Abstract
CD8+ T lymphocytes (CTL) play a role in controlling HIV/SIV infection. CTL antiviral activity is dependent on recognition of antigenic peptides associated with MHC class I molecules on infected target cells, and CTL activation can be impaired by Nef-mediated down-regulation of MHC class I molecules. We tested the ability of a series ofrhesus macaque CD8+ T-cell clones specific for the SIV Gag CM9 peptide to suppress SIV infection of autologous CD4+ T cells. We used a set of SIVmac239 viruses with either wild-type Nef or Nef mutations that impair MHC class I down-regulation. All CTL clones efficiently suppressed virus replication in cells infected with mutant viruses with altered Nef function, phenotypically MHC class Ihigh or MHC class Iintermediate. However, the ability of the clones to suppress virus replication was variably reduced in the presence of wild-type Nef (MHC Class Ilow) despite the observations that all CTL clones showed similar IFN-γ responses to titrated amounts of cognate peptide as well as to SIV-infected cells. In addition, the CTL clones showed variable CD107a (CTL degranulation marker) responses that did not correlate with their capacity to suppress virus replication. Thus, the clonal differences are not attributable to TCR avidity or typical effector responses, and point to a potential as yet unknown mechanism for CTL-mediated suppression of viral replication. These data emphasize that current assays for evaluating CTL responses in infected or vaccinated individuals do not fully capture the complex requirements for effective CTL-mediated control of virus replication.
Keywords: AIDS, Cytotoxic T cells, MHC class I, Nef, Virus suppression
Introduction
Major histocompatibility complex (MHC) Class I-restricted CD8+ T lymphocytes (CTL) are an important component of the immune response following infection of humans with the human immunodeficiency virus (HIV) (Borrow et al., 1994; Froebel et al., 1994; Gruters, van Baalen, and Osterhaus, 2002; Rowland-Jones et al., 2001) or non-human primates (NHP) with simian immunodeficiency virus (SIV) (Allen et al., 2002; Egan et al., 2000; Kuroda et al., 1999). Despite this response, the immune system ultimately fails to control the virus and virtually all untreated HIV-infected humans or SIV-infected rhesus macaques eventually develop AIDS. Several mechanisms of viral immune evasion and immune escape have been described, including mutation of major CTL epitopes (Allen et al., 2004; Allen et al., 2000; Barouch et al., 2002; Goulder et al., 2001; Johnson and Desrosiers, 2002; Leslie et al., 2004), impaired CTL responses due to upregulated expression of inhibitory receptors (Day et al., 2006; Trautmann et al., 2006) and loss of CD4+ T-cell help as a result of the direct cytopathic effects of the virus (Gandhi et al., 1998; Sousa et al., 2002). While these factors partly account for the lack of protection, alterations in the levels of expression of key molecules in the virally-infected cells necessary for effective recognition and clearance by virus-specific CTL seem to play an important role as well (Bell et al., 1998; Kestler et al., 1991; Pennington et al., 1997; Xu et al., 1997). This protection from CTL-mediated killing is thought to be mediated by a number of virus gene products, especially the accessory protein, Nef (Kestler et al., 1991; Kirchhoff et al., 1995; Rhodes et al., 2000).
Nef is a myristylated 27-kDa protein with diverse biological activities that include enhancement of viral particle infectivity and viral replication (Kestler et al., 1991; Sugimoto et al., 2003). Nef has also been shown to down-regulate the expression of a number of transmembrane molecules in host immune cells including the zeta chain of the T cell receptor (TCR) complex (Bell et al., 1998) and MHC class I on Nef transfected human T-cell lines (Brenner et al., 2006; Mangasarian et al., 1999; Munch et al., 2005; Schindler et al., 2004; Swigut et al., 2000). Given the requirement for viral peptides to be in complex with MHC class I molecules for virus-specific recognition by CD8+ CTL, Nef-mediated down-regulation of MHC class I molecules on HIV/SIV-infected cells might protect these cells from virus-specific CTL-mediated killing, while not affecting MHC class I molecules inhibiting NK cell-mediated lysis (Cohen et al., 1999; DeGottardi et al., 2008). Indeed, deletions in the Nef gene have been correlated with protection from progression to AIDS in HIV-infected individuals (Kirchhoff et al., 1995; Oelrichs et al., 2000; Salvi et al., 1998) and SIV-infected rhesus macaques (Kestler et al., 1991; Rud et al., 1994; Swigut et al., 2004). However, whether the reported slow progression to AIDS is due to the poor replicative capacity of the Nef defective virus, better CTL responses in the context of abolished MHC class I down-regulation, or both is unknown. A number of studies have suggested that the direct downregulatory effect of HIV-1 Nef on MHC class I expression by HIV-infected human cells is associated with significant loss in the virus suppressive capacity of HIV-1-specific CTL (Adnan et al., 2006; Collins et al., 1998; Fujiwara and Takiguchi, 2007; Schwartz et al., 1996; Tomiyama et al., 2002; Tomiyama et al., 2005; Ueno et al., 2008; Yang et al., 2002). The effects of SIV Nef on MHC class I expression have been mainly analyzed using human cell lines or whole human PBMC (Brenner et al., 2006; Munch et al., 2005; Swigut et al., 2000). Therefore, there is limited direct evidence of SIV Nef-mediated down-regulation of MHC class I expression on SIV-infected primary NHP CD4+ T cells (Sacha et al., 2007; Swigut et al., 2004) and its effect on CTL-mediated suppression of SIV replication.
The SIV-rhesus macaque system is an important model for understanding the pathology and immune response/control of immunodeficiency viruses. Therefore, the examination of SIV Nef-mediated MHC class I down-regulation in primary NHP CD4+ T cells and its impact on CTL-mediated recognition of virally-infected cells should help in evaluating vaccine candidates eliciting virus-specific CTL responses in NHP models of AIDS. To study this, we compared MHC class I expression on rhesus CD4+ T cells infected with wild type (WT) SIVmac239 (Nef/open) expressing a fully functional Nef (Kestler et al., 1991), a natural truncation mutant SIVmac239 (Nef/stop) (Kestler et al., 1991), or a series of engineered SIVmac239 viruses with different mutations in the Nef sequence that alter its MHC Class I down-regulatory properties (Schindler et al., 2004). We further evaluated the impact of different levels of MHC class I down-regulation mediated by these viruses on the capacity of different SIV-specific CTL clones, generated against the same Gag epitope, to suppress virus replication in infected CD4+ T cells. We found clonal differences in the ability of different virus-specific CTL clones to suppress SIV replication in target cells exhibiting Nef-mediated MHC class I down-regulation that did not correlate with typical effector parameters.
Results
SIV Nef myristylation required for Nef-mediated MHC class I down-regulation
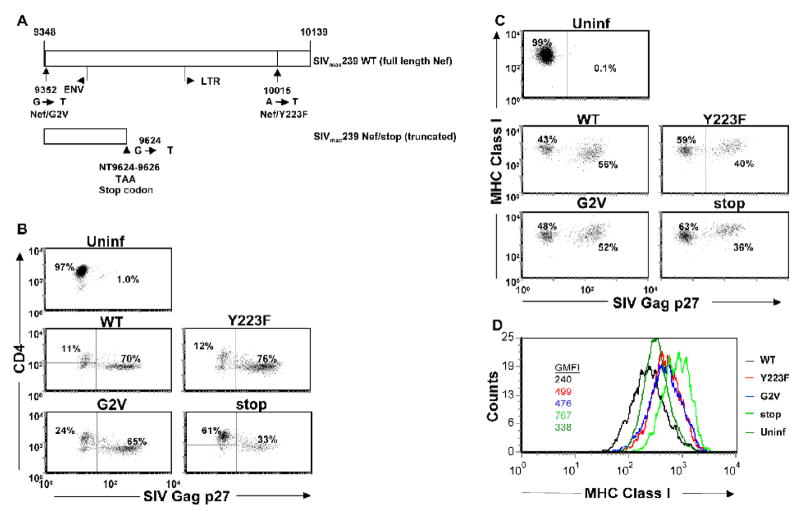
To study the dynamics of SIV Nef, MHC class I expression and CTL-mediated virus suppression in rhesus macaque T cell cultures, we constructed two Nef SIV mutants that modulated the function of Nef. To eliminate Nef function but retain Nef expression for future studies with CTL specific for Nef, we produced Nef/G2V that contains a point mutation abolishing the myristylation of Nef, so that the expressed Nef protein cannot associate with the plasma membrane. The Nef/G2V mutation has been reported to render Nef nonfunctional when transfected into human T cell lines (Schindler et al., 2004). The Y223F mutation only affects the MHC class I down-regulatory properties of Nef, allowing us to focus solely on this function and rule out other properties of Nef (Schindler et al., 2004). Noteworthy, Munch et al. reported rapid reversion of the Y223F mutant in infected rhesus macaques (Munch et al., 2002). We also included the SIV Nef/stop (truncated Nef) mutant virus in our study (Kestler et al., 1990; Kestler et al., 1991), and examined the replication and Nef-mediated MHC class I down-regulatory properties of these viruses in macaque CD4+ T-cell clones (Fig. 1A).
Figure 1.
Schematic representation of SIV Nef gene sequence present in infectious molecular clone of SIVmac239. Nef reading frame of SIVmac239 begins at nucleotide 9348 and ends at 10139 (numbering of Regier and Desrosiers, 1990) (Regier and Desrosiers, 1990). SIVmac239 Nef/stop contains a stop codon (TAA) at position 9624–9626, the 93rd codon of the gene leading to expression of a nonfunctional truncated Nef peptide. SIVmac239 Nef/Y223F contains an A to T replacement at position 10015 of the nucleic acid sequence leading to a tyrosine to phenylalanine change at position 223 of the peptide sequence (Swigut et al., 2000). SIVmac239 Nef/G2V contains a G to T replacement at position 9352 of the nucleic acid sequence leading to a glycine to valine change at position 2 of the peptide sequence (A). SIVmac239 Nef/Y223F (Y223F) and Nef/G2V (G2V) mutant viruses infect, replicate and mediate CD4 downregulation in rhesus macaque CD4+ T cells at levels comparable with WT virus invitro. CD4+ T cells from a rhesus macaque were infected with molecularly cloned WT SIVmac239, Y223F, G2V or Nef/stop mutant virus. The cells were cultured in 24-well tissue culture plates at 1 × 106 cells/well. The frequency of SIV Gag p27-expressing cells (B–C), CD4 (B) and MHC class I (C–D) expression levels were determined on day 8 PI by flow cytometry. Histograms represent MHC class I expression levels on cells within the SIV Gag p27+ gate (from SIV-infected cultures) or on CD4+ cells (from uninfected cultures). Geometric mean fluorescence intensities (GMFI) are shown. Plots are representative of 3 independent experiments.
Rhesus CD4+ T cells were infected with WT Nef/open, Y223F, G2V or Nef/stop mutants, and MHC class I expression on the infected cells was analyzed by flow cytometry. We focused the analyses on infected cells by gating on intracellular SIV Gag p27 positive cells at day 8 PI, the time point with the highest frequency of SIV Gag p27 positive cells (Minang et al., 2008). All viruses down-regulated CD4 surface expression on infected cells and most of the viruses infected the target cells to a similar degree by day 8 PI, except for the Nef/stop mutant virus with approximately half as many Gag p27 positive cells (Fig. 1B, representative experiment). We could not detect a difference in the number of infected cells between the myristylation defective G2V mutant virus and the WT or SIV Y223F mutant viruses. When gating within the Gag p27 positive population, we observed similar mean fluorescence intensities for SIV Gag p27 staining for WT and mutant virus-infected cells (data not shown), suggesting similar amounts of Gag protein expression per infected cell by the WT and mutant viruses. In addition, in a kinetic study we measured viral RNA and Gag p27 levels in the cultures on a daily basis and could not detect any consistent differences between WT and the Y223F or G2V mutants (data not shown). There was a marked decrease in MHC class I expression on cells infected with WT SIV compared to the three mutants (Fig. 1C, data from a representative experiment). By gating on SIV Gag p27 positive cells in the infected cultures and all cells for the uninfected cultures, and displaying MHC class I expression levels using histograms, reproducible but subtle differences in MHC class I expression levels between Nef/stop and Y223F or G2V mutant virus-infected cells were revealed. MHC class I expression levels on cells infected with WT virus was reduced ~3 fold compared with cells infected with virus lacking full length Nef expression (Nef/stop), confirming the role of SIV Nef in down-regulating MHC class I on infected rhesus CD4+ T cells (Schindler et al., 2004; Swigut et al., 2000). Cells infected with either the Y223F or G2V virus showed intermediate MHC class I expression compared to WT and Nef/stop (Fig 1D, inserted color coded numbers). Overall, the SIV Y223F, G2V and Nef/stop mutant virus-infected cells had higher MHC class I expression levels than uninfected cells; possibly due to the elevated protein-synthesis with increased generation of peptides in infected cells, leading to more stable expression of MHC class I molecules (Rock and Goldberg, 1999). Hence, except for the Nef/stop mutant, our Nef mutant viruses replicate in CD4+ T cells to essentially the same degree as WT SIV, but do not down-regulate MHC class I relative to WT SIV.
CTL-mediated suppression of virus replication in SIV-infected CD4+ T cells is impaired by Nef-induced down-regulation of MHC class I expression
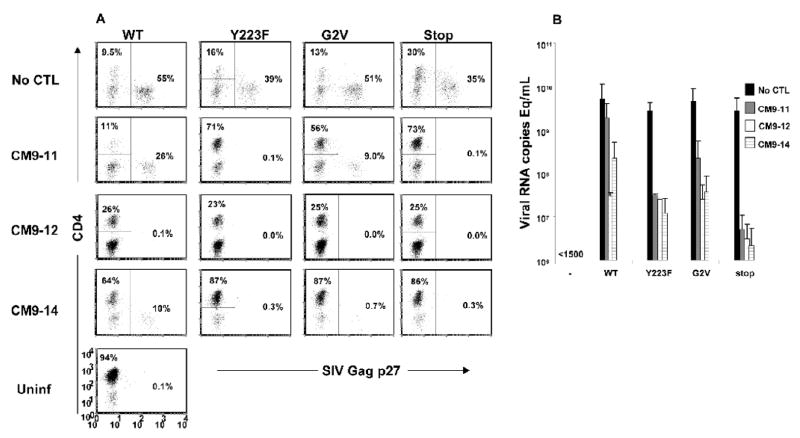
We have previously described an in vitro assay for measuring CTL-mediated SIV virus suppression (Minang et al., 2008). In a preliminary screening of a number of SIV Gag CM9-specific CTL clones generated from a SIVmac239-infected rhesus macaque, we found different levels of suppression of WT SIV replication, ranging from no effect to more than 100-fold suppression of virus production. We therefore investigated if differences in the capacity to suppress viral replication by these SIV Gag CM9-specific CTL clones were due to differences in their sensitivity to Nef-mediated MHC class I down-regulation. CD4+ T cells infected with WT (MHC class I+/−) or the Y223F and G2V (both MHC class I+++) or Nef/stop (MHC class I++++) viruses were co-cultured with different autologous SIV Gag CM9-specific CTL clones at a CD8+:CD4+ T-cell ratio of 1:1. Suppression of virus replication was assessed by measuring the frequency of SIV Gag p27 positive cells by flow cytometry and the accumulated viral RNA levels in the supernatant by quantitative RT PCR 8 days PI. As described previously, a CTL clone was arbitrarily considered to inhibit viral replication in a co-culture with infected cells when a reduction in viral RNA levels of at least 1 log was observed in culture supernatants (Minang et al., 2008) and the frequency of preserved CD4+ T cells as well as eliminated Gag p27 positive cells was at least 2-fold higher than in cultures of infected cells alone.
In the absence of virus-specific CTL, all virus-infected cultures showed massive loss of CD4+ T cells with greater than 30% of the remaining cells positive for SIV Gag p27. Little or no CD4+ T cell preservation was observed in co-cultures of WT SIV-infected cells and the CTL clone CM9-11 compared to cultures of infected cells alone. In line with this, CM9-11 had no detectable impact on the frequency of Gag p27 positive cells, taking into account the 1:1 CD8:CD4 cell ratios at the start of the co-cultures (Fig. 2A). However, CM9-11 could preserve CD4+ T cells and eliminated most Gag p27 positive cells in Y223F, G2V and Nef/stop mutant virus-infected cultures. This suggests a role of Nef-induced MHC class I down-regulation in mediating resistance to virus control by CTL. In contrast to clone CM9-11, a marked preservation of CD4+ T cells was observed in WT SIV-infected cultures in the presence of CTL clones CM9-12 and -14. This was accompanied by robust elimination of most or all Gag p27 positive CD4+ T cells. Just as clone CM9-11, CM9-12 and -14 could efficiently suppress viral replication when co-cultured with the Nef mutant virus-infected cells.
Figure 2.
SIV Gag CM9-specific CTL clones display clonal differences in their capacity to inhibit replication of WT SIVmac239 in autologous rhesus CD4+ T cell clones. CD4+ T cells infected with molecularly cloned WT SIVmac239, Y223F, G2V or the Nef/stop mutant virus were co-cultured with autologous SIV Gag CM9-specific CTL clones, CM9-11, -12 and -14, in 24-well tissue culture plates with 1×106 cells/well at a CD8+:CD4+ T cell ratio of 1:1. The frequency of SIV Gag p27+cells (A) and virion associated SIV gag RNA content of culture supernatant (B) were determined 8 days PI by flow cytometry and quantitative RT PCR, respectively. Uninfected target cells and infected CD4+ T cells cultured without CD8 effectors were included as negative and positive controls, respectively. (A) is representative of 3 independent experiments and (B) means and standard deviations of two independent experiments.
The data on CD4+ T cell preservation and Gag p27 positive cell elimination was consistent with viral RNA data. Little or no difference was observed in the viral RNA levels (<1 log reduction) in co-cultures of cells infected with WT SIV (MHC class I+/−) and CM9-11 compared to cultures of infected cells alone. On the other hand, ~2 and 1.5-logs reductions in viral RNA levels were observed in co-cultures of WT SIV-infected cells and CM9-12 and -14, respectively (Fig. 2B). A greater than 1-log reduction in viral RNA levels was observed in co-cultures of all three CTL clones and the Y223F, G2V (both MHC class I+++) and Nef/stop (MHC class I++++) mutant virus-infected cells. In line with the reduced replicative capacity of the Nef/stop mutant virus, all three CTL clones showed higher suppression of the Nef/stop mutant virus compared to the Y223F and G2V mutants. Hence, SIV Nef-mediated MHC class I down-regulation blunts the virus replication inhibitory capacity of SIV-specific CTL, with a complete abrogation seen for some clones.
Induction of IFN-γ and CD107a in SIV-specific CTL clones by SIV-infected autologous CD4+ T cells is not associated with functional avidity or ability to suppress virus
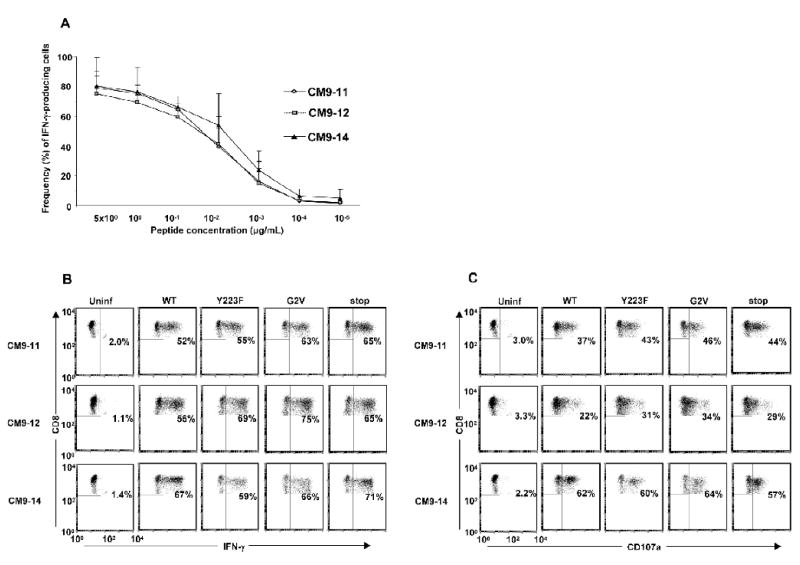
Lower levels of cell surface MHC class I would correspond to reduced presented epitope density on the surface of infected cells, and would be expected to result in preferential triggering of CTL clones with TCRs having higher functional avidity for the MHC class I-peptide complex. Having demonstrated clonal differences in the viral inhibitory capacity of different CTL clones specific for the same SIV Gag CM9 epitope, we investigated if the clonal variability in CTL suppression of WT SIV Nef/open replication was due to variable functional avidity among the clones for the cognate peptide. An autologous B-cell line pulsed with titered amounts of SIV Gag CM9 peptide or CD4+ T cells infected with WT SIV (MHC class I+/−), Y223F, G2V (both MHC class I+++) or Nef/stop mutant viruses (MHC class I++++) were used as APC to stimulate the different SIV Gag CM9-specific CTL clones, and IFN-γ expression and CD107a capture on the CTL clones was assessed by flow cytometry.
The three SIV-specific CTL clones; CM9-11, -12 and -14, showed similar maximal levels and similar dose response curves for IFN-γ expression following stimulation with APC pulsed with titered amounts of the SIV Gag CM9 peptide (Fig 3A). In addition, when tested against more physiologically relevant APC, autologous CD4+ T cells infected with WT SIV (MHC class I+/−), strong IFN-γ expression was elicited in all three CTL clones tested (Fig. 3B). In contrast to their variable viral suppression properties, the frequencies of intracellular IFN-γ positive cells as well as the levels of IFN-γ-expression per cell (mean fluorescence intensities; MFI) following stimulation were similar for the three CTL clones (Fig. 3B; data not shown). As with responses to the WT SIV-infected cells, there were no apparent differences in the IFN-γ responses to Y223F- or G2V mutant virus-infected cells (both MHC class I+++) between the three CTL clones (Fig 3B). All three CTL clones showed similar IFN-γ responses to cells infected also with the poorly replicating SIV Nef/stop mutant virus, indicating that the low number of SIV p27+ cells in these cultures was still sufficient to trigger the TCRs. Hence, the differential viral suppressive capacities of the CTL clones cannot be accounted for by any detectable differences in their functional avidity against cognate peptide or infected cells as measured by their IFN-γ response.
Figure 3.
SIV Gag CM9 peptide-pulsed APC or CD4+ T cells infected with WT or mutant SIV elicit IFN-γ and CD107a expression in SIV Gag CM9-specific CTL with high as well as low virus suppression capacities. (A) Autologous Herpesvirus papio-transformed B cells pulsed with 10-fold serial dilutions of the SIV Gag181–189CTPYDINQM (CM9) peptide (from 5 μg/mL down to 10−5 μg/mL) and (B–C) CD4+ T cells infected with WT or mutant SIV were used as APC to stimulate different SIV Gag CM9-specific CTL clones, CM9-11, -12 and -14, in a 5 h assay. The frequency of IFN-γ (A–B) and CD107a (C) positive cells were determined by flow cytometry. A) Means plus standard deviations for two independent experiments. B–C) Plots are representative of 3 independent experiments.
Lymphocyte associated membrane protein 1 (LAMP-1; CD107a) expression has been shown to directly correlate with CTL lytic capacity as measured by a flow cytometry-based killing assay (Betts et al., 2003). Given our observation of a lack of direct association between the frequency of SIV-infected cell-induced IFN-γ-expressing cells in the virus-specific CTL clonal populations and their capacity to suppress WT SIV replication, we also investigated if there was a difference in CD107a capture on the cell surface, a marker of CTL lytic capacity. All three SIV-specific CTL clones; CM9-11, -12 and -14, showed a similar CD107a staining profile in response to autologous SIV-infected CD4+ T cells, and the frequency of the CD107a positive cells within each clonal population did not correlate with the capacity of the individual CTL clones to suppress WT SIV replication (Fig 3C; data from a representative experiment). The lowest frequency of CD107a positive cells following stimulation with WT SIV-infected cells was seen with the CTL clone CM9-12, which showed the most robust suppressive capacity against WT SIV replication. A similar pattern was observed for responses to mutant virus-infected cells, with CM9-11 and CM9-14 showing slightly higher frequencies of CD107a responding cells than CM9-12 (Fig. 3C). When the CD107a responses of the three CTL clones to titrated amounts of cognate peptide were analyzed, similar responses were observed (data not shown). Hence, differences in lytic capacity, as measured by CD107a capture, could not account for the clonal differences in viral suppression capacity between the three SIV Gag CM9-specific CTL clones tested.
Subtle changes in levels of MHC class I expression have marked impact on CTL mediated viral suppression capacity
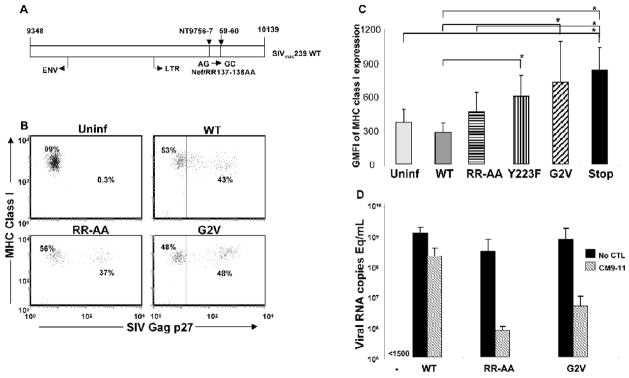
Given our observation that a 2–3 fold reduction in the expression of MHC class I molecules on the surface of WT SIV Nef/open-infected cells leads to a complete loss of virus suppression by the CTL clone CM9-11, we investigated if a less pronounced level of MHC class I down-regulation would still inhibit virus suppression by this CTL clone. To accomplish this, we constructed an SIV mutant, Nef/RR137-138AA (RR-AA; Fig. 4A), that induces a more moderate level of MHC class I down-regulation in infected cells compared with WT SIV (Schindler et al., 2004). Primary rhesus macaque CD4+ T cells were infected with WT SIV, Y223F, G2V, Nef/stop or the RR-AA mutant virus and MHC class I expression assessed on day 7 PI by flow cytometry; the RR-AA mutant virus-infected cells consistently showed MHC class I expression at levels intermediate (MHC class I+) between WT SIV (MHC class I+/−) and the Y223F and G2V mutants (both MHC class I+++) (Fig. 4B and C; data from representative experiment).
Figure 4.
Schematic representation of the Nef sequence in an engineered SIVmac239 Nef/RR137–138AA mutant virus. SIVmac239 Nef/RR137–138AA contains two AG to GC replacements at positions 9756/9757 and 9759/9760 of the Nef nucleic acid sequence leading to an arginine-arginine to alanine-alanine change at positions 137 and 138 of the peptide sequence (A). CD4+ T cells infected with SIVmac239 Nef/RR137–138AA (RR-AA) mutant virus show MHC class I expression at levels intermediate between WT SIV- and Y223F, G2V or Nef/stop mutant virus-infected cells. CD4+ T cells were infected with WT SIV, RR-AA, Y223F, G2V or Nef/stop mutant viruses and MHC class I expression levels determined 8 days PI by flow cytometry. Shown are dot plots for MHC class I levels versus SIV Gag p27 expression (B) and mean + SD of the geometric mean fluorescent intensities (GMFI) of MHC class I expression on cells within the SIV Gag p27+ gate (for SIV-infected cultures) or CD4+ cells (for uninfected cultures) (C); data are from 3 or 4 independent experiments and statistical analyses * indicates p value <0.05 for WT versus G2V, Y223F and Nef/stop as well as for RR-AA versus Nef/stop. (D) Mutant RR-AA, G2V as well as WT virus have comparable replicative capacities in rhesus CD4+ T cells but have differential susceptibility to suppression by a Gag CM9-specific CTL clone, CM9-11. CD4+ T cells infected with WT SIV, the RR-AA or G2V mutant virus were co-cultured with CM9-11 at a CD8+:CD4+ T cell ratio of 1:1 and viral RNA levels measured 8 days PI by quantitative RT PCR. Shown are means and standard deviations of two independent experiments.
Autologous CD4+ T cells infected with WT SIV (MHC class I+/−), the G2V (MHC class I+++) and RR-AA (MHC class I+) mutant viruses were then co-cultured with the CTL clone CM9-11 and viral RNA levels in culture supernatant measured by RT PCR on day 8 PI. The CM9-11 CTL clone which failed to suppress the replication of WT SIV, consistently suppressed virus replication in CD4+ T cells infected with the RR-AA mutant; > 1 log reduction in viral RNA with marked preservation of CD4+ T cells and elimination of Gag p27 positive cells (Fig. 4D; data not shown). We could not detect a consistent difference in sensitivity to suppression between CD4+ T cells infected with the RR-AA mutant compared with the G2V mutant. Thus, slightly increased MHC class I expression on target cells, as seen on cells infected with the RR-AA mutant compared with WT SIV virus is sufficient to overcome SIV Nef-induced resistance to CTL mediated suppression.
Discussion
Here we report three main findings with important implications for SIV/HIV immunobiology and HIV vaccine research: i) Different SIV specific CTL clones specific for the same epitope mediating viral suppression vary in their susceptibility to Nef-mediated MHC class I down-regulation ii) this clonal difference seems to be independent of functional avidity and iii) the difference is independent of IFN-γ responses and CTL lytic potential against virus-infected CD4+ T cells.
A central paradox in HIV/SIV immunopathology is the ability of the virus to continue to replicate and eventually overwhelm the immune system despite robust virus-specific CTL activity as measured by peptide-induced IFN-γ responses. While mutations in CTL epitopes is a powerful mechanism of viral escape, down-regulation of MHC class I by HIV-1 and SIV Nef has been proposed to protect infected cells by blunting their recognition and eventual killing by virus-specific CTL (Brenner et al., 2006; Collins et al., 1998; Munch et al., 2005; Sacha et al., 2007; Schwartz et al., 1996; Swigut et al., 2004; Swigut et al., 2000). Furthermore, data from both HIV-1 and SIV suggest that Nef selectively down-regulates MHC class I loci engaged in presenting CTL peptide epitopes, while not affecting expression of MHC class I loci shown to protect from NK-mediated lysis (Cohen et al., 1999; DeGottardi et al., 2008). In this report, we demonstrate that CTL-mediated virus suppression is inhibited by SIV Nef-dependent MHC class I down-regulation in SIV-infected primary rhesus macaque CD4+ T cells. WT SIV Nef/open-infected cells showed varying degrees of resistance to viral suppression by different SIV Gag-specific CTL clones, with one clone unable to preserve CD4+ T cells or inhibit SIV replication. Using SIV with different mutations in the Nef sequence; Y223F that selectively affects the MHC class I down-regulatory effect of Nef, and G2V, that has been described as rendering Nef non-functional (Schindler et al., 2004), we found that all tested SIV Gag-specific CTL clones preserved CD4+ T cells and inhibited the replication of the Y223F and G2V mutant viruses. Thus, SIV Nef-mediated MHC class I down-regulation can protect SIV-infected cells from CTL-mediated suppression, and render some CTL clones unable to suppress viral replication in vitro. To expand on our investigation on the differential sensitivity of the CTL clones to Nef-mediated MHC class I down-regulation, we engineered the SIVmac239 mutant virus, Nef/RR137-138AA (RR-AA) (Schindler et al., 2004), which induced MHC class I down-regulation in CD4+ T cells at levels close to but higher than WT SIV. We hypothesized that the RR-AA mutant virus-infected cells would present cognate viral peptides on their surface at levels in-between WT SIV- and G2V mutant virus-infected cells and thus also show intermediate susceptibility to SIV-specific CTL-mediated suppression of viral replication. The CM9-11 CTL clone that failed to suppress WT SIV suppressed replication of the RR-AA and G2V mutant viruses to a comparable degree. This suggests that subtle changes in MHC class I expression levels on infected cells can markedly affect the capacity of virus-specific CTL to suppress virus replication.
Interestingly, loss of myristylation has been reported to lead to a complete loss of SIV Nef function (Schindler et al., 2004), while a more subtle phenotype was reported by Chowers et al. (Chowers et al., 1994). In our model using primary rhesus macaque CD4+ T cells, we observed similar levels of CD4 down-regulation and viral replication after infection with WT SIV and the G2V mutant viruses. This is in contrast to the loss of MHC class I downregulatory effect by the G2V mutant. The lack of detectable impact of the Nef/G2V mutation on SIV replication in a primary rhesus CD4+ T-cell clone is intriguing. The discrepancy between these previous studies and our data could be due to the fact that we used SIV-infected primary CD4+ T cells while the above studies used established human cell lines as targets. In a separate study, we confirmed similar replication kinetics and CD4 downregulatory effect between WT SIV and the G2V mutant virus in primary rhesus CD4+ T cells but significantly reduced replication of the G2V mutant in a human T-cell line (manuscript in preparation) suggesting that SIV Nef differentially affects SIV replication in human cell lines and primary rhesus CD4+ T cells.
There were marked clonal differences in the abilities of clones having the same specificity to suppress replication in the context of MHC class I down-regulation. These differences were not attributable to TCR avidity as assessed by IFN-γ expression by the CTL clones following stimulation with APC pulsed with tittered amounts of peptide; IFN-γ response curves of the clones were nearly identical. This is in agreement with other reports showing similar IFN-γ responses to cognate peptide-pulsed APC by CTL clones specific for different SIV epitopes despite substantial differences in their viral suppressive capacities (Betts et al., 2003; Chung et al., 2007). A number of studies have suggested an epitope-dependent effect of HIV-1 Nef on the ability of HIV-1-specific CTL to suppress HIV-1 replication (Adnan et al., 2006; Tomiyama et al., 2005; Yang et al., 2003). We tested and compared several clones specific for the same SIV Gag epitope; CM9. Experiments with CTL clones specific to the SIV Nef165–173IW9 and Tat28–35SL8 epitopes using WT SIV and the G2V mutant virus yielded similar results i.e. clonal differences in the viral suppressive capacity of the CTL clones specific to the same epitope (data not shown). In contrast to the studies with HIV-1, our data show clear clonal differences in antiviral capacity between SIV-specific CTL clones sharing the same specificity. To further investigate any potential differences in functional avidity between the CTL clones, we assessed IFN-γ expression and CD107a capture following stimulation with SIV-infected CD4+ T cells, a more physiologically relevant target. Surprisingly, similar frequencies of IFN-γ-producing cells were seen in the three CTL clones following stimulation with autologous SIV-infected CD4+ T cells. Similar to the IFN-γ responses to virus-infected CD4+ T cells, surface caption of the CTL degranulation marker CD107a by the three tested CTL clones was not associated with their capacity to suppress SIV replication. Measurement of CD107a capture is used as a marker of antigen specific CD8+ CTL degranulation capacity (Betts et al., 2003; Dunham et al., 2006; Gauduin et al., 2006). Virus-specific CTL-mediated virus control has been suggested to involve a direct killing mechanism (granzyme and/or perforin mediated as measured by CD107a expression) or an indirect, cytokine (e.g. IFN-γ) dependent mechanism, or both (Appay et al., 2000; Appay et al., 2002; Dunham et al., 2006). Studies on HIV-1 specific CTL have suggested that senescence (Dagarag et al., 2003) or HIV-1 Nef-mediated (Tomiyama et al., 2002) virus-specific CTL dysfunction could be manifested by impaired cytolytic function with little or no effect on cytokine (IFN-γ) production capacity. Our data show that SIV Nef-mediated downregulation of MHC class I could diminish the antiviral capacity of SIV-specific CTL with no effect on the induction of either IFN-γ or CD107a and suggest that reductions in suppression can not be explained by a simple lack of activation model. These data point to a mechanism of virus control that is independent of IFN-γ, which is typically measured when analyzing HIV- and SIV-specific CTL responses, as well as CTL degranulation, and that CTL clonal variation in this yet unknown effector function is substantial, and decisive for control of SIV replication.
Our data raise important questions regarding the relevance of current CTL assays based on cognate peptide-pulsed APC. It has long been apparent that evaluating CTL responses using APCs pulsed with non-physiologic high concentrations of synthetic peptides provide an at best incomplete picture, and potentially a misleading assessment of virus-specific CD8+ T-cell responses (Yang et al., 2003). While these assays maybe very informative with regard to specificity and frequency of CTL responses, they do not necessarily estimate anti-viral capacity and should be viewed as only a first step in the evaluation of virus-specific CTL responses. Notably, our data using virus-infected APC suggest that this more physiologically appropriate assay also fails to predict the virus inhibitory potential and, thus, functional capacity of CTL clones. Our results have important implications for the evaluation of vaccine induced T cell immunity, as well as for attempts to correlate CTL responses with clinical outcomes in HIV- or SIV-infected long-term non-progressors and elite controllers (reviewed in (Walker and Burton, 2008)).
Materials and Methods
Generation of SIV Gag181–189CM9-specific CD8+ and autologous CD4+ T-cell clones
CTL clones specific to the Mamu-A*01-restricted SIV Gag181–189CM9 epitope (CM9) (O’Connor et al., 2003) were isolated from an Indian rhesus macaque, Macaca mulatta, chronically infected with SIVmac239 as described previously (Andersen et al., 2007). Briefly, lymphocytes were stimulated for 1 week with irradiated autologous PBMC pulsed with CM9 peptide (SynPep Corp., Dublin, CA). The cultures were restimulated weekly with CM9 peptide-pulsed and irradiated autologous PBMC in the presence of recombinant human IL-2 (50 IU/mL: NIH AIDS Research and Reference Reagent Program, Germantown, MD). Following 2 rounds of stimulation, the CM9-specific CTL were cloned by limiting dilution and maintained essentially as described in Riddell et al., (Riddell and Greenberg, 1990) and Berger et al., (Berger et al., 2001) using bi-weekly anti-CD3 monoclonal antibody (mAb) (30 ng/mL; clone SP34-2; BD Biosciences, San Diego, CA, USA) stimulation with irradiated human PBMC and human Epstein-Barr virus transformed B-cell lines (TM B-LCL; kindly provided by Drs. S.R. Riddell and P. D. Greenberg, FHCRC, Seattle, WA) as feeder cells, but without anti-CD28 mAb stimulation. APC and feeder cells were irradiated in a Mark I 137Cs γ-irradiator (Shepherd & Associates, San Fernando, CA) at 6000 and 12500 rad for PBMC and TM B-LCL, respectively. Positive wells were tested for antigen specificity by flow cytometry using ICS for IFN-γ production and by staining with a CM9 peptide/MHC-tetramer (Beckman Coulter, Miami, FL).
Autologous CD4+ T-cell clones were generated as described (Andersen et al., 2007). Briefly, highly enriched CD4+ T cells were isolated by negative selection using Miltenyi LD columns and anti-CD8 microbeads followed by positive selection using MS columns and anti-CD4 microbeads (Miltenyi Biotec Inc., Auburn, CA). CD4+ T-cell clones were obtained after 2-weeks expansion in limiting dilution cultures containing irradiated human PBMC, IL-2 and anti-CD3 mAb and maintained as described above for CD8+ T-cell clones. Animal care was according to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, and the Health and Human Services guidelines “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996, National Academy Press, Washington, D.C.), under an Institutional Animal Care and Use Committee approved protocol.
Virus stocks
SIVs used in this study were derived from the plasmid molecular clone pSIV239spxfl (kind gift of Ronald Desrosiers, New England Primate Research Center, Harvard Medical School, Southborough, MA) SIVmac239 (Genbank accession no. M33262.1). These were SIVmac239 Nef/open (WT), SIVmac239 Nef/stop (stop; truncated Nef mutant) (Kestler et al., 1990; Kestler et al., 1991) and three other SIVmac239 mutant viruses constructed by PCR-mediated overlap extension (Horton et al., 1990): Nef/Y223F (Y223F), pSIV239spxfl with a A-to-T change at nucleotide (nt) 10015 resulting in a tyrosine (Y) to phenylalanine (F) change in Nef codon 223; Nef/G2V (G2V), pSIV239spxfl with a G-toT change at nt 9352 resulting in a glycine (G) to valine (V) change in Nef codon 2; and Nef/RR137-138AA (RR-AA), pSIV239spxfl with a pair of AG-to-GC changes at nts 9756/7 and 9759/60 resulting in arginine (R) to alanine (A) changes in Nef codons 137 and 138 (Schindler et al., 2004). Virus stocks were produced by transfection of HEK293T cells with WT or mutant SIVmac239 using TransIt 293 reagent (Mirus Corporation, Madison, WI) according to the manufacturer’s recommendations. HEK293T cells were maintained in high glucose or Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 50 U of penicillin per mL and 50 μg of streptomycin per mL (Invitrogen corp., Carlsbad, CA, USA).
Infection of CD4+ T cells with SIVmac239
A clonal population of CD4+ T cells was activated with anti-CD3 mAb (T-25 flask-bound: 5 μg/mL) and IL-2 (50 IU/mL) for 48 h and infected by incubating with aliquots of virus stock for 2–3 h using the Viromag magnetofection reagents with a ratio of 7.5 μl of beads per ml of clarified transfection supernatant according to manufacturer’s recommendation (OzBiosciences, Marseille, France). Virus stocks with ~1 × 109 viral RNA copies Eq/mL in a volume of 250 μL were added per 1×106 CD4+ T cells. Incubations were carried out on a magnetic plate at 37°C in a humidified atmosphere of 5% CO2 and the virus-exposed CD4+ T cells washed twice with PBS to remove residual non-incorporated viral material prior to use in assays.
In vitro viral replication inhibition assay
Virus replication inhibition assays were set up as described previously (Minang et al., 2008). CD4+ T cells were freshly infected with either WT or mutant SIV, plated at 5 × 105 cells per well in 24-well plates and co-cultured with autologous CTL clones specific to the SIV Gag CM9 epitope at a CD8+:CD4+ T-cell ratio of 1:1. Uninfected and SIV-infected CD4+ T cells were cultured without CTL effectors at 1 × 106 cells per well as negative and positive controls, respectively, for virus infectivity. The cultures were maintained for 7 to 9 days with IL-2 addition every 2–3 days at a final concentration of 50 IU/mL.
Intracellular staining for SIV Gag p27 expression by virus-infected CD4+ T cells
Staining for surface CD4, MHC class I and intracellular SIV Gag p27 expression was performed to assess the levels of SIV infection at different time points post infection (PI). All antibodies were obtained from BD Biosciences (San Diego, CA, USA) unless otherwise indicated. Cells were washed once in FACS surface buffer(PBS, 1% rat sera, 1% mouse sera, 0.05% sodium azide) and stained with antihuman-CD4-PerCP-Cy5.5 (clone L200) and anti-HLA-ABC-PE (clone G46-2.6) conjugated mAbs. Cells were incubated for 30 min at 4°C, washed with sterile PBS and then fixed with 4% paraformaldehyde (PFA), 500 μL/sample tube for 30 min at 4°C. The cells were permeabilized (PBS, 0.1% saponin, 1% rat sera, 1% mouse sera and 0.05% sodium azide), 1 mL/sample tube for 5 min, washed (0.1% saponin in PBS) and incubated with FITC conjugated anti-SIV Gag p27 mAb (Clone 55-2F12, NIH AIDS Research and Reference Reagent Program). A final wash was performed and the cells resuspended in 0.1% PFA. Samples were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) and subsequent data analyses performed using FCS Express Version 3 (De Novo Software, Thornhill, Ontario, Canada). Dead cells were excluded from the analyses based on forward versus side scatter gating, and at least 100,000 live cell events collected for each sample.
Measurement of IFN-γ and CD107a in SIV-specific CTL clones in response to peptide-pulsed or SIV-infected target cells
SIV Gag CM9 peptide-pulsed autologous herpesvirus papio-transformed B-cell lines were used as APC to stimulate SIV Gag CM9-specific CTL clones in 5 mL polypropylene tubes. The APC were pulsed with peptide titrated from 5 μg/mL down to 10−5 μg/mL. The SIV Gag CM9-specific CTL clones were also stimulated with autologous CD4+ T cells infected with WT SIV, the Y223F, G2V or Nef/stop mutant virus. The CD4+ T cells had been infected for 7 or 8 days prior to use as APC; in our hands, the incubation period needed to achieve peak frequencies of SIV Gag p27 expressing (>30%) target cells (Minang et al., 2008). A CD8+:CD4+ T cell ratio of 1:1 with a total of 1 × 106 cells in a final volume of 0.5 ml per tube was used and non-pulsed autologous B cells or uninfected autologous CD4+ T cells were included as negative control APC. PE conjugated mAb specific to the CTL degranulation marker CD107a (LAMP-1) (clone H4A3) was added at the start of culture as described (Betts et al., 2003). Monensin, 20 μL/test of a 1:20 dilution, (Golgi stop™; BD Bioscience) was added after 1 h of incubation and the cultures incubated for additional 4 h. Cells were washed, stained for surface CD8 (PerCP-Cy5.5 conjugated anti-human CD8 mAb (clone SK1)) and intracellular IFN-γ (FITC conjugated antihuman IFN-γ mAb (clone 4S.B3)) expression. Data were acquired and analyzed as described above.
Viral RNA measurements
To monitor viral replication in vitro, culture supernatant was collected at defined time points PI and viral RNA extracted from the supernatant essentially as described previously (Cline et al., 2005). Viral replication was quantified using a real time RT-PCR assay essentially as described (Cline et al., 2005; Lifson et al., 2001).
Statistical analyses
The means and standard deviations for viral RNA levels were computed for duplicate wells of two or more experiments for cultures of virus-infected or uninfected CD4+ T cells alone or co-cultures of infected CD4+ T cells and autologous virus-specific CTL. As described previously (Minang et al., 2008), a CTL clone was arbitrarily considered to inhibit viral replication when a reduction in viral RNA levels of at least 1 log was observed, and the frequency of preserved CD4+ T cells as well as eliminated Gag p27 positive cells was at least 2-fold higher than in cultures of infected cells alone. Differences in geometric mean fluorescent intensities (GMFI) of surface MHC class I expression by CD4+ T cells infected with WT SIVmac239 compared with different Nef mutants were computed using the two-tailed unpaired student’s t test. Statistical significance was set at p<0.05.
Acknowledgments
The authors thank Dr Jeffrey Lifson for helpful discussions. The authors also thank Kelli Oswald and Lakeisha Galloway for help with the viral load (QPCR) analyzes. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: IL-2 from Hoffman-La Roche Inc., NJ; SIVmac p27 Hybridoma (55-2F12) from Dr Niels Pedersen. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract numbers N01-CO-12400 and HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adnan S, Balamurugan A, Trocha A, Bennett MS, Ng HL, Ali A, Brander C, Yang OO. Nef interference with HIV-1-specific CTL antiviral activity is epitope specific. Blood. 2006;108 (10):3414–9. doi: 10.1182/blood-2006-06-030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, Altfeld M, Yu XG, O’Sullivan KM, Lichterfeld M, Le Gall S, John M, Mothe BR, Lee PK, Kalife ET, Cohen DE, Freedberg KA, Strick DA, Johnston MN, Sette A, Rosenberg ES, Mallal SA, Goulder PJ, Brander C, Walker BD. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J Virol. 2004;78 (13):7069–78. doi: 10.1128/JVI.78.13.7069-7078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, Jing P, Calore B, Horton H, O’Connor DH, Hanke T, Piekarczyk M, Ruddersdorf R, Mothe BR, Emerson C, Wilson N, Lifson JD, Belyakov IM, Berzofsky JA, Wang C, Allison DB, Montefiori DC, Desrosiers RC, Wolinsky S, Kunstman KJ, Altman JD, Sette A, McMichael AJ, Watkins DI. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J Virol. 2002;76 (20):10507–11. doi: 10.1128/JVI.76.20.10507-10511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, O’Connor DH, Jing PC, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, Kunstman KJ, Wang XC, Allison DB, Hughes AL, Desrosiers RC, Altman JD, Wolinsky SM, Sette A, Watkins DI. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407(6802):386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- Andersen H, Barsov EV, Trivett MT, Trubey CM, Giavedoni LD, Lifson JD, Ott DE, Ohlen C. Transduction with human telomerase reverse transcriptase immortalizes a rhesus macaque CD8(+) T cell clone with maintenance of surface marker phenotype and function. AIDS Res Hum Retroviruses. 2007;23(3):456–65. doi: 10.1089/aid.2006.0194. [DOI] [PubMed] [Google Scholar]
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ, Rowland-Jones SL. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192(1):63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Papagno L, Spina CA, Hansasuta P, King A, Jones L, Ogg GS, Little S, McMichael AJ, Richman DD, Rowland-Jones SL. Dynamics of T cell responses in HIV infection. J Immunol. 2002;168(7):3660–6. doi: 10.4049/jimmunol.168.7.3660. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Kunstman J, Kuroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gorgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415(6869):335–9. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart TA. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J Gen Virol. 1998;79 (Pt 11):2717–27. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- Berger C, Huang ML, Gough M, Greenberg PD, Riddell SR, Kiem HP. Nonmyeloablative immunosuppressive regimen prolongs In vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J Virol. 2001;75 (2):799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+T cells by a flow cytometric assay for degranulation. Journal of Immunological Methods. 2003;281 (1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68 (9):6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Munch J, Schindler M, Wildum S, Stolte N, Stahl-Hennig C, Fuchs D, Matz-Rensing K, Franz M, Heeney J, Ten Haaft P, Swigut T, Hrecka K, Skowronski J, Kirchhoff F. Importance of the N-distal AP-2 binding element in Nef for simian immunodeficiency virus replication and pathogenicity in rhesus macaques. J Virol. 2006;80 (9):4469–81. doi: 10.1128/JVI.80.9.4469-4481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, Guatelli JC. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68 (5):2906–14. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C, Lee W, Loffredo JT, Burwitz B, Friedrich TC, Giraldo Vela JP, Napoe G, Rakasz EG, Wilson NA, Allison DB, Watkins DI. Not all cytokine-producing CD8+ T cells suppress simian immunodeficiency virus replication. J Virol. 2007;81 (3):1517–23. doi: 10.1128/JVI.01780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34 (5–6):303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10 (6):661–71. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391 (6665):397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Dagarag M, Ng H, Lubong R, Effros RB, Yang OO. Differential impairment of lytic and cytokine functions in senescent human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J Virol. 2003;77 (5):3077–83. doi: 10.1128/JVI.77.5.3077-3083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443 (7109):350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- DeGottardi MQ, Specht A, Metcalf B, Kaur A, Kirchhoff F, Evans DT. Selective downregulation of rhesus macaque and sooty mangabey major histocompatibility complex class I molecules by Nef alleles of simian immunodeficiency virus and human immunodeficiency virus type 2. J Virol. 2008;82(6):3139–46. doi: 10.1128/JVI.02102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl JN, Lawson B, Garg S, McClure HM, Xu YX, Ibegbu C, Easley K, Katz N, Pandrea I, Apetrei C, Sodora DL, Staprans SI, Feinberg MB, Silvestri G. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108(1):209–17. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MA, Charini WA, Kuroda MJ, Schmitz JE, Racz P, Tenner-Racz K, Manson K, Wyand M, Lifton MA, Nickerson CE, Fu T, Shiver JW, Letvin NL. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J Virol. 2000;74 (16):7485–95. doi: 10.1128/jvi.74.16.7485-7495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froebel KS, Aldhous MC, Mok JY, Hayley J, Arnott M, Peutherer JF. Cytotoxic T lymphocyte activity in children infected with HIV. AIDS Res Hum Retroviruses. 1994;10(Suppl 2):S83–8. [PubMed] [Google Scholar]
- Fujiwara M, Takiguchi M. HIV-1-specific CTLs effectively suppress replication of HIV-1 in HIV-1-infected macrophages. Blood. 2007;109(11):4832–8. doi: 10.1182/blood-2006-07-037481. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 directly kills CD4(+) T cells by a Fas-independent mechanism. Journal of Experimental Medicine. 1998;187 (7):1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauduin MC, Yu Y, Barabasz A, Carville A, Piatak M, Lifson JD, Desrosiers RC, Johnson RP. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J Exp Med. 2006;203(12):2661–72. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Brander C, Tang Y, Tremblay C, Colbert RA, Addo MM, Rosenberg ES, Nguyen T, Allen R, Trocha A, Altfeld M, He S, Bunce M, Funkhouser R, Pelton SI, Burchett SK, McIntosh K, Korber BT, Walker BD. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001;412 (6844):334–8. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- Gruters RA, van Baalen CA, Osterhaus AD. The advantage of early recognition of HIV-infected cells by cytotoxic T-lymphocytes. Vaccine. 2002;20 (15):2011–5. doi: 10.1016/s0264-410x(02)00089-0. [DOI] [PubMed] [Google Scholar]
- Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8(5):528–35. [PubMed] [Google Scholar]
- Johnson WE, Desrosiers RC. Viral persistence: HIV’s strategies of immune system evasion. Annual Review of Medicine. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248(4959):1109–12. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65 (4):651–62. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332(4):228–32. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- Kuroda MJ, Schmitz JE, Charini WA, Nickerson CE, Lifton MA, Lord CI, Forman MA, Letvin NL. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162 (9):5127–33. [PubMed] [Google Scholar]
- Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10(3):282–9. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Piatak M, Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. Journal of Virology. 2001;75 (21):10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangasarian A, Piguet V, Wang JK, Chen YL, Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73 (3):1964–73. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minang JT, Barsov EV, Yuan F, Trivett MT, Piatak M, Jr, Lifson JD, Ott DE, Ohlen C. Efficient inhibition of SIV replication in rhesus CD4+ T-cell clones by autologous immortalized SIV-specific CD8+ T-cell clones. Virology. 2008;372 (2):430–41. doi: 10.1016/j.virol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Munch J, Janardhan A, Stolte N, Stahl-Hennig C, Ten Haaft P, Heeney JL, Swigut T, Kirchhoff F, Skowronski J. T-cell receptor: CD3 down-regulation is a selected in vivo function of simian immunodeficiency virus Nef but is not sufficient for effective viral replication in rhesus macaques. J Virol. 2002;76(23):12360–4. doi: 10.1128/JVI.76.23.12360-12364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch J, Schindler M, Wildum S, Rucker E, Bailer N, Knoop V, Novembre FJ, Kirchhoff F. Primary sooty mangabey simian immunodeficiency virus and human immunodeficiency virus type 2 nef alleles modulate cell surface expression of various human receptors and enhance viral infectivity and replication. J Virol. 2005;79 (16):10547–60. doi: 10.1128/JVI.79.16.10547-10560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, Dodds E, Loffredo J, Martin S, McDermott AB, Allen TM, Wang C, Doxiadis GG, Montefiori DC, Hughes A, Burton DR, Allison DB, Wolinsky SM, Bontrop R, Picker LJ, Watkins DI. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol. 2003;77 (16):9029–40. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelrichs RB, Shrestha IL, Anderson DA, Deacon NJ. The explosive human immunodeficiency virus type 1 epidemic among injecting drug users of Kathmandu, Nepal, is caused by a subtype C virus of restricted genetic diversity. J Virol. 2000;74 (3):1149–57. doi: 10.1128/jvi.74.3.1149-1157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DJ, Jenkins SA, Brady HJ, Miles CG, Dzierzak EA, Abraham DJ. HIV-I Nef severely impairs thymocyte development and peripheral T-cell function by a CD4-independent mechanism. Genes Funct. 1997;1 (5–6):321–35. doi: 10.1046/j.1365-4624.1997.00029.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Desrosiers RC. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6 (11):1221–31. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Rhodes DI, Ashton L, Solomon A, Carr A, Cooper D, Kaldor J, Deacon N. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. Australian Long-Term Nonprogressor Study Group. J Virol. 2000;74 (22):10581–8. doi: 10.1128/jvi.74.22.10581-10588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128 (2):189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–79. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones SL, Pinheiro S, Kaul R, Hansasuta P, Gillespie G, Dong T, Plummer FA, Bwayo JB, Fidler S, Weber J, McMichael A, Appay V. How important is the ‘quality’ of the cytotoxic T lymphocyte (CTL) response in protection against HIV infection? Immunol Lett. 2001;79 (1–2):15–20. doi: 10.1016/s0165-2478(01)00261-9. [DOI] [PubMed] [Google Scholar]
- Rud EW, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke BE. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75 (Pt 3):529–43. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- Sacha JB, Chung C, Reed J, Jonas AK, Bean AT, Spencer SP, Lee W, Vojnov L, Rudersdorf R, Friedrich TC, Wilson NA, Lifson JD, Watkins DI. Pol-specific CD8+ T cells recognize simian immunodeficiency virus-infected cells prior to Nef-mediated major histocompatibility complex class I downregulation. J Virol. 2007;81 (21):11703–12. doi: 10.1128/JVI.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R, Garbuglia AR, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72 (5):3646–57. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M, Munch J, Brenner M, Stahl-Hennig C, Skowronski J, Kirchhoff F. Comprehensive analysis of nef functions selected in simian immunodeficiency virus-infected macaques. J Virol. 2004;78 (19):10588–97. doi: 10.1128/JVI.78.19.10588-10597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2(3):338–42. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RMM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. Journal of Immunology. 2002;169 (6):3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- Sugimoto C, Tadakuma K, Otani I, Moritoyo T, Akari H, Ono F, Yoshikawa Y, Sata T, Izumo S, Mori K. nef gene is required for robust productive infection by simian immunodeficiency virus of T-cell-rich paracortex in lymph nodes. J Virol. 2003;77 (7):4169–80. doi: 10.1128/JVI.77.7.4169-4180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigut T, Alexander L, Morgan J, Lifson J, Mansfield KG, Lang S, Johnson RP, Skowronski J, Desrosiers R. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J Virol. 2004;78 (23):13335–44. doi: 10.1128/JVI.78.23.13335-13344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigut T, Iafrate AJ, Muench J, Kirchhoff F, Skowronski J. Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility complex antigen expression. J Virol. 2000;74 (12):5691–701. doi: 10.1128/jvi.74.12.5691-5701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama H, Akari H, Adachi A, Takiguchi M. Different effects of Nef-mediated HLA class I down-regulation on human immunodeficiency virus type 1-specific CD8(+) T-cell cytolytic activity and cytokine production. J Virol. 2002;76 (15):7535–43. doi: 10.1128/JVI.76.15.7535-7543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama H, Fujiwara M, Oka S, Takiguchi M. Cutting Edge: Epitope-dependent effect of Nef-mediated HLA class I down-regulation on ability of HIV-1-specific CTLs to suppress HIV-1 replication. J Immunol. 2005;174 (1):36–40. doi: 10.4049/jimmunol.174.1.36. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8(+) T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Ueno T, Motozono C, Dohki S, Mwimanzi P, Rauch S, Fackler OT, Oka S, Takiguchi M. CTL-mediated selective pressure influences dynamic evolution and pathogenic functions of HIV-1 Nef. J Immunol. 2008;180 (2):1107–16. doi: 10.4049/jimmunol.180.2.1107. [DOI] [PubMed] [Google Scholar]
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320 (5877):760–4. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- Xu XN, Screaton GR, Gotch FM, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott JE, McMichael AJ. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186(1):7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Nguyen PT, Kalams SA, Dorfman T, Gottlinger HG, Stewart S, Chen IS, Threlkeld S, Walker BD. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J Virol. 2002;76 (4):1626–31. doi: 10.1128/JVI.76.4.1626-1631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Sarkis PT, Trocha A, Kalams SA, Johnson RP, Walker BD. Impacts of avidity and specificity on the antiviral efficiency of HIV-1-specific CTL. J Immunol. 2003;171 (7):3718–24. doi: 10.4049/jimmunol.171.7.3718. [DOI] [PubMed] [Google Scholar]