfavorable response to chemotherapy was defined as > 10% viable tumor cells.
PET Imaging
Detailed methods for PET imaging of sarcoma patients have been previously published [17,18]. Briefly, patients fasted for at least two hours before imaging. Patients received 7−10 mCi of FDG intravenously over two minutes. Blood glucose level was recorded in 30 of the 40 patients before administration of FDG and was < 120 mg/dl in all patients. Intra-patient blood glucose levels before injection of FDG varied by < 20% in 24 out of 30 patients. After a 45 minute equilibration period during which the patient rested, both emission and transmission scans were obtained to generate attenuation corrected images over the known tumor site using a GE Advance Positron Tomograph (General Electric, Waukesha, Wisconsin). Typically, the tumor extent was captured in two adjacent 15 cm fields of view. Reconstructed data was rendered into three-dimensional images using a Hanning filter at a resolution of 4.2 mm. The three dimensional image sets were available for review in slice thicknesses of 4.2 to 12.0 mm. Regions of interest for determination of tumor SUV were then hand drawn around the area of tumor uptake, using plain film, MR, and CT scan for reference. The FDG PET image was visually inspected for heterogeneity in tumor uptake. The tumor SUV was automatically calculated by the tomograph software, and, after careful assessment of the SUV values throughout the tumor, the maximum tumor SUV, rather than the average SUV, was recorded for analysis. SUV1 was defined as the maximal SUV obtained before neoadjuvant chemotherapy, SUV2 was defined as the maximal SUV obtained after neoadjuvant chemotherapy, and SUV2:1 was defined as the ratio of SUV2 to SUV1.
Statistical analysis
Statistical analysis of PFS was performed using the Kaplan-Meier method for calculating survival curves and 95% confidence intervals (CI) [19]. PFS was defined as the time from initial diagnosis to either disease progression or death from any cause. Patients who had not developed progression or died were censored at the date of last contact. Differences in PFS among groups defined by patient or treatment characteristics were analyzed using the log-rank test [20]. PFS data were analyzed as of June 17, 2008 using SPSS for Windows version 13.0 (SPSS Inc, Chicago, IL).
RESULTS
The clinical characteristics of 40 OS patients are presented in Table 1. Included in this series are clinical and histologic response data from 8 patients previously reported [13]. All patients had surgical resection of the primary tumor. Thirty patients had SUV2 obtained less than two weeks before surgery. Only five patients received additional chemotherapy courses after SUV2 before surgical resection, in each case due to problems with surgical scheduling or availability of an appropriate limb-salvage implant. Four patients received one course and one received two courses.
Table 1.
Patient characteristics and timing of FDG PET, surgery
| Patient characteristic | Number of patients/value |
|---|---|
| Median age, years (range) | 15.1 (7.1−31.0) |
| Anatomic site | |
| Femur | 20 |
| Tibia | 13 |
| Humerus | 5 |
| Fibula | 2 |
| Metastases at diagnosis | |
| Present | 6 |
| Absent | 34 |
| Chemotherapy treatment | |
| CDM | 27 |
| CDI | 7 |
| CDMI | 5 |
| CDMIE | 1 |
| Median weeks from SUV1 to SUV2 (range) | 10.3 (6.3−15.7) |
| Median weeks from SUV2 to surgery (range) | 1.1 (0.1−11.3) |
| Median therapy courses from SUV1 to SUV2 (range) | 2 (2−4) |
| Median therapy courses from SUV2 to surgery (range) | 0 (0−2) |
Abbreviations: FDG PET: [F-18]-fluorodeoxy-D-glucose positron emission tomography; C, cisplatin; D, doxorubicin; M, methotrexate; I, ifosfamide; E, etoposide; SUV1, maximum standard uptake value prior to chemotherapy; SUV2, maximum standard uptake value after chemotherapy
Tables 2 and 3 summarize the FDG PET imaging and histologic response data. Only one patient had a higher SUV2 than SUV1. Neoadjuvant chemotherapy resulted in a favorable response to chemotherapy (≤ 10% viable tumor) in 48% of patients. Favorable FDG PET response (SUV2 < 2.5 or SUV2:1 ≤ 0.5) were concordant with favorable histologic response (≤ 10% viable tumor) in 58% and 68% of patients, respectively.
Table 2.
SUV1, SUV2, SUV2:1, and percent viable tumor by tumor histology
| Clinical feature | Value |
|---|---|
| Pre-chemotherapy | |
| Median SUV1 (range) | 6.8 (3−24) |
| Post-chemotherapy | |
| Median SUV2 (range) | 2.3 (1.2−12.8) |
| Reduction in SUV | |
| Median SUV2:1 (range) | 0.36 (0.12−1.10) |
| Histologic evaluation | |
| Favorable (≤ 10% viable tumor), n | 19 |
| Unfavorable (> 10% viable tumor), n | 21 |
Abbreviations: SUV1, maximum standard uptake value prior to chemotherapy; SUV2, maximum standard uptake value after chemotherapy; SUV2:1, ratio of SUV2 to SUV1.
Table 3.
Histologic response compared to SUV2 and SUV2:SUV1
| ≤ 10% viable tumor | > 10% viable tumor | |
|---|---|---|
| SUV2 | ||
| ≥ 2.5 | 7 | 11 |
| < 2.5 |
12 |
10 |
| SUV2:1 | ||
| > 0.5 | 2 | 10 |
| ≤ 0.5 | 17 | 11 |
Abbreviations: SUV1, maximum standard uptake value prior to chemotherapy; SUV2, maximum standard uptake value after chemotherapy; SUV2:1, ratio of SUV2 to SUV1.
All patients resumed chemotherapy post-operatively. Four patients received alternative post-operative chemotherapy: two patients with tumor progression prior to surgery (both confirmed to have poor histologic response after receiving cisplatin, doxorubicin, methotrexate, and (in one case) ifosfamide pre-operatively), one patient treated with escalated cumulative dose of doxorubicin (600 mg/m2) because of poor histologic response by protocol design, and one patient treated with ifosfamide in place of cisplatin post-operatively due to severe hearing loss and poor histologic response. All 36 other patients had no modification of post-operative chemotherapy due to histologic response.
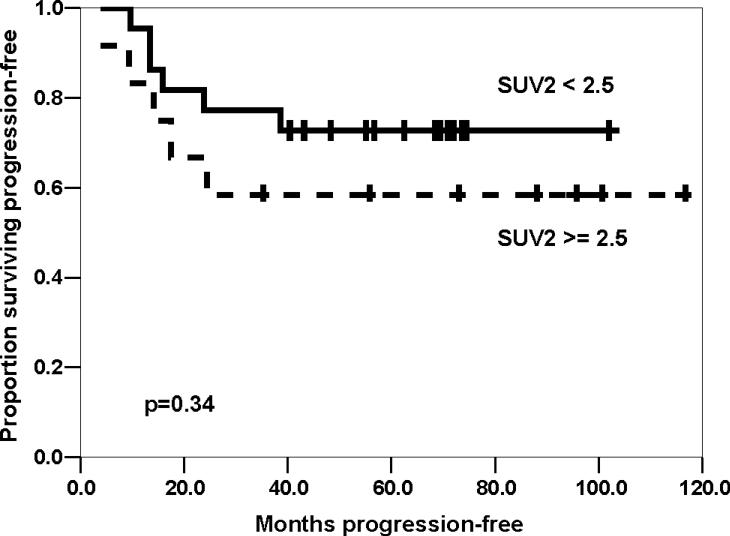
Seventeen of the 40 patients have experienced disease recurrence, including thirteen at metastatic sites only, two with combined metastatic and local sites, and two local sites only. The median SUV2 for patients with local only or combined recurrence was 2.1 (range 1.6−4.4). One patient died from secondary acute myeloid leukemia without osteosarcoma relapse; no other patient died prior to disease recurrence. The median follow-up for patients who survived without progression was 69 months (range 35−117 months). The four-year PFS for all patients was estimated to be 57% (95% CI, 41−73%, Table 4). Univariate analysis of potential prognostic factors (Table 4) demonstrated that improved PFS was associated with non-metastatic disease at initial diagnosis and with SUV2 < 2.5 for all patients. However, when metastatic patients were excluded, the difference between PFS by SUV2 was not statistically significant. Neither SUV1 > 6 nor SUV2:1 ≤ 0.5 were associated with PFS among all patients or among patients with only localized disease (Table 4). A strong trend towards improved PFS was seen with favorable histologic response (≤ 10% viable tumor): four-year PFS 74% versus 42%, p=0.061. When patients with metastases at diagnosis were excluded, favorable histologic response showed a modest trend toward improved PFS (four-year PFS 78% versus 56%, p=0.18).
Table 4.
Univariate analysis of progression-free survival
| Variable | Number of patients | 4-year PFS (95% CI) | p value |
|---|---|---|---|
| Overall | 40 | 57% (41−73%) | |
| Initial stage | |||
| Localized | 34 | 67% (51−83%) | < 0.001 |
| Metastatic | 6 | 0% (0−46%) | |
| Histologic response | |||
| ≤ 10% viable tumor | 19 | 74% (54−94%) | 0.061 |
| > 10% viable tumor | 21 | 42% (20−62%) | |
| Histologic response (Localized only) | |||
| ≤ 10% viable tumor | 18 | 78% (58−98%) | 0.18 |
| > 10% viable tumor | 16 | 56% (31−81%) | |
| SUV2 | |||
| < 2.5 | 22 | 73% (54−92%) | 0.021 |
| ≥ 2.5 | 18 | 39% (16−62%) | |
| SUV2 (Localized only) | |||
| < 2.5 | 22 | 73% (54−92%) | 0.34 |
| ≥ 2.5 | 12 | 58% (30−86%) | |
| SUV2:1 | |||
| ≤ 0.5 | 28 | 57% (36−78%) | 0.9 |
| > 0.5 | 12 | 58% (30−86%) | |
| SUV2:1 (Localized only) | |||
| ≤ 0.5 | 23 | 69% (49−89%) | 0.62 |
| > 0.5 | 11 | 64% (35−93%) | |
| SUV1 | |||
| ≤ 6 | 19 | 63% (41−85%) | 0.41 |
| > 6 | 21 | 52% (30−74%) | |
| SUV1 (Localized only) | |||
| ≤ 6 | 19 | 63% (41−85%) | 0.6 |
| > 6 | 15 | 73% (40−96%) | |
Abbreviations: PFS, progression-free survival; CI, confidence interval; SUV1, maximum standard uptake value prior to chemotherapy; SUV2, maximum standard uptake value after chemotherapy; SUV2:1, ratio of SUV2 to SUV1.
DISCUSSION
Histologic response to neo-adjuvant chemotherapy is one of the strongest prognostic factors for OS [3-7, 10]. Histologic response has significant limitations, including the ability to assess only once and only after 10−15 weeks of initial chemotherapy. We have reported the utility of FDG PET imaging as a non-invasive method to assess response [13] to neoadjuvant chemotherapy and predicting the probability of recurrence for soft sarcomas [14,21] and ESFT [15]. The current report expands upon our previous series of pediatric bone sarcomas [13], with 32 additional patients including adults, and shows an association between SUV2 and PFS for extremity OS. In contrast to our observation in ESFT, SUV2 did not remain prognostically significant when patients with metastatic disease were excluded [15]. However, neither categorization of response (histologic or FDG PET) was completely predictive of patient outcome in our dataset. We have previously reported an association between SUV1 > 6 [22] and SUV2:1 in soft tissue sarcomas [14]. Neither association was observed in this cohort of OS patients. We previously observed an increase in SUV2 over SUV1 in 22% of soft tissue sarcoma patients [14]. In contrast, only 3% of OS patients experienced an increase in SUV2 over SUV1 after neoadjuvant chemotherapy, illustrating that FDG PET imaging characteristics may be histology-specific.
To our knowledge, the current report is the first to describe an association between FDG PET imaging and PFS for extremity OS. Our analysis is clearly limited by its relatively small sample size. The association between SUV2 and PFS will need confirmation in a larger, prospective study, particularly to determine if SUV2 < 2.5 is associated with improved PFS in patients with localized OS. The difference in predictive value of SUV2 in localized OS versus ESFT patients [15] suggests that FDG PET may be less useful in OS. Our small study population also prevents a multivariate analysis of other potential prognostic factors, such as tumor site and size [5]. Another limitation of our study was the diversity of treatment characteristics (including neo-adjuvant chemotherapy duration) of the study population. Minor differences in chemotherapy regimens or surgery could have influenced patient outcome, confounding the predictive value of FDG PET.
The concordance between histologic (≤ 10% viable tumor) and SUV2 (< 2.5) was less robust than our prior combined analysis of pediatric bone sarcoma patients [13]. We have previously speculated [15] on the potential explanations for the discordance between SUV2 and histologic assessments of response, including non-specific (but bioenergetically intense) inflammatory response and scarring around necrotic tumor. Histologic response is an averaged assessment in a representative plane, in contrast to the three-dimensional assessment of maximal remaining tumor activity measured by FDG PET. It is likely that histologic and FDG PET assessments of response are evaluating a similar biologic process (residual tumor cells after therapy) but with different methodologic limitations, generating related but non-concordant results.
The optimal timing of FDG PET to determine response remains to be defined. Changes in FDG metabolism can occur within 24 hours after administration of imatinib in gastrointestinal stromal tumor [23] and within 3−5 days after administration of AP23573, an inhibitor of the mammalian target of rapamycin, in OS and other sarcomas [24]. We have reported a preliminary analysis of a prospective study of high-grade soft tissue sarcoma patients by obtaining FDG PET imaging after six weeks (two courses) of therapy and correlating the results with those obtained immediately prior to surgery (after four courses), suggesting that FDG PET imaging changes are apparent after only two courses, and FDG PET imaging results after two and four courses of therapy are essentially concordant [21]. We are currently conducting two prospective studies with FDG PET imaging after only one course of treatment to explore the rapidity of FDG metabolism change in soft tissue and bone sarcomas. The Children's Oncology Group is also performing a similar study with intermediate-risk rhabdomyosarcoma (ARST0531), assessing FDG PET response after 3 weeks of chemotherapy.
FDG PET could have several potential clinical uses. First, orthopedic oncologist could identify patients who are more likely to have a favorable histologic response to neo-adjuvant chemotherapy. Such patients would be candidates for less aggressive limb-sparing resections. Patients predicted to have an unfavorable histologic response could be considered for more aggressive local surgery. However, our current data suggest that SUV2 is weakly correlated with histologic response. In contrast SUV2:1 was more useful, particularly in identifying patients with poor histologic response. Second, chemotherapy resistant OS could be identified earlier in the neo-adjuvant period by an unfavorable FDG PET response, leading to an alteration in systemic chemotherapy. The success of FDG PET-guided therapy modification assumes that FDG PET imaging changes occur early in treatment (as discussed above) and that alteration of systemic chemotherapy will improve the outcome of chemotherapy resistant OS. Non-randomized clinical trials have suggested that augmentation of chemotherapy in response to poor histologic response can improve outcome [7, 25]. However, others have refuted this observation [6, 26]. The current EURAMOS-1 trial addresses the benefit of response-adapted therapy with the randomized comparison of continued standard adjuvant chemotherapy versus standard adjuvant chemotherapy with the addition of ifosfamide and etoposide. It is possible that response-adapted chemotherapy would be more successful if FDG PET was able to identify chemotherapy resistant OS after only 3−5 weeks of neo-adjuvant chemotherapy rather than after 10 weeks of neoadjuvant and 4 weeks of adjuvant chemotherapy (as done on EURAMOS-1). Finally, FDG PET could be a surrogate marker of response for patients with recurrent disease or non-resectable metastases, such as distant bone sites. This would be particularly useful in phase II studies, where uni- or bi-dimensional measurements of response may underestimate the activity of a novel chemotherapy agent. The lack of radiographic response yet clear clinical benefit associated with FDG metabolic response with imatinib in the treatment of GIST clearly illustrated the limitations of anatomic imaging to assess response in sarcomas.
In summary, SUV2 < 2.5 after neoadjuvant chemotherapy for OS was associated with an improved PFS. The association was less robust than we observed with ESFT and lost statistical significance when metastatic OS patients were excluded. In our small series, neither SUV2 nor histologic response were precisely predictive of PFS. A larger prospective study is required to determine whether FDG PET imaging can complement histologic response as a prognostic factor for OS and determine whether FDG PET is a prognostic factor independent of initial stage. Additional areas of uncertainty include the optimal timing FDG PET and modification of treatment for patients at higher risk for disease recurrence.
Figure 1.
Kaplan-Meier estimated progression-free survival by maximal standard uptake value after chemotherapy (SUV2), for all patients.
Figure 2.
Kaplan-Meier estimated progression-free survival by maximal standard uptake value after chemotherapy (SUV2), excluding patients with metastases at diagnosis.
Acknowledgments
This manuscript is written in support of NIH/NCI Grants: CA87721 and CA65537
Footnotes
Financial disclosures: None All patients (or parents for minors) provided written informed consent.
REFERENCES
- 1.Gurney JG, Swenson AR, Bulterys M. Malignant bone tumors. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975−1995. National Cancer Institute, SEER Program; Besthesda: 1999. pp. 99–110. [Google Scholar]
- 2.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–989. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 3.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 4.Meyers P, Schwartz C, Krailo M, et al. Osteosarcoma: a randomized, prospective study of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremity or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of non-metastastic osteosarcoma of the extremity with pre- and postoperative chemotherapy, CCG-782: a report from the Children's Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Picci P, Ferrari S, et al. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities: results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993;72:3227–3238. doi: 10.1002/1097-0142(19931201)72:11<3227::aid-cncr2820721116>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma. Pathologic aspects in 20 patients after treatment with chemotherapy, en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14–18. [PubMed] [Google Scholar]
- 9.Salzer-Kuntschik M, Delling G, Beron G, et al. Morphological grades of regression in osteosarcoma. J Cancer Res Clin Oncol. 1983;106:21–24. doi: 10.1007/BF00625047. [DOI] [PubMed] [Google Scholar]
- 10.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12:423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
- 11.Picci P, Sangiorgi L, Rougraff BT, et al. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 12.Murphy WA. Imaging bone tumors in the 1990's. Cancer. 1991;67:1169–1176. doi: 10.1002/1097-0142(19910215)67:4+<1169::aid-cncr2820671511>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins DS, Rajendran JG, Conrad EU, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18] fluorodeoxyglucose positron emission tomography. Cancer. 2002;94:3277–3284. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 14.Schuetze SM, Rubin BP, Vernon C, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005;103:339–348. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins DS, Schuetze SM, Butrynski JE, et al. [F-18]-fluorodeoxy-D-glucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 16.Zalupski MM, Rankin C, Ryan JR, et al. Adjuvant therapy of osteosarcoma: a phase II trial, Southwest Oncology Group Study 9139. Cancer. 2004;100:818–825. doi: 10.1002/cncr.20021. [DOI] [PubMed] [Google Scholar]
- 17.Eary JF, Conrad EU, Bruckner JD, et al. Quantitative [F-18] fluorodeoxyglucose positron emission tomography in pretreatment evaluation and grading of sarcoma. Clin Cancer Res. 1998;4:1215–1220. [PubMed] [Google Scholar]
- 18.Folpe AL, Lyles RH, Sprouse JT, et al. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res. 2000;6:1279–1287. [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: I-analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuetze SM, Griffith KA, Rubin BP, et al. FDG PET but not RECIST agrees with histologic response of soft tissue sarcoma to neoadjuvant chemotherapy. Proc Am Soc Clin Oncol. 2005;23:817s. [Google Scholar]
- 22.Eary JF, O'Sullivan F, Powitan Y, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med 29. 2002:1149–1154. doi: 10.1007/s00259-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 24.Sankhala KK, Chawla SP, Iagaru A, et al. Early response evaluation of therapy with AP23573 (an mTOR inhibitor) using [18F]2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) scan. Proc Am Soc Clin Oncol. 2005;23:823s. [Google Scholar]
- 25.Rosen G, Marcove RC, Caparros B, et al. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Meyer PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]