Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) are believed to be critically involved in ethanol-related behaviors as well as in neurochemical responses to ethanol. However, discernment of nAChR contribution to ethanol reinforcement and consumption remains incomplete. The current studies examined the influence of the nAChR antagonist mecamylamine (MEC) on operant ethanol self-administration using a procedure that independently assessed appetitive and consumptive processes, and compared these findings to effects of MEC on sucrose self-administration.
Male C57BL/6J (B6) mice were trained to respond for 30-min access to a retractable drinking tube containing either 10% v/v ethanol (10E) or 5% w/v sucrose (5S). Once trained, mice were habituated to saline injection and then treated with a series of MEC doses (0 - 8 mg/kg; i.p.) in a within-subject design. In a separate cohort, MEC was evaluated for its influence on locomotor activity.
MEC dose-dependently reduced 10E and 5S self-administration. The suppression in ethanol intake was attributable to a reduction in bout frequency, whereas the attenuation in sucrose intake was due to a decrease in bout size. Doses of MEC (6 - 8 mg/kg) that altered drinking patterns were also found to impair locomotor activity.
Although MEC non-selectively reduced 10E and 5S intakes in mice, there was some specificity in alterations of the underlying drinking pattern for each reinforcer. Assessment of drinking topography within an operant self-administration procedure may provide useful insights regarding the role of nAChR function in the regulation of ethanol consumption.
Keywords: Alcohol, Nicotinic acetylcholine receptor, Reinforcement, Intake pattern, Preference drinking, Reward
1. Introduction
Ethanol consumption and tobacco smoking are highly correlated behaviors. Greater than 80% of alcoholics are smokers (DiFranza and Guerrera, 1990; Istvan and Matarazzo, 1984), and approximately 35% of alcoholics exhibit nicotine co-dependence (Grant et al., 2004). Conversely, nicotine-dependent smokers are 2.7 times more likely to be dependent on ethanol when compared to non-smokers (Breslau, 1995). Genetic analyses have yielded a robust correlation (r = 0.68) between the two disorders (True et al., 1999), and the genetic risk factors that are associated with increased alcoholism risk are similar to those associated with nicotine dependence (Koopmans et al., 1997). The high prevalence of co-dependence on ethanol and tobacco (i.e., nicotine) is suggestive of a shared neurobiological mechanism(s) mediating reinforcement and reward for these drugs (Little, 2000; Funk et al., 2006).
Nicotine exerts its behavioral and psychopharmacological effects via pentameric neuronal nicotinic receptors (nAChRs), whereas ethanol interacts with multiple neurotransmitter systems. However, ethanol (Imperato and Di Chiara, 1986; Larsson et al., 2002), nicotine (Imperato et al., 1986; Corrigall et al., 1992; Nisell et al., 1996; Zhou et al., 2001) or their combination (Clark and Little, 2004) increase extracellular dopamine levels within the nucleus accumbens, suggesting a common mechanism of action within the neurocircuitry underlying reward. Studies examining the ability of nAChRs to modify ethanol's neurochemical and behavioral effects have been limited by the availability of nAChR ligands that exhibit receptor isoform specificity and readily traverse the blood-brain barrier to exert central effects. The most commonly employed antagonist in ethanol studies, mecamylamine hydrochloride (MEC), is a non-competitive nAChR antagonist that penetrates the blood-brain barrier, but interacts with multiple receptor isoforms that contain a combination of α and β subunits (Papke et al., 2001). Additional antagonists investigated to date include methyllycaconitine (MLA), dihydro-β-erythroidine (DHβE), and α-conotoxin M II (αCTX) which selectively antagonize α7 subunits (i.e., homomeric), α4β2-containing receptors, and α6β2β3-containing receptors, respectively (Larsson and Engel, 2004; Salminen et al., 2005). Varenicline, a partial agonist at α4β2-containing nAChRs, also has been studied (Steensland et al., 2007).
The ability of nAChR antagonism to alter ethanol-stimulated accumbal dopamine levels, locomotor activity, and the discriminative stimulus properties of ethanol has yielded mixed results. Ethanol-stimulated (2.0 - 2.5 g/kg) dopamine levels in the rodent nucleus accumbens was inhibited by systemic MEC (1-2 mg/kg) and intra-ventral tegmental area (VTA) αCTX (5 nmol), but not by systemic DHβE (0.5 mg/kg) or MLA (2 mg/kg) (Blomqvist et al., 1993; Larsson et al., 2002, 2004). At identical doses and routes of administration, MEC and αCTX also blocked ethanol-induced (1.75 - 2.00 g/kg) locomotor stimulation in mice, whereas DHβE and MLA were ineffective (Larsson et al., 2002, 2004). MEC (0.5 - 6.0 mg/kg) did not alter the discriminative stimulus properties of ethanol in rats (Bienkowski et al., 1998). In contrast, studies conducted with healthy men and women demonstrated that MEC attenuated ratings of stimulation following ethanol as well as the self-reported desire to drink (Blomqvist et al., 2002; Chi and de Wit, 2003; Young et al., 2005).
Relevant to the current study, evidence suggests that some nAChR antagonists and a partial agonist reduce ethanol consumption in rodents. Systemic MEC (2-4 mg/kg) decreased ethanol intake in some (Blomqvist et al., 1996; Kuzmin et al., 2009; Le et al., 2000), but not all (Dyr et al., 1999) studies, whereas DHβE (1-8 mg/kg) was ineffective (Kuzmin et al., 2009; Le et al., 2000). While intra-VTA MEC (100 μM) or αCTX (5 nmol) significantly decreased ethanol intake (Ericson et al., 1998; Kuzmin et al., 2009; Soderpalm et al., 2000; Larsson et al., 2004), intracranial MEC did not alter ethanol intake when applied to the lateral ventricle (Katner et al., 1997) or nucleus accumbens (Nadal et al., 1998). Systemic varenicline (1 - 2 mg/kg) also significantly decreased ethanol consumption (Steensland et al., 2007). Thus, evidence to date suggests that nAChR antagonism (MEC and αCTX) or partial agonism (varenicline) may be suitable pharmacological strategies to reduce ethanol self-administration.
The present series of experiments were conducted with the purpose of extending existing results to determine the selectivity and specificity of MEC's effects in mouse and to compare the effects on ethanol (and sucrose) intake in two different models of self-administration. With this in mind, the goals addressed in the current experiments were four-fold. First, this work examined the influence of systemic MEC on ethanol reinforcement using an operant-based procedure that permitted separate inspection of appetitive versus consumptive processes. Unlike self-administration examined under standard fixed ratio (FR) schedules, this procedural variation allows for the determination of MEC effects on operant (appetitive) responding without the confound from the pharmacological onset of ethanol (that is inherent in animals consuming ethanol while responding on a standard FR schedule). Second, the current work assessed the specificity of MEC by testing the effects of this antagonist on an alternate reinforcer (i.e., sucrose). Although multiple studies have evaluated the effects of MEC on operant ethanol self-administration, none to date have examined the influence of the same doses on another behavioral reinforcer. Third, this work sought to compare the effects of MEC across operant self-administration and two-bottle choice drinking procedures. Fourth, this study determined whether concomitant changes in locomotor activity occur at MEC doses that impact self-administration behavior. Although operant self-administration studies frequently measure a generalized suppression of activity indirectly via the degree of responding on an inactive lever, the interpretation of this measure is often confounded by the presence of ethanol and by a floor effect (inactive responding is often already minimally expressed and further changes are difficult to detect). In the current report, activity levels were evaluated by both inactive lever responding and by an independent locomotor assessment. Furthermore, this work is the first to report the effects of MEC in mice run in an operant-based self-administration procedure, as all previous examinations with this nAChR antagonist have been performed in rats.
2. Methods
2.1. Animals
Male C57BL/6J (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The mice weighed 22.3 ± 0.2 grams upon arrival and were individually housed in Thoren cages (Maxi Mizer model #1; 19.6 cm W × 30.9 cm L × 12 cm H; Plexiglas) and acclimated for a minimum of 7 days to a 12hr/12hr light/dark cycle (lights on at 0600 h, except when noted below). Ad libitum access to food was provided in the home cage, and water was freely available (except when noted below). Animals were weighed and handled daily. In the conduct of this research, all efforts were made to minimize animal suffering, to reduce the number of mice used, and to explore available alternatives to in vivo techniques. The local Institutional Animal Care and Use Committee approved all procedures in accordance with the guidelines described in the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council of the National Academies, 2003).
2.2. Operant self-administration
2.2.1. Experimental design and apparatus
Self-administration behavior was assessed with the `sipper' procedure, which our laboratory has adapted for use in mice (Ford et al., 2007a, 2007b) and which required the completion of a single FR of responding on the active lever to gain 30 min of continuous access to the reinforcer, a schedule that is termed a response requirement (RR). The advantage of the 'sipper' procedure is that it allows for a separate evaluation of appetitive versus consumptive processes that underlie operant ethanol self-administration (Samson et al., 1998, 2000), and permits mice to exhibit a spontaneous pattern of drinking that more closely models the human condition (refer to Mello and Mendelson, 1970). Daily sessions were carried out in modular operant conditioning chambers (21.6 × 17.8 × 12.7 cm) with stainless steel rod floors (Med-Associates Inc., St Albans, VT). Each chamber was outfitted with two ultra-sensitive retractable levers (requiring approximately 2 grams or greater of force; located 2.2 cm above floor), two stimulus lights, a retractable sipper apparatus for controlling access to the sipper tube, and a house light. One wall of the chamber contained the retractable levers 11 cm apart (with a stimulus light positioned directly above each lever). The access portal for the retractable sipper tube was located on the opposing wall. Modified graduated pipettes with double ball-bearing metal sippers permitted fluid measurements to the nearest 0.05 ml. Drinking patterns were monitored via a lickometer circuit wired between each sipper and rod floor. These circuits were then interfaced to a computer running MED-PC IV software (Med-Associates Inc.). Each chamber was positioned within a sound-attenuating cabinet (61 × 38 × 33 cm) and was ventilated by an exhaust fan.
2.2.2. Lever acquisition training
Mice were initially trained to respond on an active lever to obtain 30-sec access to a 10% w/v sucrose solution (10S) on a fixed ratio 1 (FR1) reinforcement schedule throughout 30-min sessions. When the active lever was pressed, both levers retracted, the house light turned off as a stimulus light positioned above the active lever illuminated for a 5-sec period, and the retractable sipper was extended into the operant chamber. Following 30-sec of sipper access, the sipper retracted and the two levers were extended back into the chamber. Responding on the inactive lever was recorded, but had no scheduled consequence. The position of the active lever was counterbalanced across subjects between the left and right sides of the chamber wall. Ten to twelve sessions were required for the majority of mice to achieve a minimum criterion of ten sipper presentations in 30-min. Each mouse was exposed to a single overnight session (15 h duration; starting at dark phase onset) during the first week of the lever training to facilitate acquisition of responding. Further, to elevate motivational state, mice were water restricted for 16 h prior to each of these initial training sessions. Water was then provided ad libitum during all subsequent experimental phases.
2.2.3. Sucrose fading and reinforcement schedule manipulations
A modified sucrose fading procedure was utilized to initiate ethanol self-administration, as previously described (Samson, 1986; Ford et al., 2007a, 2007b). Throughout fading, mice were maintained on a FR1 reinforcement schedule during 30-min sessions. For ethanol-reinforced mice, 10% v/v ethanol (10E) was added to the 10S solution, and then sucrose was faded out in a step-wise manner to eventually yield 10E alone (2-3 sessions each with 10S/10E, 5S/10E, and 2.5S/10E solutions). In contrast, sucrose-reinforced mice remained ethanol-naïve during fading to a final concentration of 5S (2-3 sessions each with 10S, 8.5S, and 7S solutions). Sessions were conducted 5-6 days per week throughout the fading procedure and during subsequent experimental phases.
After sucrose fading, the schedule of reinforcement was increased from FR1 to FR8 over a 3-week period. After maintenance on the FR8 schedule for two weeks, the appetitive and consummatory phases of operant self-administration were procedurally separated, as previously described in a rat model (Samson et al., 1998, 2000) and recently replicated in mice (Ford et al., 2007a). Briefly, the completion of 8 responses on the active lever (response requirement 8; RR8) resulted in 30-min of continuous access to the reinforcer solution. While this could be considered as completion of a single FR8, the difference from the FR schedule was that reoccurring cycles of additional responding were neither required nor permitted by the animals Therefore, once reinforcer access was achieved, the animal regulated its rate of oral consumption for the remainder of the session (i.e., 30-min of limited access), ensuring that measurement of appetitive drive (responding) was not influenced by the onset of pharmacology resulting from ongoing ethanol consumption. A 20-min time limit was imposed for completion of the RR schedule. If the RR was not completed, the session was terminated and mice were denied access to the reinforcer. Throughout a subsequent 2-week period, the RR was incrementally increased from 8 to 16 responses.
2.2.4. Injection habituation and mecamylamine (MEC) treatment
Following a 6-week maintenance period on the RR16 schedule, mice were habituated to vehicle injection (saline i.p.; 10 ml/kg body weight) over 5 consecutive sessions. Injections were administered 10-min prior to session start. Animal weights averaged 28.7 ± 0.3 g immediately prior to testing, with no significant differences between the 5S and 10E groups. Fourteen 5S-reinforced and ten 10E-reinforced mice were then administered a series of MEC doses (2, 4, 6, and 8 mg/kg) in random order throughout a 3-week period. Baseline responding and consumption were re-established between tests, with a minimum of two vehicle injection sessions conducted between treatments. MEC doses were selected based on previous reports examining MEC effects on ethanol-related behavioral endpoints (Blomqvist et al., 1992; Ericson et al., 2000; Larsson et al., 2002; Larsson and Engel, 2004).
2.3. Locomotor activity
Locomotor chambers with automated activity monitoring (Accuscan; Columbus, OH) were used as previously described (Phillips et al., 2005; Ford et al., 2007b). Briefly, photocell beam breaks were recorded by the Accuscan analyzer, and were subsequently translated to total horizontal distance traveled (cm). Activity was evaluated for 50-min to match the longest possible time course of an operant self-administration session (20-min maximum for completing the RR16 plus 30-min sipper access).
A separate cohort of 48 mice was used for activity testing. A 3-day procedure was executed: `habituation' to saline injection (day 1), `baseline' for saline injection (day 2), and `treatment' with MEC (day 3). Each day mice were acclimated to the procedure room for 60-min, weighed, injected with saline or MEC (i.p.), returned to their home cage for 10-min, and then placed into the center of the activity chamber floor (40 × 40 cm) for the session start. On the `treatment' day, each mouse received one of four MEC doses (0, 2, 6, or 8 mg/kg) in a between-group design. Twelve mice were assigned per dose group, and groups were counterbalanced for `baseline' activity levels. Due to experimental demand for the locomotor chambers, only one dose of MEC could be tested per group.
2.4. Two-bottle choice tests
Following locomotor testing, the same cohort of mice were allowed a 2-week washout period, during which time they were acclimated both to a reverse light/dark schedule (lights on at 2200 h) and to double ball-bearing drinking tubes in their home cage (identical to those described above). A red light remained on in the procedure room at all times to facilitate the procedures carried out in the dark. Two bottle choice tests, including the order of fluid presentations, were conducted as previously described (Belknap et al., 1993; Yoneyama et al., 2008). Two-hour limited access sessions were started at 1200 h (2 hr into the dark phase), with one tube always containing water and a second tube containing a 10E solution or subsequently, a 0.033% w/v saccharin solution (0.033Sac). The 0.033Sac solution was chosen for its non-caloric nature. Volume measurements were taken at the start and end of each session. Two sets of control tubes were placed on vacant cages to monitor volume loss from the drinking tubes (loss was typically negligible). The drug-containing tube was counterbalanced between the left and right sides across subjects.
The effect of MEC on 10E preference drinking was assessed first, followed by an evaluation of its effect on 0.033Sac preference one week later. Once stable consumption for 10E was achieved (≤ 15% variability during 3 consecutive sessions), mice were habituated to saline injections administered 10-min prior to session start. Following the re-establishment of baseline consumption (typically 2-3 sessions), mice were injected with one of four MEC doses (0, 2, 6, and 8 mg/kg MEC; i.p.) in a between-subject design. Treatment groups (n = 11-12) were balanced for `baseline' g/kg intakes that occurred during the 2 sessions immediately prior to testing. After acute treatment with MEC in conjunction with 10E drinking, mice were then provided a 0.033Sac solution during subsequent 2-bottle choice sessions. Mice rapidly achieved stable 0.033Sac intake within 3-4 sessions, were re-habituated to saline injections, and were then injected with one of same MEC doses tested with 10E (see above). For this second round of testing, mice were assigned to treatment groups (n = 11-12), which were balanced for `baseline' mg/kg saccharin intake irrespective of prior group assignment during the earlier testing with 10E. Body weights and home cage water consumption between sessions (22 h/day) were closely monitored to detect any residual effects of MEC on consumptive behaviors.
2.5. Drugs
Ethanol solutions (v/v) were made with ethyl alcohol (200 proof) mixed in tap water. Sweetener solutions (w/v) were constituted with sucrose or saccharin dissolved in tap water. MEC (mecamylamine hydrochloride; Sigma, St. Louis, MO) was dissolved in sterile saline. With the exception of the ethyl alcohol (Pharmco Products, Inc.; Brookfield, CT), all other reagents were acquired from Sigma-Aldrich Co. (St. Louis, MO).
2.6. Drinking patterns, response criteria, and statistical analyses
Ethanol and sucrose intakes (grams per kilogram of body weight; g/kg) were determined from the volume of solution depleted and pre-session body weights. Saccharin intakes were reported as mg/kg. For the two-bottle choice experiment, preference ratios for 10E and 0.033Sac were calculated as the test solution volume divided by total fluid volume (test solution plus water). For the operant study, cumulative records of lever and sipper (i.e., lick) responding were recorded by MED-PC IV software (MED Associates, Inc.). In rodent models, assessment of the drinking topography in the form of bout size and frequency can be used in an analogous fashion to the quantity-frequency measures used in humans (see Samson and Hodge, 1996). Measures of appetitive (response rate, latency to first press, latency to first bout) and consummatory (bout frequency, bout size, bout duration, bout lick rate) processes were derived from these cumulative records via a custom data analysis program written for the online software R Project for Statistical Computing (http://www.r-project.org). Based on previous work, an ethanol or a sucrose bout was defined as ≥ 20 licks with < 60-sec pause between successive licks (Finn et al., 2008; Ford et al., 2002; 2007a). Bout lick rate was calculated from the average rate of all bouts expressed, and did not include the time elapsed between bouts. Since appetitive and consummatory measures following baseline saline injection sessions were not statistically different across the treatment time course (3 weeks), they were collapsed and reported as 0 mg/kg MEC (saline control).
All statistical analyses were performed using the SigmaStat version 2.0 software package (Jandel Scientific; San Rafael, CA). All response measures, intakes, and bout pattern variables for the operant study were analyzed by one-way repeated measures ANOVA (factor: Dose). For the analysis of consumption variables in the two-bottle choice experiment and for overall locomotor activity (50-min total), two-way repeated measures ANOVA (factors: Treatment, Dose) were used, with Treatment as the repeated factor. Additional two-way repeated measures ANOVA (factor: Dose, Interval) were conducted for the temporal distribution of licks (10-min intervals) and for the temporal analysis of locomotor activity (10-min intervals). In the event that a significant Dose × Interval or Treatment × Interval interaction occurred, then a subsequent Simple Main Effects analysis of Dose or Treatment within each interval was separately carried out. Correlations between variables were evaluated by the Pearson product moment test. In all cases, statistical significance was set at P ≤ 0.05.
3. Results
3.1. Operant self-administration
3.1.1. Ethanol- and sucrose-reinforced responding
Fourteen of sixteen 5S-reinforced and ten of sixteen 10E-reinforced mice exhibited reliable completion of the response requirement on the active lever (i.e., RR16) following operant conditioning. All mice completed the RR following pretreatment with saline or 2, 4 and 6 mg/kg MEC. Two mice, one 5S-reinforced and one 10E-reinforced, were unable to complete the RR consequent to the 8 mg/kg MEC dose. These two mice were excluded from the analysis of consumptive measures for this treatment session because sipper access was never realized.
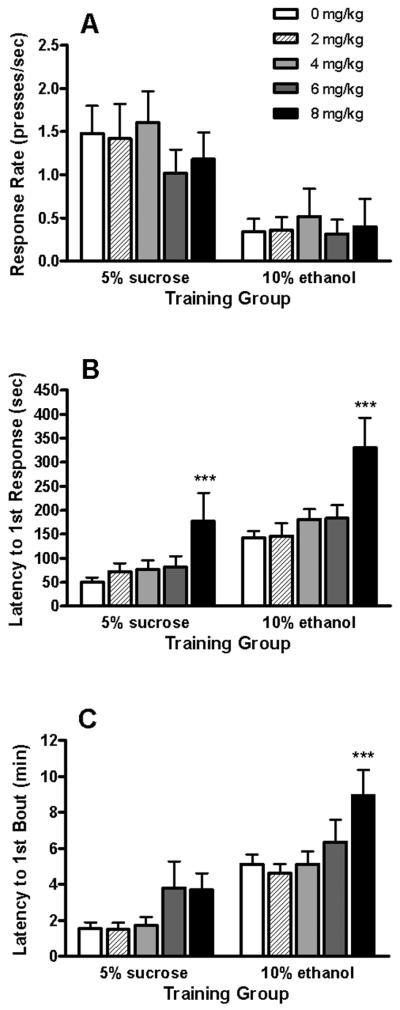
MEC did not alter inactive lever response frequency (means of 0.45 ± 0.25 and 1.25 ± 0.29 for 5S- and 10E-reinforced mice, respectively) or total response frequency summed from both levers (data not shown). MEC also had no effect on active lever response rates (Fig. 1A). However, significant main effects of MEC dose on the latency to 1st response on the active lever were noted for both the sucrose- [F(4,51) = 4.90; P < 0.01] and ethanol-reinforced [F(4,36) = 7.29; P < 0.001] mice. The 8 mg/kg MEC dose significantly augmented response latencies by 3.6- and 2.3-fold in the 5S- and 10E-trained animals (Ps < 0.001), respectively, when compared to saline baseline values (Fig. 1B). Another appetitive measure evaluated was the latency to initiate the first drinking bout following sipper presentation (Fig. 1C). MEC treatment significantly increased latency to the 1st bout in 10E-reinforced mice [F(4,33) = 4.88; P < 0.01], with the 8 mg/kg dose eliciting a 75% increase in this measure (P < 0.001) versus saline treatment. There also was a tendency for MEC to augment these latencies in sucrose-reinforced mice [F(4,48) = 2.17; P = 0.09].
Fig. 1. Effects of MEC on appetitive measures in 5S- and 10E-reinforced mice.
All mice were trained to complete a response requirement of 16 (RR16) active lever presses to gain 30-min continuous access to the reinforcer solution. The response rate on the active lever (panel A), the latency to first active lever response (panel B), and latency from RR completion to initiation of the first drinking bout (panel C) are shown for each operant group. Only mice completing the RR16 were included in the analyses. Data represent the mean ± SEM for 5S-reinforced (n = 14) and 10-reinforced (n = 10) mice. ***P < 0.001 versus within-group saline treatment (i.e., 0 mg/kg) values; Fisher Least Square Difference (LSD) post-hoc test.
3.1.2. Intakes and bout micro-architecture for ethanol and sucrose
Significant positive correlations were found between sipper contacts and the g/kg of solution consumed for both the 5S- (r = 0.79, P < 0.001, n = 66) and 10E-reinforced (r = 0.95, P < 0.001, n = 49) mice, indicating that the cumulative licks recorded accurately reflected the consumption that occurred. MEC pretreatment significantly decreased the amount of sucrose consumed [F(4,48) = 10.29; P < 0.001], with the 6 and 8 mg/kg MEC doses significantly attenuating this measure by 25-40% (Ps < 0.001) versus saline administration (Fig 2A). Ethanol intake [F(4,35) = 19.23; P < 0.001] was also significantly influenced by MEC treatment (Fig. 2A), with the 6 and 8 mg/kg MEC doses markedly reducing 10E consumption by 40-65% (Ps < 0.001) when compared to saline injection.
Fig. 2. Effects of MEC on the intake and bout micro-architecture of 5S and 10E solutions.
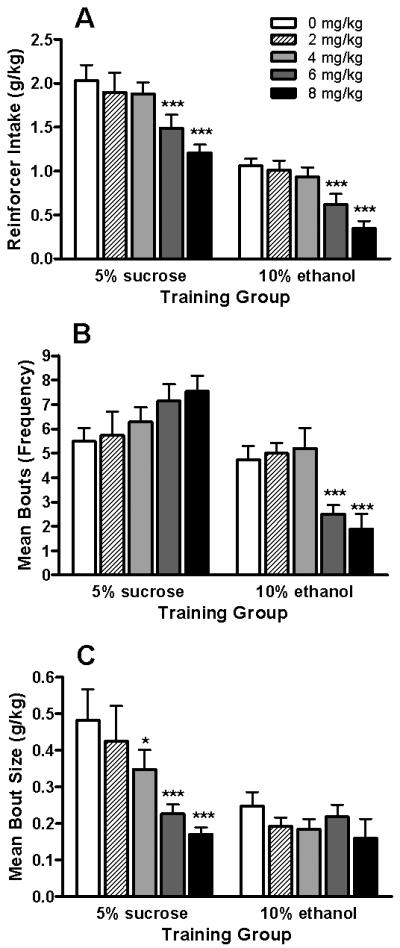
Mice acquired 30-min continuous access to a reinforcer solution following completion of a RR16 schedule. The g/kg intake (panel A), mean bout frequency (panel B), and mean bout size (panel C) are depicted for each operant group. Only mice completing the response requirement were included in the analyses. Vertical bars represent the mean ± SEM for 5S-reinforced (n = 14) and 10-reinforced (n = 10) mice. *P < 0.05, ***P < 0.001 versus within-group saline treatment (i.e., 0 mg/kg) values; Fisher Least Square Difference (LSD) post-hoc test.
Although MEC non-selectively decreased 5S and 10E intake (Fig. 2A), the bout dynamics underlying 10E consumption were uniquely modulated by MEC when compared to 5S drinking patterns. The MEC-elicited reduction in 10E intake was attributable to decreases in bout frequency [F(4,35) = 15.59; P < 0.001], with the 6 and 8 mg/kg MEC doses significantly diminishing bout frequency by 60-65% (Ps < 0.001). In contrast, the 5S-reinforced mice exhibited a non-significant 30-35% increase in bout frequency following these two highest MEC doses (Fig. 2B). Furthermore, the MEC-elicited decrease in 5S consumption was primarily due to an attenuation in bout size [F(4,48) = 7.56; P < 0.001]. Following pretreatment with 4, 6, and 8 mg/kg MEC, the mean bout size in 5S-reinforced mice was significantly reduced by 30% (P = 0.05), 55% (P < 0.001), and 65% (P < 0.001), respectively, when compared to saline administration (Fig. 2C). MEC exhibited no effect on bout size in 10E-reinforced mice [F(4,33) = 1.17; P = 0.34]. In addition, significant main effects of MEC on bout lick rate [F(4,48) = 8.64; P < 0.001] and bout duration [F(4,48) = 7.11; P < 0.001] were observed only for 5S consumption. The 6 and 8 mg/kg doses significantly elevated 5S lick rates by 2.0- to 2.2-fold (Ps < 0.001) while suppressing the duration of sucrose bouts by 60-65% (Ps < 0.001) versus saline baseline (data not shown).
3.1.3. Temporal distribution of cumulative licks for ethanol and sucrose
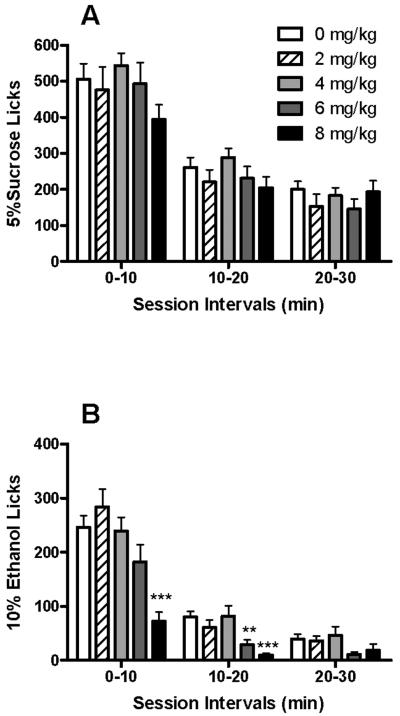
The temporal distribution of 5S licks (Fig. 3A) was significantly influenced by session interval [F(2,26) = 167.15; P < 0.001], with a significant dose × interval interaction [F(8,104) = 2.44; P < 0.001]. There was a trend for a main effect of MEC dose on 5S licks [F(4,52) = 2.05; P = 0.10). Evaluation of simple main effects within session intervals revealed a significant influence of MEC dose only at the 10-20 min interval [F(4,52) = 2.93; P < 0.05]. Despite this effect on 5S licks during minutes 10-20, no pair-wise differences versus vehicle treatment were apparent (Fig. 3A). The analysis of the temporal distribution of 10E licks revealed significant effects of MEC dose [F(4,36) = 25.49; P < 0.001], session interval [F(2,18) = 90.90; P < 0.001], and a dose × interval interaction [F(8,72) = 8.82; P < 0.001]. Simple main effects of MEC dose on 10E licks were subsequently examined, with statistical significance occurring at the 0-10 min [F(4,36) = 19.66; P < 0.001] and 10-20 min [F(4,36) = 9.89; P < 0.001] session intervals. The 6 mg/kg dose of MEC significantly reduced 10E licks during the 10-20 min interval by 65% (P < 0.01) whereas 8 mg/kg MEC significantly attenuated this measure during the 0-10 and 10-20 min intervals (Ps < 0.001) by 71% and 87%, respectively, when compared to saline administration (Fig. 3B).
Fig. 3. Effects of MEC on the temporal distribution of 5S and 10E licks.
Operant sessions included a variable length appetitive responding phase followed by a fixed length 30-min sipper access period. The mean ± SEM lick numbers for 5S-reinforced mice (panel A; n = 14) and 10E-reinforced mice (panel B; n = 10) are depicted. *P < 0.05, **P < 0.01, and ***P < 0.001 versus saline treatment (0 mg/kg) values within the respective session interval; Tukey post-hoc test.
3.2. Assessment of locomotor activity
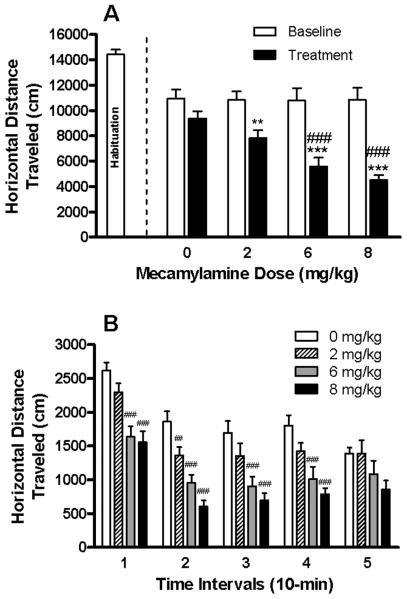
In a separate cohort of mice, the influence of MEC on locomotor activity was assessed. MEC dose-dependently reduced horizontal distance traveled [F(3,43) = 14.04; P < 0.001] during the 50-min session, with 6 and 8 mg/kg MEC significantly decreasing this measure by 40% (P < 0.001) and 52% (P < 0.001), respectively, when compared to the saline-treated control group (Fig. 4A). To determine the duration and time course of MEC's impact on activity, a subsequent analysis of horizontal distance traveled per 10-min interval was conducted (see Fig. 4B). There was a significant main effect of MEC dose [F(3,43) = 14.04; P < 0.001] and interval [F(4,12) = 58.73; P < 0.001], and a significant dose × interval interaction [F(12,172) = 1.81; P = 0.05]. Simple main effects analyses revealed that distance traveled was significantly altered by MEC dose [F(3,43) ≥ 8.60; Ps < 0.001], with 6 and 8 mg/kg MEC persistently attenuating this activity measure throughout the first four 10-min intervals (Ps < 0.001). The activity suppressing effects of MEC began to dissipate during minutes 40-50 of the locomotor assessment. <<Insert Fig. 4 here>>
Fig. 4. Effects of MEC on locomotor activity.
Activity subsequent to MEC treatment is illustrated as both the total horizontal distance traveled throughout the 50-min session (panel A) and per 10-min interval (panel B). In panel A, values for the first activity session (habituation) are shown for reference only, and are not included in the statistical analyses. Baseline and treatment sessions represented the second and third consecutive days of activity monitoring, respectively. The mean ± SEM for n = 11-12 per treatment group is shown. **P < 0.01 and ***P < 0.001 versus within-group baseline (panel A only); ##P < 0.01 and ###P < 0.001 versus saline (i.e., 0 mg/kg) group on treatment day (panels A-B); Fisher Least Square Difference (LSD) post-hoc test.
3.3. Assessment of two-bottle choice tests for ethanol and saccharin
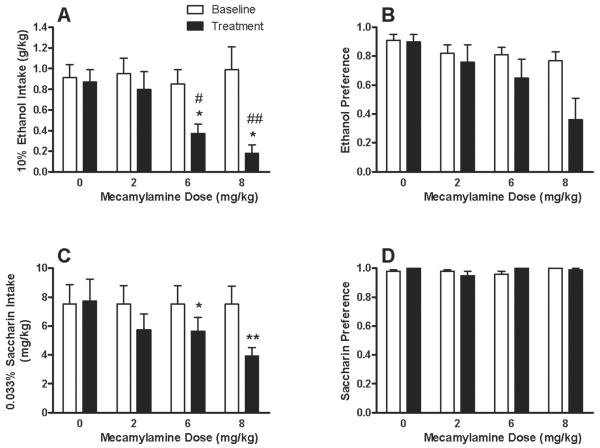
Although MEC non-selectively reduced consumption of ethanol and sucrose solutions under an operant condition, it was not known whether MEC would similarly affect drinking under a two-bottle choice procedure. A two-way repeated measures ANOVA revealed a significant main effect of treatment [F(1,40) = 18.16; P < 0.001] and a dose × treatment interaction [F(3,40) = 3.41; P < 0.05] for g/kg ethanol consumed (Fig. 5A). Consistent with MEC's influence on operant ethanol self-administration (refer to Fig. 2A), the 6 and 8 mg/kg doses significantly reduced ethanol intake by 56% (P < 0.01) and 75% (P < 0.01), respectively, when compared to their respective within-subject baseline measure (Fig. 5A). Furthermore, 6 mg/kg (P < 0.05) and 8 mg/kg (P < 0.01) MEC significantly reduced 10E intake versus the saline control group. Although a significant main effect of MEC dose on ethanol preference was observed [F(1,40) = 6.64; P < 0.05], a treatment × dose interaction was absent. Notably, treatment with 8 mg/kg MEC was associated with a 50% decrease in the ethanol preference ratio (Fig. 5B).
Fig. 5. Effects of MEC on 10E and 0.033Sac preference drinking.
The g/kg intake of 10E (panel A) and the mg/kg intake of 0.033Sac (panel C) are shown along with the corresponding preference ratios for each solution (panels B and D, respectively) during 2-hr drinking sessions. Baseline measures represented a 2-day average of all treatment groups immediately prior to MEC testing. The mean ± SEM for n = 11-12 per treatment group is depicted. *P < 0.01 and **P < 0.01 versus within-group baseline; #P < 0.05 and ##P < 0.01 versus saline (i.e., 0 mg/kg) group on treatment day; Tukey post-hoc test.
A subsequent evaluation of preference drinking with a 0.033Sac solution determined that mg/kg intakes were significantly influenced by MEC treatment [F(1,41) = 17.76; P < 0.001], and that a dose × treatment interaction [F(3,41) = 3.53; P < 0.05] was also present. Although no pair-wise differences were identified between treatment groups, the 6 mg/kg (P < 0.05) and 8 mg/kg (P < 0.01) dose groups did exhibit significant reductions in 0.033Sac intake when compared to their respective within-group baseline levels (Fig. 5C). In contrast to ethanol preference, no effect of MEC on 0.033Sac preference was observed (Fig. 5D). Mice demonstrated high preference ratios for the 10E and 0.033Sac solutions (Fig. 5, panels B & D), and the volume of water consumed during the 2-hr limited access sessions was typically at or below the limit of detection (0.05 ml). Thus, effects of MEC on total fluid intake (data not shown) mirrored the observed changes in 10E and 0.033Sac intakes. Body weights and between-session water intakes (over 22-hrs) were monitored daily, and neither were affected following MEC exposure (data not shown).
4. Discussion
The present series of studies examined the influence of systemic nAChR antagonism (i.e., MEC) on ethanol intake with two different procedures, one of which allowed for the separation of appetitive and consummatory processes. Parallel assessments with another positive reinforcer (e.g., 5S and 0.033Sac) and another behavioral endpoint (e.g., locomotor activity) determined the degree of MEC selectivity in suppressing ethanol self-administration. MEC exhibited only a subtle influence on the appetitive responding required to gain access to either an ethanol or a sucrose solution, with the highest MEC dose tested (8 mg/kg) producing a non-selective, significant increase in the latency to the first active lever press in 10E- and 5S-reinforced mice. In contrast to this modest effect on appetitive behavior, 6 and 8 mg/kg MEC significantly attenuated the amount of 10E (by up to 67%) or 5S (by up to 40%) consumed during the 30-min consumption phase of the operant session. Notably, MEC differentially altered the bout microarchitecture underlying ethanol and sucrose consumption. The MEC-induced reduction in 10E intake was attributable to commensurate decreases in bout frequency, whereas the attenuation in 5S consumption was due to a decrease in bout size. A separate analysis of MEC effects on two-bottle choice drinking in the home cage also revealed a non-selective reduction in 10E and 0.033Sac intakes following pretreatment with 6 and 8 mg/kg MEC. Finally, the 6 and 8 mg/kg doses of MEC significantly suppressed locomotor activity. In summary, MEC significantly attenuated the self-administration of ethanol and sweet solutions in mice, an effect that was likely attributable, in part, to a concomitant suppression in locomotor activity.
MEC pretreatment in the current set of experiments dose-dependently decreased ethanol intake by 5-70% in mice. Although ethanol preference was not significantly altered, the highest MEC dose led to a 50% reduction in ethanol preference ratio. The suppressive influence of MEC on the amount of ethanol consumed was comparable across the procedures performed (operant and 2-bottle choice) or the length of access provided (30-min and 120-min), and was consistent with the majority of earlier reports in rats. A recent report by Kuzmin and colleagues (2009) demonstrated that 2.5 and 5 mg/kg MEC reduced the frequency of ethanol deliveries obtained by male Wistar rats performing under a FR-1 schedule by 72% and 94%, respectively. Similar to the current work, these investigators found no evidence that MEC influenced the level of inactive lever responding. However, in both cases, baseline inactive lever responding was already minimal, so the absence of a MEC effect on this measure may have been due to a floor effect. Secondly, 2 mg/kg MEC provided twice daily for 3 days led to an approximate 40% reduction in ethanol intake in high-preferring, but not low-preferring, rats over a 6-hr period (Blomqvist et al., 1996). In this study, no changes in ethanol preference were found, despite a significant suppression of total fluid intake in both lines. In a separate report, two consecutive days of treatment with either 2 or 4 mg/kg MEC produced a dose-dependent attenuation in ethanol consumption by 40-70% during 60-min sessions (Le et al., 2000). Although concomitant water intakes were not altered by MEC treatment in this study by Le and colleagues, baseline consumption of water was purportedly undetectable. In the current work, a similar limitation was met in that water intakes were at or below the level of detection, making it difficult to draw definitive conclusions regarding MEC's impact on ethanol preference. Lastly, intra-VTA infusion of MEC (100 μM) immediately prior to a 60-min access session decreased ethanol intake by 50-60% while concomitantly enhancing water intake by 40-50% (Ericson et al., 1998). However, the observation that MEC simultaneously attenuated both ethanol consumption and ethanol preference could have stemmed from the 23-hr fluid deprivation imposed prior to the limited access to ethanol in that study, which would have enhanced overall fluid intake. A separate study in rats determined that 1.5 mg/kg MEC did not alter home cage water intake (Clarke and Kumar, 1984). Collectively, these findings confirm that MEC consistently suppresses ethanol intake, but that the dose of MEC required for the reduction of ethanol consumption in mice (6-8 mg/kg) is considerably higher than those doses previously demonstrated to decrease ethanol intake in rats (2-4 mg/kg). The variable effects on ethanol preference may have been due to procedural differences, such as the degree of satiation, which influenced the assessment of fluid intake.
To date, parallel effects of MEC pre-treatment on an alternate reinforcer (i.e., a sweet solution) have not been investigated within an operant self-administration procedure. Thus, the current studies addressed the selectivity of MEC-induced alterations in ethanol self-administration by examining sucrose-reinforced responding and saccharin preference drinking. Consistent with the findings for ethanol reinforcement, MEC exhibited no influence on the rate of responding under an operant condition of sucrose self-administration, but did increase the latency to first response. Treatment with 6 and 8 mg/kg MEC suppressed the amount of sucrose and saccharin consumed by up to 40% and 50%, respectively. Although it should be noted that prior history of 10E preference testing may have confounded the subsequent evaluation of 0.033Sac drinking in the same cohort of mice, the consistency of findings between the operant and two-bottle choice experiments regarding the influence of MEC on sweetener intake would suggest that this drinking history had minimal impact on the results. The MEC treatment effects on sweet solutions were less than the observed declines in ethanol intake (maximal 65% decrease for operant study and 70% decrease for two-bottle choice study). The greater magnitude of suppression in g/kg ethanol versus sucrose intake was consistent with the significant shift in the temporal patterns of licks that were observed only in ethanol-reinforced mice (see Fig. 3). However, the two highest doses of MEC tested resulted in non-selective reductions in the self-administration of ethanol and sweet solutions. Collectively, these findings suggest that MEC-elicited decreases in ethanol intake may generalize to multiple consummatory behaviors.
By using the `sipper' method in the current study, drinking patterns were assessed under a contingency that permitted mice to regulate their own rate of consumption (see Samson et al., 1998, 2000; Ford et al., 2007a). In humans, quantity-frequency measures of ethanol intake are used to estimate habitual use and can help to diagnose individuals in which a regulatory loss of control has occurred (Feunekes et al., 1999). Thus, the examination of drinking patterns can provide vital information on the mechanism of altered drinking topography subsequent to a change in access conditions or following a pharmacological intervention. For instance, Hölter and colleagues (1998) demonstrated that a period of ethanol abstinence can result in a relapse-like effect that is characterized by a significant elevation in the frequency of drinking bouts. Furthermore, it has been suggested that the efficacy of naltrexone as a pharmacotherapy for alcoholism may be contingent upon a particular pattern of drinking that is susceptible to change (Anton et al., 2004). Despite the overall absence of MEC selectivity in the present work, one key finding was that MEC differentially altered the bout micro-architecture of self-administered ethanol versus sucrose (i.e., decrease in bout frequency versus bout size, respectively). Since MEC is known to interact with multiple nAChR isoforms, it is theoretically feasible that an antagonist with greater receptor subunit specificity could be designed to selectively reduce ethanol bout frequency while sparing the bout sizes of alternate reinforcers like sucrose. An alternate strategy could be the application of a nAChR partial agonist, as was recently employed by Steensland and colleagues (Steensland et al., 2007). These investigators demonstrated that varenicline, which exhibits greater affinity but is less efficacious at α4β2-containing nAChRs than full agonists like acetylcholine, decreased ethanol responding without significantly affecting sucrose responding or water consumption. Additional work will have to be conducted to determine which strategy is best suited to selectively modulate ethanol-self administration patterns.
Previous reports on MEC-induced alterations in activity, cognitive function, and memory support the claim that non-selective effects of MEC contributed to the observed changes in ethanol, sucrose, and saccharin drinking described in this work. Whereas a 10 mg/kg MEC dose disrupted Morris water maze performance (Decker and Majchrzak, 1992) and induced an amnesic effect within a passive avoidance procedure (Rush and Streit, 1992), an 8 mg/kg dose was found to decrease locomotor activity by 40% in mice (Blomqvist et al., 1992). In another report, sizeable yet non-significant decreases in baseline locomotion were noted following pretreatment with 4 and 6 mg/kg MEC in multiple mouse strains (Kamens and Phillips, 2008). In the present study, the 6 and 8 mg/kg MEC doses suppressed locomotor activity by 40-52% , and the 8 mg/kg MEC dose prolonged the latency to the first active lever response by 3.6-fold and 2.3-fold in the 5S- and 10E-trained mice, respectively. However, the total number of responses (on both levers) and the concomitant response rates were not significantly influenced by MEC treatment, and the differential modulation of ethanol versus sucrose drinking patterns following MEC injection would not have been expected if treatment effects were solely due to a locomotor suppression. While the present findings collectively suggest a dissociation of MEC effects on goal-directed behavior and drinking patterns versus its influence on locomotor activity, it is likely that the MEC-induced suppression of locomotor activity contributed to the pronounced reduction in ethanol, sucrose, and saccharin consumption.
Future studies will be required to further delineate the role of nAChRs in ethanol reinforcement and self-administration. A combination of pharmacological and genetic strategies holds promise in achieving this goal. For example, the peripherally-active nAChR antagonist, hexamethonium, exhibited no effect on ethanol intake or preference (Blomqvist et al., 1996), suggesting that centrally-located nAChRs are necessary for mediating reductions in consumption. Furthermore, preliminary examination of additional nAChR antagonists indicate that regulation of ethanol self-administration will require receptor isoform specificity, as the competitive antagonist DHβE, which is specific for α4β2 subunit-containing receptors, was without influence on ethanol consumption (Kuzmin et al., 2009; Le et al., 2000) whereas αCTX, an antagonist with preference for α6β2β3-containing receptors, exhibited efficacy in reducing drinking (Kuzmin et al., 2009; Larsson et al., 2004). Unfortunately, assessments of selectivity in the manipulation of ethanol intakes have yet to be undertaken for these antagonists, as have been reported in the current study and in the previous work with the partial agonist, varenicline (Steensland et al., 2007). Although not yet tested for ethanol consumption, α7 null mutant mice exhibit sensitized responses to ethanol-induced locomotion, hypothermia and loss of righting reflex (Bowers et al., 2005), thereby indicating that this and other genetically modified animal models may prove useful for unraveling the relationship between nAChRs and ethanol-related behaviors (Balogh et al., 2002). Assessment of pharmacological and genetic manipulations of nAChRs within an operant self-administration procedure for ethanol that accounts for drinking patterns could provide valuable insights into reinforcement mechanisms as well as the regulatory processes governing ethanol intake, thereby opening new avenues of pharmacotherapeutic treatment strategies for ethanol abuse.
Acknowledgements
This work was supported in part by the Department of Veteran Affairs VA Merit Review grant (DAF) and National Institute of Health grants AA16849 (MMF), AA13478 (DAF), and DA14639 (GPM). The authors would like to thank Dr. Tamara Phillips for the use of her activity monitor chambers, for her advice on designing the locomotor activity study, and for the gift of mecamylamine hydrochloride.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Owens JC, Butt CM, Wehner JM, Collins AC. Animal models as a tool for studying mechanisms of co-abuse of alcohol and tobacco. Alcohol. Clin. Exp. Res. 2002;26:1911–1914. doi: 10.1097/01.ALC.0000040847.98115.6D. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Piasecki J, Koros E, Stefanski R, Kostowski W. Studies on the role of nicotinic acetylcholine receptors in the discriminative and aversive stimulus properties of ethanol in the rat. Eur. Neuropsychopharmacol. 1998;8:79–87. doi: 10.1016/s0924-977x(97)00052-7. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Söderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur. J. Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur. J. Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol. Clin. Exp. Res. 2002;26:326–331. [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res. Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol. Clin. Exp. Res. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav. Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol. Clin. Exp. Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Clark A, Little HJ. Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend. 2004;75:199–206. doi: 10.1016/j.drugalcdep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. Some effects of nicotine on food and water intake in undeprived rats. Br. J. Pharmacol. 1984;82:233–239. doi: 10.1111/j.1476-5381.1984.tb16463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Decker MW, Majchrzak MJ. Effects of systemic and intracerebroventricular administration of mecamylamine, a nicotinic cholinergic antagonist, on spatial memory in rats. Psychopharmacology. 1992;107:530–534. doi: 10.1007/BF02245267. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J. Stud. Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Dyr W, Koros E, Bienkowski P, Kostowski W. Involvement of nicotinic acetylcholine receptors in the regulation of alcohol drinking in Wistar rats. Alcohol Alcohol. 1999;34:43–47. doi: 10.1093/alcalc/34.1.43. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur. J. Pharmacol. 1998;358:189–96. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Ericson M, Engel JA, Soderpalm B. Peripheral involvement in nicotine-induced enhancement of ethanol intake. Alcohol. 2000;21:37–47. doi: 10.1016/s0741-8329(99)00099-3. [DOI] [PubMed] [Google Scholar]
- Feunekes GI, van `t Veer P, Van Staveren WA, Kok FJ. Alcohol intake assessment: the sober facts. Am. J. Epidemiol. 1999;150:105–112. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology. 2008;201:423–433. doi: 10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol. Clin. Exp. Res. 2002;26:635–643. [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA. Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol. 2007a;41:21–29. doi: 10.1016/j.alcohol.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav. Brain. Res. 2007b;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Lê AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res. Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hölter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav. Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol. Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Katner SN, McBride WJ. Involvement of CNS cholinergic systems in alcohol drinking of P rats. Addict. Biol. 1997;2:215–224. doi: 10.1080/13556219772769. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, van Doornen LJ, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: a bivariate genetic analysis. Alcohol. Clin. Exp. Res. 1997;21:537–546. [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology. 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci. Biobehav. Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Larsson A, Jerlhag E, Svensson L, Söderpalm B, Engel JA. Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol. 2004;34:239–350. doi: 10.1016/j.alcohol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol. Clin. Exp. Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Little HJ. Behavioral mechanisms underlying the link between smoking and drinking. Alcohol Res. Health. 2000;24:215–224. [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programmed and spontaneous drinking. J. Pharmacol. Exp. Ther. 1970;173:101–116. [PubMed] [Google Scholar]
- Nadal R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol. Clin. Exp. Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- National Research Council of the National Academies . Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J. Pharmacol. Exp. Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, McKinnon CS, Cunningham CL. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav. Neurosci. 2005;119:892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Rush DK, Streit K. Memory modulation with peripherally acting cholinergic drugs. Psychopharmacology. 1992;106:375–382. doi: 10.1007/BF02245421. [DOI] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol. Clin. Exp. Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol. Clin. Exp. Res. 2000;24:766–773. [PubMed] [Google Scholar]
- Samson HH, Hodge CW. In: Neurobehavioral regulation of ethanol intake, in Pharmacological Effects of Ethanol on the Nervous System. Deitrich RA, editor. CRC Press; New York: 1996. pp. 203–226. [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol. Clin. Exp. Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav. Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc. Natl. Acad. Sci. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch. Gen. Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol. Clin. Exp. Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]