Abstract
Background
Recent reports have suggested that angioplasty +/− stenting (PTA/S) may have lower perioperative mortality than open surgery for revascularization of acute and chronic mesenteric ischemia (AMI and CMI). It is unclear if there has been nationwide adoption of this methodology or whether there is in fact a mortality benefit.
Methods
We identified all patients undergoing mesenteric revascularization, either surgical (bypass, endarterectomy, or embolectomy) or PTA/S from the Nationwide Inpatient Sample from 1988–2006. A diagnosis by ICD-9 coding of AMI or CMI was required for inclusion. We evaluated trends in management over this time period and compared in-hospital mortality and complications between surgical bypass and PTA/S for the years 2000–2006.
Results
From 1988 to 2006 there were 6,342 PTA/S and 16,071 open surgical repairs overall. PTA/S increased steadily over time surpassing all surgery for CMI in 2002. PTA/S for AMI has also increased and surpassed bypass in 2002 but has not surpassed all surgical procedures for AMI even in 2006. Mortality was lower after PTA/S than bypass for both CMI (3.7% vs 13%, P<0.01) and AMI (16% vs 28%, P<0.01). Bowel resection was more common after bypass than PTA/S for CMI (7% vs 3%, P<0.01) and this subgroup showed an increased in-hospital mortality for both repair types (54% and 25%).
Conclusion
PTA/S in being utilized with increasing frequency for revascularization of both CMI and AMI. Based on lower in-hospital mortality for patients as they are currently being selected, PTA/S is appropriate therapy for selected patients with CMI. Longitudinal data are needed to determine the durability of this benefit. The greater proportion of patients undergoing bowel resection with bypass for AMI suggests a more advanced level of ischemia in this group making comparison with PTA/S difficult. However, PTA/S may be useful in selected patients with AMI and appropriate anatomy. Further data with greater detail regarding symptomatology and anatomy will clarify appropriate patient selection.
Introduction
Mesenteric ischemia requiring revascularization is associated with high mortality in both chronic and acute forms. Open surgical treatment has been the standard for many years with bypass, endarterectomy, or embolectomy. Revascularization for chronic mesenteric ischemia is typically performed in elderly patients with extensive atherosclerotic disease and malnutrition. Given the rarity of the disease there are few reported series with large numbers of patients undergoing surgery for chronic mesenteric ischemia (CMI). Results from these relatively high volume centers of excellence have operative mortality up to 12%.1–7 Derrow et al reported national outcomes for surgical revascularization of CMI from the Nationwide Inpatient Sample (NIS) revealing an in-hospital mortality of 15%.8 There are also few reports with large numbers of patients with acute mesenteric ischemia (AMI) undergoing revascularization. Revascularization for AMI is typically associated with mortality rates over 50%.1, 9–11
There has been an increasing number of small reports of the use of angioplasty with or without stenting (PTA/S) for treatment of CMI with mortality rates similar to surgery in some reports and lower than surgery in others.12–17 Several small case series have described using PTA/S for treatment of AMI.18–22 The national adoption of PTA/S for CMI and AMI is largely unknown. Additionally, it is unclear if there is a short term mortality benefit with PTA/S compared to surgery.
To address this, we evaluated trends in management of CMI and AMI using either surgery or PTA/S over the time period 1988–2006 and compared in-hospital outcomes in the most recent years using a national hospital administrative database.
Methods
The Nationwide Inpatient Sample was used for this study. The NIS is maintained by the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality. The database is a 20% all-payer sample of hospital stays and contains sampling weights to allow for stratified calculation of total population estimates. The years 1988 to 2006 were used for trend analysis whereas comparisons between PTA/S and surgery were limited to the years 2000 to 2006 when PTA/S became commonplace.
Queries were performed with SAS statistical software (version 9.1, SAS Institute, Cary, NC) using ICD-9 diagnosis and procedure codes. Initial case selection required a primary admission diagnosis of acute (557.0) or chronic mesenteric ischemia (557.1) combined with a procedure code of mesenteric angioplasty with and without stent placement (39.50, 39.90) or open mesenteric surgical procedures including mesenteric bypass (39.26, 38.36, 38.46), mesenteric embolectomy (38.06), or mesenteric endarterectomy (38.16). Patients undergoing aortic reconstruction and patients less than 18 years were excluded from this analysis. Total population estimates were calculated per year of hospitalization. The primary outcome was in-hospital mortality. Secondary outcomes included need for bowel resection and complications including acute renal failure, acute myocardial infarction, and others as defined by ICD-9 coding for complications (Appendix I, online only).
Appendix.
Table of ICD-9 codes used for comorbidities and complications
| ICD-9 Codes | |
|---|---|
| Comorbidities | |
| Hypertension | 401–405 |
| Peripheral Vascular Disease | 440, 443 |
| Coronary Artery Disease | 412–414 |
| Cardiac Dysrthmias | 427 |
| Afib/flutter | 427.3 |
| Prior Myocardial Infarction | 412 |
| Congestive Heart Failure | 428 |
| Diabetes Mellitus | 250 |
| Chronic Obstructive Pulmonary Disease | 491, 491, 492, 496 |
| Chronic renal disease | 585, 586, V45.1, V56 |
| Cerebrovascular Disease | 433–438 |
| Bowel Resection | 45.5, 45.6, 45.7, 45.8, 45.9, 46.1, 46.2, 48.5 |
| Complications | |
| Acute Renal Failure | 584 |
| Acute Myocardial Infarction | 410 |
| 996–999 | 996–999 |
| Stroke | 997.02 |
| Cardiac | 997.1 |
| Peripheral Vasccular | 997.2 |
| Respiratory | 997.3 |
| Hemorrhage | 998.11 |
Statistical Analysis
Statistical analysis was performed using survey analysis programs with Stata (Stata Statistical Software: Release 8. College Station, TX: StataCorp LP). Population estimates are reported by applying the sampling weight for each observation within Stata calculations. Means and standard deviations are reported for parametric data and median values and ranges for non-parametric data. Statistical significance was assigned as a p-value of <.01. Comparisons between cohorts were carried out using the Wilcoxon-ranked sum for nonparametric continuous data, Student’s t-test for parametric continuous data, and chi-square for categorical and count data. Groups were stratified by diagnosis (AMI versus CMI) as well as by revascularization method. Univariate and multivariate logistic regression was performed using all demographic and comorbidity data as potential risk factors. Procedure complications were not included in multivariate analyses. The need for a bowel resection was included as an independent predictor variable in analysis of AMI (but not CMI) since this was felt more likely to be a reflection of the pre-existing extent of ischemia at the time of revascularization rather than a complication of the revascularization procedure. Multivariate analysis was performed by backwards selection of risk factors meeting statistical significance at the P <.10 level on univariate analysis.
Study approval was obtained from the Institutional Review Board at Beth Israel Deaconess Medical Center. Data use agreements for use of the NIS data were made with HCUP.
Results
OVERALL
From 1988 to 2006 there were 6,342 PTA/S and 16,071 open surgical repairs overall. Between 2000–2006, the majority of patients diagnosed with AMI underwent open surgery (64.5%) versus PTA/S (35.5%), while patients with chronic mesenteric ischemia more often were treated with PTA/S (61.9% vs 38.1%). Overall revascularization for CMI has increased over time (Figure 1) while for AMI there has been relatively little change (Figure 2). PTA/S increased over time for both diagnoses and surpassed surgery for CMI by 2002 and more than doubled it by 2005. For AMI, PTA/S has surpassed bypass and embolectomy individually, but surgical revascularization in general remains the predominant treatment. Of note, the proportion of patients with atrial fibrillation or atrial flutter decreased over time from a maximum or 38% in 1994 to 24% in 2006.
Figure 1.
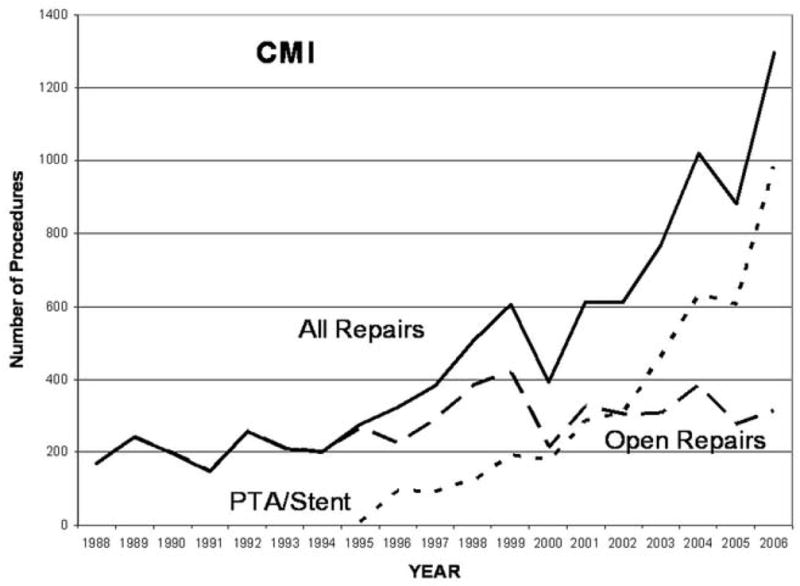
Procedure volume for chronic mesenteric ischemia revascularization 1988–2006.
Figure 2.
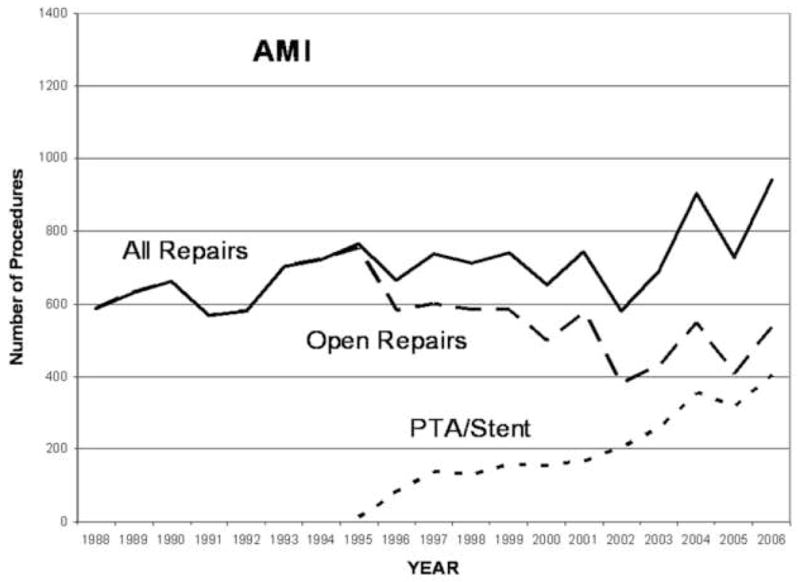
Procedure volume for acute mesenteric ischemia revascularization 1988–2006.
Over time, there was an overall decrease in mortality for both CMI and AMI (Test of trend, P <.001) (Figure 3 and 4). After the year 2000, when endovascular therapy was more commonly utilized, the mortality after repair for either indication was significantly lower than prior to this time period (CMI 8% vs 15%, P <.001; AMI 30 % vs 49 %, P <.001).
Figure 3.
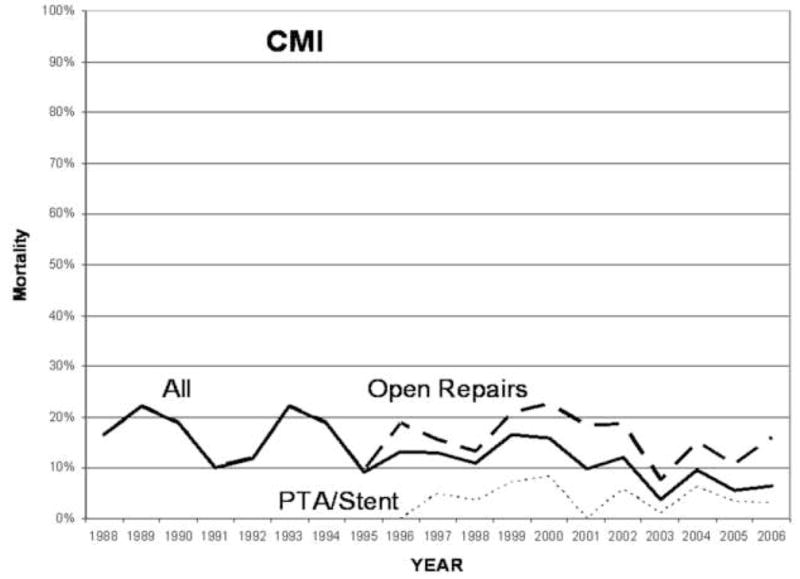
Mortality after angioplasty with or without stenting or surgical repair for chronic mesenteric ischemia from 1988–2006.
Figure 4.
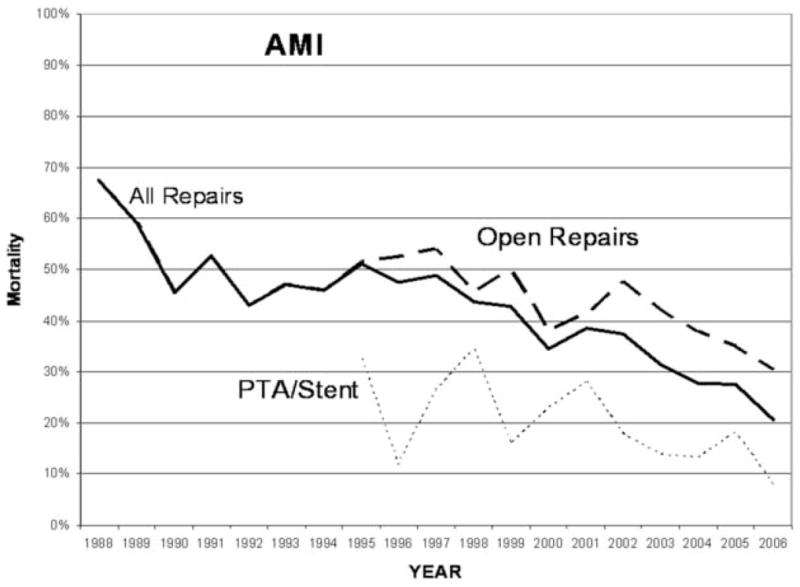
Mortality after angioplasty with or without stenting or surgical repair for acute mesenteric ischemia from 1988–2006.
CMI 2000–2006
For CMI, from 2000–2006, patients undergoing revascularization in general were older women with multiple comorbidities. However, those undergoing PTA/S were older, more likely male, and had higher rates of comorbidities (Table I). The surgical procedure performed for CMI was overwhelmingly bypass (93%) compared to endarterectomy (4%) or embolectomy (3%). Mortality was 3.7% after PTA/S and 15% after open surgical repair (P <.001). Mortality after PTA/S was significantly lower when limiting the comparison to bypass (13% P <.001), and excluding the higher mortality seen in those undergoing endarterectomy (31%) or embolectomy (69%). There were no significant mortality differences for gender for PTA/S (Male vs Female 4% vs 4%, P =.72) or open repairs (14% vs 16%, P =.66).
Table I.
Baseline characteristics of patients undergoing angioplasty with or without stenting or surgical repair for chronic and acute mesenteric ischemia from 2000–2006.
| Chronic Mesenteric Ischemia | Acute Mesenteric Ischemia | |||||
|---|---|---|---|---|---|---|
| Angioplasty with/without stent N=3,455, 61.9% | Bypass, endarterectomy, or embolectomy N=2,128, 38.1% | P- value | Angioplasty with/without stent N=1,857, 35.5% | Bypass, endarterectomy, or embolectomy N=3,380, 64.5% | P-value | |
| Age, y (median, range) | 74, 24–97 | 68, 29–99 | <.001 | 72, 26–96 | 72, 21–99 | .53 |
| <60 | 15% | 32% | <.001 | 24% | 26% | .34 |
| 60–69 | 23% | 28% | <.05 | 25% | 22% | .36 |
| 70–79 | 37% | 30% | <.01 | 31% | 33% | .62 |
| ≥80 | 25% | 11% | <.001 | 21% | 19% | .52 |
| Female | 74% | 79% | <.05 | 70% | 66% | .14 |
| Comorbidities | ||||||
| Hypertension | 66% | 51% | <.001 | 56% | 46% | <.01 |
| Peripheral Vascular Disease | 40% | 32% | <.01 | 33% | 13% | <.001 |
| Coronary Artery Disease | 39% | 26% | <.001 | 34% | 19% | <.001 |
| Atrial Fibrillation/Flutter | 16.5% | 14.9% | .49 | 23.6% | 38.7% | <.001 |
| Prior Myocardial Infarction | 8.3% | 6.0% | .17 | 6.4% | 4.7% | .23 |
| Congestive Heart Failure | 17.5% | 10.5% | <.01 | 22.1% | 22.6% | .85 |
| Diabetes Mellitus | 19% | 12% | <.01 | 18% | 17% | .73 |
| Chronic Obstructive | ||||||
| Pulmonary Disease | 25% | 27% | .40 | 29% | 23% | .06 |
| Chronic renal disease | 6.3 | 1.2 | <.001 | 9.8 | 3.5 | <.001 |
| Cerebrovascular Disease | 6.9% | 7.7% | .61 | 4.7% | 5.9% | .41 |
| Charlson (mean +/− SD) | 1.3 +/− 1.1 | 1.0 +/− 1.0 | <.001 | 1.4 +/− 1.3 | 0.9 +/− 1.1 | <.001 |
| Bowel Resection | 28.1% | 47.8% | <.001 | |||
SD Standard Deviation
Overall morbidity was also lower after PTA/S compared to bypass (20% vs. 38%, P <.001) with significant differences in cardiac and respiratory complications (Table III). PTA/S had approximately half the rate of acute renal failure and need for bowel resection. Mortality in those undergoing bowel resection was significantly increased for all patients regardless of revascularization method compared to those without a bowel operation (PTA/S 25% vs 3.1%, P <.001 and bypass 54% vs 10%, P <.001). Length of stay was shorter after PTA/S than bypass (Median (range) 5 (0–94) vs 11 (1–135) days, P <.001). Discharge to home was more common after PTA/S vs bypass (87% vs 79%, P <.01).
Table III.
Perioperative complications and length of stay after angioplasty with or without stenting or surgical repair for chronic and acute mesenteric ischemia from 2000–2006.
| Chronic Mesenteric Ischemia | Acute Mesenteric Ischemia | |||||
|---|---|---|---|---|---|---|
| Angioplasty with/without stent | Bypass, endarterectomy, or embolectomy (Bypass only) | P-value | Angioplasty with/without stent | Bypass, endarterectomy, or embolectomy (Bypass only) | P-value | |
| Any Complication | 20.2% | 39.7%(38.4%) | <.001(<001) | 36.7% | 48.2%(49.9%) | <.001(<.001) |
| Bowel Resection | 3.0% | 8.0%(6.6%) | <.001(<.05) | |||
| Acute Renal Failure | 6.0% | 10.5%(9.7%) | <.01(<.05) | 11.4% | 18.4%(16.8%) | <.01(<.05) |
| Acute Myocardial | ||||||
| Infarction | 3.0% | 4.8%(3.6%) | .13(.61) | 5.0% | 7.6%(4.4%) | .10(.73) |
| Cardiac | 0.7% | 5.9%(5.6%) | <.001(<.001) | 2.1% | 7.2%(9.3%) | <.001(<.001) |
| Stroke | 0% | 0.7%(0.8%) | <.05(<.05) | 1.0% | 0.3%(0.3%) | .11(.24) |
| Peripheral Vasccular | 0.8% | 0.2%(0.3%) | .25(.28) | 0.5% | 0.3%(0.7%) | .58(.79) |
| Respiratory | 0.3% | 5.3%(5.7%) | <.001(<.001) | 1.1% | 5.7%(8.8%) | <.001(<.001) |
| Hemorrhage | 1.3% | 3.4%(3.4%) | <.05(<.05) | 2.4% | 2.7%(3.1%) | .74(.59) |
| LOS,d (median,range) | 5, 0–94 | 11, 1–135 (11, 1–135) | <.001 (<.001) | 9, 0–104 | 13, 0–198 (14, 1–127) | <.001 (<.001) |
LOS Length of Stay
On multivariate analysis, mortality was still 5 to 6-fold higher with bypass compared to PTA/S (Table IV). Increasing age was also a predictor of mortality with a 50% increased risk per decade of life. Comorbidities that were predictive of mortality included congestive heart failure (CHF) and atrial fibrillation/flutter with a greater than 2-fold increased risk for both. Hypertension was protective.
Table IV.
Multivariate predictors of mortality after angioplasty with or without stenting or mesenteric bypass for chronic and acute mesenteric ischemia from 2000–2006. A) Without comorbidities. B) With comorbidities
| OR | 95% | CI | P-Value | |
|---|---|---|---|---|
| Chronic Mesenteric Ischemia | ||||
| Bypass (vs. PTA/S) | 5.1 | 3.1 | 8.4 | <.001 |
| Age (per decade) | 1.6 | 1.2 | 2.0 | <.001 |
|
| ||||
| Acute Mesenteric Ischemia | ||||
| Bypass (vs. PTA/S) | 2.2 | 1.5 | 3.3 | <.001 |
| Age (per decade) | 1.3 | 1.1 | 1.6 | <.001 |
| OR | 95% | CI | P-Value | |
|
| ||||
| Chronic Mesenteric Ischemia | ||||
| Bypass (vs. PTA/S) | 5.7 | 3.3 | 9.8 | <.001 |
| Age (per decade) | 1.5 | 1.1 | 2.0 | <.01 |
| Hypertension | 0.4 | 0.2 | 0.7 | <.001 |
| Atrial Fibrillation/Flutter | 2.5 | 1.4 | 4.5 | <.01 |
| Congestive Heart Failure | 2.8 | 1.5 | 5.3 | <.01 |
|
| ||||
| Acute Mesenteric Ischemia | ||||
| Bypass (vs. PTA/S) | 2.2 | 1.5 | 3.4 | <.001 |
| Age (per decade) | 1.3 | 1.02 | 1.5 | <.05 |
| Bowel Resection | 3.6 | 2.4 | 5.4 | <.001 |
| Atrial Fibrillation/Flutter | 2.2 | 1.3 | 3.4 | <.001 |
AMI 2000–2006
Surgical revascularization for AMI consisted of 49% embolectomy, 44% bypass, and 7% endarterectomy. Patients undergoing revascularization for AMI also tended to be older women with multiple comorbidities. Those undergoing PTA/S for AMI also had higher rates of comorbidities compared to those undergoing open surgical repair including hypertension, peripheral vascular disease, coronary artery disease, atrial fibrillation/flutter, and chronic renal failure (Table I). Atrial fibrillation and flutter were more common for AMI than CMI as expected (33% vs 16%, P <.001). Bowel resection occurred in 28% of patients undergoing PTA/S and 37% of those undergoing bypass ( P <.05). In-hospital mortality was 16% after PTA/S and 39% after surgical repair for AMI (P <.001). Mortality was highest for embolectomy at 49% and was 28% for bypass and 35% for endarterectomy. When limiting the comparison of PTA/S to bypass, mortality was still significantly lower with PTA/S (P <.001). There was a trend for males to have a lower mortality than females after open repair (34% vs 41%, P =.07), however mortality by gender was similar after PTA/S (16% vs 15%, P =.85). Mortality was again increased for both groups when bowel resection was performed during the same admission (Bypass 45% vs 17%, P <.001; PTA/S 29% vs 11%, P <.001).
For AMI, overall morbidity was higher after bypass compared to PTA/S (Table III). Specifically, cardiac and respiratory complications were again significantly higher. Length of stay was shorter after PTA/S than bypass (Median (range) 9 (0–104) vs 14 (1–127) days, P <.001). Discharge disposition was similar for PTA/S and bypass (Home 72% vs 68%, P =.37).
Multivariate predictors of mortality for AMI included bypass vs PTA/S and age (Table IV). With comorbidities included, the need for a bowel resection and atrial fibrillation/flutter were also significant predictors. Bypass was associated with a greater than 2-fold increased risk of mortality while the need for a bowel resection carried a greater than 3.5-fold increased risk. Bowel resection was included in the multivariate analysis for AMI (but not CMI) as a coexisting condition. Gender was not a significant predictor of mortality.
Discussion
Revascularization for CMI and AMI is increasingly being performed with PTA/S. For CMI, PTA/S is associated with lower in-hospital mortality, shorter length of stay, and fewer bowel resections. For AMI, bypass was typically employed in more advanced cases requiring bowel resection making comparison with PTA/S difficult. However, after controlling for the need for bowel resection, there was a lower mortality with PTA/S. Revascularization procedures for CMI are increasing overall with a dramatic rise in PTA/S and a constant rate of surgical procedures, while revascularization for AMI is relatively constant with PTA/S apparently replacing surgery in some cases.
The increase in revascularization procedures for CMI may be due to many reasons. There may be increasing awareness of the disease particularly now that a minimally invasive treatment is available and being more widely utilized. The advancing age of the population may be contributing although this would also be expected to demonstrate a rise in AMI as well unless the increase in elective procedures is preventing AMI. With the less invasive approach there may expansion of treatment indications to include those previously considered to be at prohibitive risk for surgery. In this series PTA/S patients were older with greater comorbidity. Additionally, indications may have expanded to include treatment of those with symptoms that are less severe or those in whom the diagnosis is in question. If this is the case, this may bias the results in favor of PTA/S while treatment of those at prohibitive risk for surgery would potentially bias in favor of surgery. Finally, it is well documented that PTA/S is associated with a greater risk of restenosis and recurrent symptoms.15 This is certainly playing some role in the increase in overall CMI interventions. Additionally, patients undergoing re-intervention for restenosis may have a different risk for inhospital mortality which could again influence the comparison of PTA/S with surgery. Clearly, there is a need for longitudinal follow-up to determine whether there is any long term benefit to PTA/S over surgery. It has been suggested that a strategy of initial PTA/S would allow for correction of the malnourished state so that the patient may more safely undergo surgical revascularization after the early onset of recurrent stenosis and symptoms prior to a severe deterioration in nutritional status.12, 23
The decline in surgical procedures for AMI appears to be primarily in those undergoing embolectomy. This is likely due to an increasing utilization of anticoagulation for dysrhythmias such as atrial fibrillation. A recent study from the Mayo clinic found an increase in rates of anticoagulation and a decrease in embolic strokes in patients with atrial fibrillation between 1980–2000.24
A recent review by Kougias et al summarized the largest series of PTA/S for CMI.15 They found a 30-day mortality rate of 3%, and a restenosis rate of 28% at a mean follow up of 2 years with 27% undergoing repeat PTA/S. Mortality from large series of open revascularization for CMI range from 0% to 12%.1–7, 13, 14 The broad range reflects the small number of patients undergoing revascularization for CMI even at large referral centers. Derrow et al reviewed the NIS database for surgical revascularization of CMI from 1993–1997 and found a mortality rate of 14.7% which corresponds to our findings from a broader period using the same database.8 Therefore, the numbers from the current study better reflect the true risk of open surgical revascularization and demonstrate the potential benefit of PTA/S.
Mortality with revascularization for AMI is typically >50%. 1, 5, 9, 10 We found that surgical revascularization mortality was highest with embolectomy and lowest with bypass. This is likely due to the lack of an established collateral circulation with embolization. Since PTA/S is felt by most to be inappropriate treatment for most cases of embolism we focused on comparison of PTA/S with bypass. We found that the need for bowel resection was the primary predictor of mortality with AMI more so than the use of PTA/S versus bypass. Unlike CMI, the need for bowel resection with AMI likely reflects the severity of ischemia at the time of vascular consultation rather than a complication of revascularization.9, 10 Given that resection was required in a much larger proportion of those undergoing bypass it is likely that the two cohorts are dissimilar and this biases the comparison in favor of PTA/S. There is general agreement that in most cases of AMI there is a need to inspect the bowel viability and perform resection as needed. The diagnosis of AMI is often made at laparotomy in an operating room setting that may not be amenable for angiography and intervention which may limit the broader application of this technology. Retrograde stenting of the SMA via an arteriotomy has been advocated as a means to provide rapid revascularization through the open abdomen at the time of laparotomy.18, 21, 22 We do not have the ability to determine whether percutaneous antegrade or open retrograde PTA/S was employed in patients undergoing laparotomy in the NIS database and cannot comment on the potential merits of this approach. With the expansion of operating room endovascular suites and the increasing use of rapid multi-row detector computed tomographic angiography for diagnosis, PTA/S may be utilized with greater frequency when the diagnosis is entertained prior to laparotomy. However, PTA/S would not be expected to obviate the need to inspect bowel viability.
Our study has several limitations. This is a retrospective study with clear selection bias in the choice of therapy. This is most evident in AMI where more patients with advanced ischemia underwent bypass than PTA/S. There may have been other factors in addition to the need for bowel resection that were unmeasured that impacted the decision to choose bypass rather than PTA/S. We do not have anatomic details about number of vessels treated, lesion length, or stenosis versus occlusion which are likely to impact revascularization strategy and could impact outcome. The large numbers in this study (20% sample of non-federal hospitals) obtained from hospital discharge data are the strength of the study. This is evidenced by comparison to the recent review of the literature of mesenteric PTA/S by Kougias et al with a total of 292 patients that would represent a <5% sample of patients undergoing this procedure. However, discharge databases such as NIS are subject to coding errors. This is highlighted by the 3% of patients who were coded as undergoing embolectomy for CMI. A nearly 3-fold higher percentage of CMI patients undergoing embolectomy had atrial fibrillation/flutter compared to those undergoing another method of repair. Additionally, the diagnosis of CMI is often a difficult one and may be made without a complete workup particularly when percutaneous therapy is attempted. We also cannot identify patients undergoing repeat mesenteric procedures. The increasing number of endovascular procedures may be reflective of either of these and bias mortality in favor of PTA/S. The inability to definitively state whether a bowel resection is done at the time of mesenteric operation, during a “second look” procedure, before revascularization, or as a complication of the mesenteric operation is one more limitation of the NIS database. There is a variable for day of operation, but this was infrequently recorded and thus was an unreliable method to further clarify this.
Conclusion
There is a clear trend toward increasing utilization of PTA/S for CMI and AMI. PTA/S appears to be reasonable first line therapy in selected patients, particularly in CMI, based on significantly lower in-hospital mortality and complications. Longitudinal data are needed to determine if this benefit is maintained over time. Further data with greater detail regarding symptomatology and anatomy will clarify appropriate patient selection.
Table II.
Mortality after angioplasty with or without stenting or surgical repair for chronic and acute mesenteric ischemia from 2000–2006.
| Chronic Mesenteric Ischemia | Acute Mesenteric Ischemia | |||||
|---|---|---|---|---|---|---|
| Angioplasty with/without stent | Bypass, endarterectomy, or embolectomy | P-value | Angioplasty with/without stent | Bypass, endarterectomy, or embolectomy | P-value | |
| 15.4% | <.001 | 38.6% | <.001 | |||
| All Patients | 3.7% | (13.1% with bypass) | (<.001) | 15.6% | (27.6% with bypass) | (<.001) |
| Mortality within subgroups | ||||||
| Age | ||||||
| <60 | 0.7% | 8.8% | <.01 | 14.4% | 22.3% | .13 |
| 60–70 | 2.4% | 14.8% | <.001 | 12.9% | 28.2% | <.01 |
| 70–80 | 3.8% | 14.1% | <.001 | 12.5% | 46.1% | <.001 |
| ≥80 | 6.8% | 39.2% | <.001 | 24.8% | 59.9% | <.001 |
| Female | 3.6% | 15.8% | <.001 | 15.4% | 41.0% | <.001 |
| Male* | 4.2% | 13.9% | <.01 | 16.2% | 34.0% | <.001 |
| Hypertension | 2.9% | 9.2% | <.001 | 13.1% | 41.3% | <.001 |
| Peripheral Vascular | ||||||
| Disease | 1.8% | 13.7% | <.001 | 10.2% | 39.7% | <.001 |
| Coronary Artery Disease | 2.2% | 16.4% | <.001 | 10.2% | 45.0% | <.001 |
| Atrial Fibrillation/Flutter | 11.5% | 35.9% | <.001 | 28.9% | 49.2% | <.001 |
| Prior Myocardial | ||||||
| Infarction | 1.8% | 3.7% | .59 | 12.5% | 39.1% | <.05 |
| Congestive Heart Failure | 9.9% | 38.8% | <.001 | 28.2% | 44.6% | <.05 |
| Diabetes Mellitus | 4.1% | 12.6% | <.05 | 11.6% | 38.0% | <.001 |
| Chronic Obstructive | ||||||
| Pulmonary Disease | 5.0% | 17.8% | <.001 | 16.3% | 41.3% | <.001 |
| Chronic renal disease | 4.9% | 17.9% | .26 | 19.4% | 49.0% | <.05 |
| Cerebrovascular Disease | 2.3% | 23.9% | <.01 | 11.6% | 32.2% | .11 |
| Bowel Resection | 24.6% | 55.3% (53.9% with bypass) | <.05 (<.05) | 28.8% | 46.5% (45.4% with bypass) | <.01 (<.05) |
P=NS for mortality of males vs females for each repair type.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cho JS, Carr JA, Jacobsen G, Shepard AD, Nypaver TJ, Reddy DJ. Long-term outcome after mesenteric artery reconstruction: a 37-year experience. J Vasc Surg. 2002;35:453–60. doi: 10.1067/mva.2002.118593. [DOI] [PubMed] [Google Scholar]
- 2.Park WM, Cherry KJ, Jr, Chua HK, et al. Current results of open revascularization for chronic mesenteric ischemia: a standard for comparison. J Vasc Surg. 2002;35:853–9. doi: 10.1067/mva.2002.123753. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez JG, Huber TS, Ozaki CK, et al. Durability of antegrade synthetic aortomesenteric bypass for chronic mesenteric ischemia. J Vasc Surg. 2002;35:1078–84. doi: 10.1067/mva.2002.124377. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham CG, Reilly LM, Rapp JH, Schneider PA, Stoney RJ. Chronic visceral ischemia. Three decades of progress. Ann Surg. 1991;214:276–87. doi: 10.1097/00000658-199109000-00010. discussion 287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston KW, Lindsay TF, Walker PM, Kalman PG. Mesenteric arterial bypass grafts: early and late results and suggested surgical approach for chronic and acute mesenteric ischemia. Surgery. 1995;118:1–7. doi: 10.1016/s0039-6060(05)80002-9. [DOI] [PubMed] [Google Scholar]
- 6.McAfee MK, Cherry KJ, Jr, Naessens JM, et al. Influence of complete revascularization on chronic mesenteric ischemia. Am J Surg. 1992;164:220–4. doi: 10.1016/s0002-9610(05)81074-8. [DOI] [PubMed] [Google Scholar]
- 7.Foley MI, Moneta GL, Abou-Zamzam AM, Jr, et al. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg. 2000;32:37–47. doi: 10.1067/mva.2000.107314. [DOI] [PubMed] [Google Scholar]
- 8.Derrow AE, Seeger JM, Dame DA, et al. The outcome in the United States after thoracoabdominal aortic aneurysm repair, renal artery bypass, and mesenteric revascularization. J Vasc Surg. 2001;34:54–61. doi: 10.1067/mva.2001.115596. [DOI] [PubMed] [Google Scholar]
- 9.Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467–74. doi: 10.1016/j.jvs.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 10.Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054–62. doi: 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 11.Park WM, Gloviczki P, Cherry KJ, Jr, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35:445–52. doi: 10.1067/mva.2002.120373. [DOI] [PubMed] [Google Scholar]
- 12.Brown DJ, Schermerhorn ML, Powell RJ, et al. Mesenteric stenting for chronic mesenteric ischemia. J Vasc Surg. 2005;42:268–74. doi: 10.1016/j.jvs.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Atkins MD, Kwolek CJ, LaMuraglia GM, Brewster DC, Chung TK, Cambria RP. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162–71. doi: 10.1016/j.jvs.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Kasirajan K, O’Hara PJ, Gray BH, et al. Chronic mesenteric ischemia: open surgery versus percutaneous angioplasty and stenting. J Vasc Surg. 2001;33:63–71. doi: 10.1067/mva.2001.111808. [DOI] [PubMed] [Google Scholar]
- 15.Kougias P, El Sayed HF, Zhou W, Lin PH. Management of chronic mesenteric ischemia. The role of endovascular therapy. J Endovasc Ther. 2007;14:395–405. doi: 10.1583/07-2102.1. [DOI] [PubMed] [Google Scholar]
- 16.Lee RW, Bakken AM, Palchik E, Saad WE, Davies MG. Long-term outcomes of endoluminal therapy for chronic atherosclerotic occlusive mesenteric disease. Ann Vasc Surg. 2008;22:541–6. doi: 10.1016/j.avsg.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Sarac TP, Altinel O, Kashyap V, et al. Endovascular treatment of stenotic and occluded visceral arteries for chronic mesenteric ischemia. J Vasc Surg. 2008;47:485–491. doi: 10.1016/j.jvs.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Wyers MC, Powell RJ, Nolan BW, Cronenwett JL. Retrograde mesenteric stenting during laparotomy for acute occlusive mesenteric ischemia. J Vasc Surg. 2007;45:269–75. doi: 10.1016/j.jvs.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Demirpolat G, Oran I, Tamsel S, Parildar M, Memis A. Acute mesenteric ischemia: endovascular therapy. Abdom Imaging. 2007;32:299–303. doi: 10.1007/s00261-006-9074-3. [DOI] [PubMed] [Google Scholar]
- 20.Gartenschlaeger S, Bender S, Maeurer J, Schroeder RJ. Successful percutaneous transluminal angioplasty and stenting in acute mesenteric ischemia. Cardiovasc Intervent Radiol. 2008;31:398–400. doi: 10.1007/s00270-006-0147-z. [DOI] [PubMed] [Google Scholar]
- 21.Moyes LH, McCarter DH, Vass DG, Orr DJ. Intraoperative retrograde mesenteric angioplasty for acute occlusive mesenteric ischaemia: a case series. Eur J Vasc Endovasc Surg. 2008;36:203–6. doi: 10.1016/j.ejvs.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Sonesson B, Hinchliffe RJ, Dias NV, Resch TA, Malina M, Ivancev K. Hybrid recanalization of superior mesenteric artery occlusion in acute mesenteric ischemia. J Endovasc Ther. 2008;15:129–32. doi: 10.1583/07-2210.1. [DOI] [PubMed] [Google Scholar]
- 23.Biebl M, Oldenburg WA, Paz-Fumagalli R, McKinney JM, Hakaim AG. Endovascular treatment as a bridge to successful surgical revascularization for chronic mesenteric ischemia. Am Surg. 2004;70:994–8. [PubMed] [Google Scholar]
- 24.Miyasaka Y, Barnes ME, Gersh BJ, et al. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community-based study. Stroke. 2005;36:2362–6. doi: 10.1161/01.STR.0000185927.63746.23. [DOI] [PubMed] [Google Scholar]


