Abstract
Epidemiological and clinical evidence has suggested that increased dietary intake of fish oil containing w-3 fatty acids including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) may be associated with a reduced risk of asthma. However, interventional studies on these effects have been equivocal and controversial. Free radical oxidation products of lipids and cyclooxygenases-derived prostaglandins are believed to play an important role in asthma, and fish oil supplementation may modulate the levels of these critical lipid mediators. We employed a murine model of allergic inflammation produced by sensitization to ovalbumin (OVA) to study the effects of fish oil supplementation on airway inflammation. Our studies demonstrated that ω-3 fatty acids were dose-dependently incorporated into mouse lung tissue after dietary supplementation. We examined the oxidative stress status by measuring the levels of isoprostanes (IsoPs), the gold standard for oxidative stress in vivo. OVA challenge caused significant increase of F2-IsoPs in mouse lung, suggesting an elevated level of oxidative stress. Comparing to the control group, fish oil supplementation led to a significant reduction of F2-IsoP (from arachidonic acid) with a concomitant increase of F3-IsoPs (from EPA) and F4-IsoPs (from DHA). Surprisingly, however, fish oil supplementation enhanced production of pro-inflammatory cytokine IL-5 and IL-13. Furthermore, fish oil supplementation suppressed the production of pulmonary protective PGE2 in the bronchoalveolar lavage (BAL) while level of urinary metabolite of the PGE2 was increased. Our data suggest that augmented lung inflammation after fish oil supplementation may be due to the reduction of PGE2 production in the lung and these dichotomous results bring into question the role of fish oil supplementation in the treatment of asthma.
Keywords: fish oil, ω-3 polyunsaturated fatty acids, asthma, free radicals, lung inflammation, mass spectrometry, Isoprostanes, prostaglandins, ovalbumin (OVA)
Introduction
Asthma is a chronic inflammatory disorder of the respiratory tract, involving variable airflow obstruction and increased airway hyperresponsiveness (AHR) to a variety of stimuli [1, 2]. Mounting evidence has suggested that oxidative stress plays an important role in the pathophysiology of asthma [3-5]. Many substances including allergens, gaseous pollutants, chemicals, drugs, bacteria, and viruses can cause recruitment and activation of inflammatory cells in asthmatic airways. The activated inflammatory cells generate reactive oxygen species (ROS) and release them into surrounding cells. When the ROS overwhelm the host antioxidant defense, oxidative stress causes many detrimental effects on airway functions including airway smooth muscle contraction, induction of AHR, mucus hypersecretion, epithelial shedding, and vascular exudation [3]. It is well established that membrane lipids containing polyunsaturated fatty acids (PUFAs) are primary targets for ROS attack and an array of oxidation products can be generated [6]. Isoprostanes (IsoPs), isomers of cyclooxygenase (COX)-derived prostaglandins (PGs), are one of the major classes of lipid peroxidation products generated from the membrane lipids by free radical reactions [7, 8]. Analysis of F2-IsoPs by gas chromatography-mass spectrometry (GC-MS) has been regarded as the gold standard to assess oxidative stress status [9-11]. Elevated levels of F2-IsoPs have been observed in animal models of asthma and in asthmatic patients [12-14]. Levels of these IsoPs in urine, plasma, and breath condensate of asthma patients correlate well with the severity of asthma [15-17]. Furthermore, some of these IsoPs, such as 15-F2t-IsoP (8-iso-PGF2α) and 15-E2t-IsoP (8-iso-PGE2), have been found to cause potent vasoconstriction in human and guinea pig airways, cause airway obstruction and airway plasma exudation in guinea pigs in vivo [10, 18, 19]. These findings suggest that oxidative stress may be involved in the onset and progression of asthma and thus antioxidants may be beneficial in asthma treatment through attenuation of the oxidative stress in the airway [5].
PGs, lipid mediators derived from COXs, also play a critical role in allergic lung inflammation [20]. In the mouse model, inhibition of PG production by COX inhibitors results in increased allergic inflammation, suggesting that the overall effects of PGs during the allergen sensitization and challenge process is to restrain allergic inflammation [21-24]. Current in vivo animal studies suggest that PGD2 and Thromboxane A2 (TXA2) both increase allergic lung inflammation whereas the PGE2 and PGI2 restrain the allergen-induced inflammatory response. More specifically, PGE2 suppresses allergic inflammation through the EP3 receptor pathways and inhibits eosinophil trafficking through EP2 receptors [25, 26]. Furthermore, selective PGE2 receptor agonists are being evaluated as therapeutic agents for the treatment of asthma [27].
Although medication and environmental manipulation play an important role in the treatment of asthma, dietary intervention appears to be an alternative therapy. Evidence from some epidemiological studies has suggested that consumption of ω-3 fatty acids such as EPA and DHA may reduce the incidence of asthma while dietary intake of a high fat diet rich in ω-6 fatty acids is associated with a higher risk of asthma [28-30]. However, clinical data of interventional studies on the effects of fish oil intake has been equivocal and controversial [31, 32]. While some interventional studies have observed clinical improvements, other studies have not demonstrated improvements in asthmatic symptoms following ω-3 PUFAs supplementation [1, 33-35]. Recent reports suggested that anti-inflammatory effects and other biologically relevant properties of ω-3 fatty acids may be due, in part, to the generation of various bioactive oxidation products [1, 28, 36, 37]. For example, EPA-derived resolvin E1 dampens airway inflammation and hyperresponsiveness in a mouse model of ovalbumin (OVA)-induced allergic lung inflammation while similar effects have been observed for protectin D1, an enzymatic product generated from DHA, in asthmatic patients and in this OVA model [38, 39].
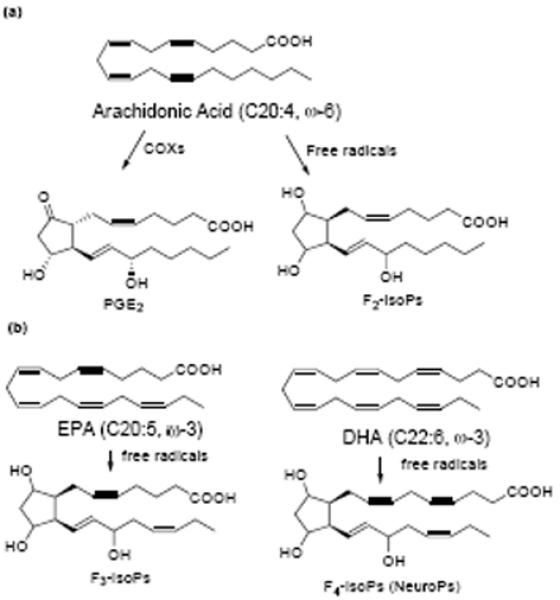
Our research group has extensively studied mechanisms of free radical-initiated peroxidation of PUFAs in vitro and in vivo and has developed a number of analytical techniques based on MS to analyze the oxidation products [40-42]. We have determined that oxidation of PUFAs generates an array of peroxidation products and F2-IsoPs, F3-IsoPs and F4-IsoPs are one of the major classes of oxidation products generated from AA, EPA and DHA respectively (scheme 1) [41, 43-45]. We contend that supplementation of ω-3 PUFAs may alter the production of oxidation compounds, such as F2-IsoPs, F3-IsoPs and F4-IsoPs, as well as the PGs. The modulation of the levels of these lipid mediators may have profound impact on allergic lung inflammation. In this study, we employed the OVA model to study the effects of fish oil supplementation on the levels of PGs and IsoPs and correlated these levels to airway inflammation. Our studies showed that OVA sensitization and challenge caused increased production of F2-IsoPs in mouse lung and fish oil supplementation significantly reduced the F2-IsoP levels with the concomitant increase of F3- and F4-IsoPs. Moreover, the production of PGE2 in the lung was suppressed. Alteration of these lipid mediators by dietary fish oil supplementation resulted in unexpected augmentation of lung inflammation as measured by the elevated levels of cytokine IL-5 and IL-13.
scheme 1.
Materials and methods
Reagents
Pentafluorobenzyl (PFB) bromide, diisopropylethylamine and OVA (Chicken, grade V) were obtained from Sigma (St. Louis, MO). Dimethylformamide, and undecane were obtained from Aldrich (Milwaukee, WI). N,O-bis(trimethylsilyl)-trifluoroacetamide (BSTFA) was obtained from Supelco Inc. (Bellefonte, PA). C18 and silica Sep-Paks were purchased from Waters Associates (Milford, MA). Thin layer chromatography (TLC) was performed on silica gel 60ALK6D plates (Whatman International Ltd., Maidstone, UK). All fatty acids were purchased from Nu-Chek Prep Inc. (Elysian, MN). D4-15-F2t-IsoP (8-iso-PGF2α) was purchased from Cayman Chemical Co. (Ann Arbor, MI). 17-F4c-neuroprostanes (17-F4c-NP) was chemically synthesized, and 18O exchange was performed according to a procedure reported previously [46, 47]. The unlabeled blank of the standard is 2 parts/thousand. Interleukin-5 (IL-5) and IL-13 were obtained from R&D Systems (Minneapolis, MN). AIN-93 diet was purchased from Dyets Inc (Bethlehem, PA).
Animals
Pathogen-free 8-week-old female BALB/c mice were purchased from Charles River (Wilmington, MA). Control mice were fed with an AIN-93 diet containing 4% (by weight) olive oil. Fish oil treated mice were fed with an AIN-93 diet containing either 2% or 4% (by weight) menhaden fish oil. In caring for animals, the investigators adhered to the Guide for the Care and Use of Laboratory Animals prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (revised 1996).
Study protocol
The time course for the animal studies was illustrated in Figure 1. Two protocols were designed to study the timing of fish oil supplementation and effects on inflammatory response. In the first protocol, the mice were fed 4% fish oil for ten weeks before OVA sensitization. For sensitization, mice were injected intraperitoneally with 0.1 ml (10 μg) of OVA (Chicken OVA, grade V; Sigma-Aldrich) complexed with 20 mg of Al(OH)3 on Day 0. After two weeks, the mice were challenged by exposing to aerosols of 1% OVA diluted in sterile phosphate-buffed saline using an ultrasonic nebulizer (Ultraneb 99; De Vilbis, Somerset, PA) for 40 min each day for four days. In the second protocol, fish oil feeding started two days after OVA sensitization. After 4 weeks feeding, the two groups of mice were challenged with OVA followed the same procedure as in the first protocol. Blood samples were obtained by retro-orbital bleeding and collected in heparinized tubes. Plasma was separated by centrifugation at 4 °C and samples were stored at -80 °C until analysis. Lung tissues were removed and frozen in liquid nitrogen and stored at -80 °C.
Figure 1.
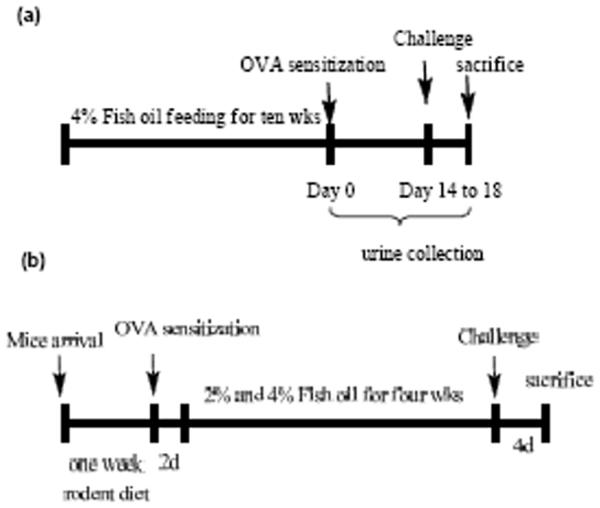
Time line of experimental protocols. (a) Fish oil feeding before OVA sensitization. (b) OVA sensitization prior to fish oil supplementation.
Urine and BAL collection
After the OVA challenge, mice were placed in metabolic cages and urine was collected. After the animals had been given a lethal dose injection of pentobarbital, BAL were performed by instilling 800 μl of normal saline through the tracheostomy tube and then withdrawing the fluid with gentle suction via the syringe. The typical BAL fluid return was 500-600 μl.
Fatty acids analysis by Liquid Chromatography (LC)-MS
Tissue (~ 50 mg) was homogenized in 5 ml ice-cold mixture of chloroform : methanol (2:1, v/v) containing 0.005% butylated hydroxyl toluene (BHT) to prevent auto-oxidation and placed at room temperature with occasional shaking for 1 h. Tridecanoic acid was added as internal standard. After addition of 2 ml of 0.9% NaCl, the sample was then vigorously vortexed and centrifuged. The organic layer was separated and dried under a stream of nitrogen. Then the residue was reconstituted in 1 ml methanol containing BHT. Phospholipids were hydrolyzed using chemical saponification by adding 1 ml 1 M aqueous potassium hydroxide. The incubation was carried out at 37°C for 30 min under nitrogen. The sample was acidified to pH 3 with 1 M HCl and extracted by heptane. Then the samples were dried under nitrogen and re-dissolved in methanol for LC-MS. MS was conducted using a Thermo TSQ Quantum Ultra instrument (San Jose, CA) equipped with a Finnigan Surveyor Autosampler Plus. Reversed phase HPLC was performed on a Phenomenex Luna C18(2) column (150 × 2 mm) with an isocratic of methanol/0.01% acetic acid at a flow rate of 200 μl/min. The MS was operated in the negative ion mode using electrospray ionization (ESI) in the selective reaction monitoring (SRM) mode. MS parameters were optimized for tridecanoic acid to achieve maximal response. Collision induced dissociation (CID) was optimized at 5 eV for under 1.0 mTorr of argon. Data acquisition and analysis were performed using Xcalibur software, version 2.0 (San Jose, CA).
Measurement of IsoPs by GC-MS
The purification and derivatization schemes for the analysis of F2-IsoPs, F3-IsoPs and F4-IsoPs were reported recently and utilized herein [46, 48, 49]. Briefly, tissue samples (~ 100 mg) were homogenized in 5 ml ice-cold chloroform : methanol (2:1, v/v) containing 0.005% BHT. Esterified IsoPs in phospholipids were isolated and hydrolyzed using chemical saponification by adding 1 M aqueous potassium hydroxide. The samples were acidified to pH 3 and diluted to 12 ml with pH 3 H2O. 1 ng d4-15-F2t-IsoP and 1 ng 18O 17-F4c-NP standards were then added to the samples and the samples were purified by silica and C18 Sep-Pak extraction. Purified IsoPs were converted to PFB esters and trimethylsilyl ether derivatives and analyzed by GC coupled to negative ion chemical ionization MS. GC-MS was performed using a Hewlett-Packard HP5989A GC-MS instrument interfaced with an IBM Pentium III computer system. GC was performed using a 15 m, 0.25 mm diameter, 0.25 μm film thickness, DB1701 fused silica capillary column (J & W Scientific, Folsom, CA). The column temperature was programmed from 190 to 300 °C at 15 °C/min. Methane was used as the reagent gas at a flow rate of 1 ml/min. Ion source temperature was 250 °C, electron energy was 70 eV, and filament current was 0.25 mA. For analysis, compounds were dissolved in 10 μl of undecane which was dried over a bed of calcium hydride.
Measurement of PGE2 in BAL and urinary metabolite PGE-M
Analysis of PGE2 in BAL and urinary metabolite of PGE2 (PGE-M) was performed using the methods reported previously [50] [51].
Quantitation of IL-5 and IL-13 in Lung Tissues
Levels of IL-5 and IL-13 in lung tissues of the three groups of mice were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocols and reported previously [52].
Statistical analysis
Data were expressed as mean ± standard deviation. The p values were calculated using either unpaired Student's t test for two group comparison or ANOVA with Bonferroni's post test for multi-group comparison using a commercial package (GraphPad Prism 3). Data were considered to be significant if P<0.05.
Results
Fish oil feeding alters lipid composition in mouse tissue
Before we performed the OVA experiments, time course of ω-3 fatty acid incorporation into various mouse tissues including lung was studied. In our experiment, mice were randomly divided into three groups: control, 2%, and 4% fish oil groups. The control mice were fed with olive oil 4% by weight, while fish oil-treated mice were fed with menhaden fish oil either at 2% or 4% by weight. Four mice from each group were sacrificed for fatty acid analysis each week for ten weeks. We developed a LC-MS method to analyze the fatty acids in mouse tissues. A representative chromatogram of the major PUFAs is shown in Fig. 2a. This LC-MS method has advantages over the conventional GC or GC-MS methods in that the free acids were directly analyzed after hydrolysis from the phospholipids. Formation of methyl ester or PFB ester is required for conventional GC or GC-MS analysis. The fatty acid composition in mouse lung after four weeks feeding was illustrated as an example in Fig. 2b. It is evident that fish oil supplementation dose-dependently decreased the level of arachidonic acid (C20:4) whereas the levels of EPA (20:5) and DHA (C22:6) was significantly increased (p< 0.05 comparing to the control). It should be noted that fish oil supplementation does not significantly change the level of linoleic acid (C18:2, ω-9). We also observed significant reduction of AA after two weeks. But it took at least three weeks to reach the steady-state for ω-3 fish oil in mouse lung tissue. The profile of fatty acids in mouse lung did not change significantly after four weeks (data not shown).
Figure 2.
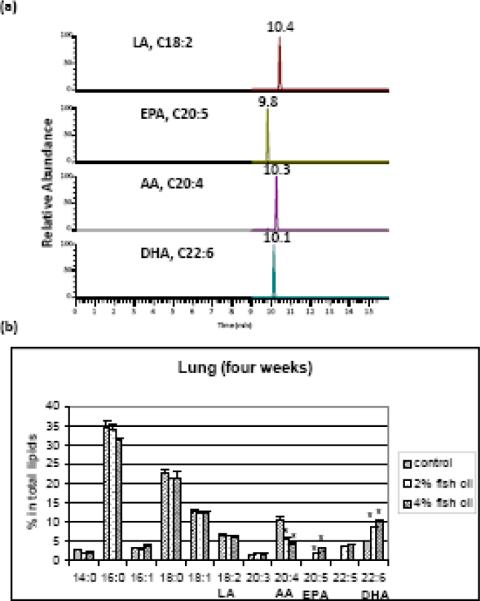
Lipid composition analysis in mice lung tissue by LC-MS. (a) Representative chromatogram of major PUFAs in mouse lung tissue. LA, linoleic acid (C18:2); EPA, eicosapentaenoic acid (C20:5); AA, arachidonic acid (C20:4); DHA, docosahexaenoic acid (C22:6). (b) Lipid composition in mouse lung after four-week fish oil feeding. Control mice were fed with an AIN-93 diet containing 4% (by weight) olive oil. Fish oil treated mice were fed with an AIN-93 diet containing either 2% or 4% (by weight) menhaden fish oil. *, p< 0.05 (comparing to the control). The data are expressed as means ± standard error of the mean (n = 4).
Effects of fish oil supplementation on IsoPs formation in mouse lung tissue
Oxidative stress has been implicated in pathogenesis of asthma. Elevated levels of F2-IsoPs, the gold standard for lipid oxidation and oxidative stress in vivo, have been reported in animal models and asthmatic patients [5, 14]. Analogous to F2-IsoPs, F3-IsoPs, and F4-IsoPs are one of the major classes of oxidation products generated from EPA and DHA respectively [41, 43-45]. Although two experimental protocols were designed for the feeding studies (Figure 1), we did not observe differences for the levels of the lipid mediators and inflammatory response between these two protocols. As shown in Figure 3, OVA challenge caused significant increase of F2-IsoP level from 1.9 ± 0.4 ng/g lung tissue at basal level to 25.1 ± 0.9 ng/g lung tissue (p < 0.0001). Both the basal and post OVA challenge F2-IsoPs levels were significantly suppressed after 4% Fish oil feeding (Figure 3A). We also examined the effects of fish oil supplementation on EPA and DHA-derived IsoPs formation in mouse lung tissue and observed significant increase of F3-IsoPs and F4-IsoPs in mouse lung tissue after 4% fish oil feeding for ten weeks even without OVA challenge. Further increase of these oxidation products was observed after OVA challenge and levels of F4-IsoPs derived from DHA were shown in Figure 3b. EPA-derived F3-IsoPs followed similar trend (data not shown).
Figure 3.
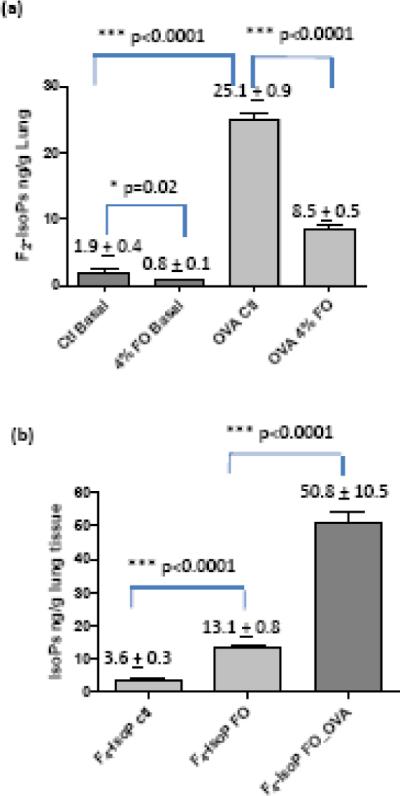
Levels of free radical oxidation products in mouse lung tissue after fish oil feeding and OVA challenge. (A) Levels of F2-IsoPs, in mice lung tissue. Control basal (Ctl Basal), basal level of F2-IsoP in mouse lung fed with control diet; 4% FO Basal, basal level of F2-IsoPs after 4% fish oil (FO) feeding; OVA control (OVA Ctl), F2-IsoP level of control mice after OVA challenge; OVA 4% FO, F2-IsoP levels in mice after 4% fish oil feeding and OVA challenge. (B) Levels of F4-IsoPs derived from DHA in mice lung tissue after fish oil feeding and OVA challenge. F4-IsoP Ctl, F4-IsoP level in control mice; F4-IsoP FO, F4-IsoP level fish oil group; F4-IsoP FO_OVA, level of F4-IsoP after fish oil feeding and OVA challenge. *, P< 0.05; ***, P < 0.0001, t test. The data are expressed as means ± standard error of the mean (n = 10).
Levels of PGE2 in BAL and urinary PGE-M
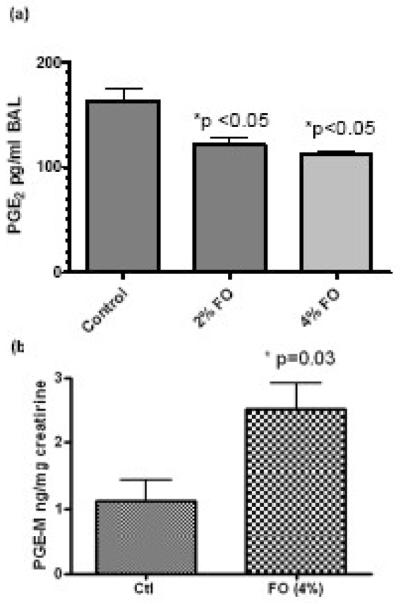
The level of PGE2 was measured in BAL and fish oil feeding with either 2% or 4% both dose-dependently decreased the production of PGE2 after OVA challenge (Figure 4a). However, the level of urinary metabolite of PGE2, PGE-M, was significantly increased after fish oil feeding (Figure 4b). PGE-M level is a marker of the total body production of the PGE2, which may be different from the level that is produced in the lung. We also attempted to measure the level of PGD2 in the BAL, but it was below the detection limit. Recent reports showed that PGD2 and PGE2 were differentially produced by human lung alveolar cell line A549 and macrophages [53]. Moreover, PGD2 is much less stable than PGE2 in vivo or in cell media.
Figure 4.
Levels of PGE2 in BAL and urinary metabolite PGE-M after fish oil feeding and OVA challenge. (a) Levels of PGE2 in mouse BAL analyzed by GC-MS. (b) Level of urinary PGE2 metabolite, PGE-M, analyzed by LC-MS method. Ctl, control mice; 2% FO, 2% fish oil treated mice; 4% FO, 4% fish oil treated mice. The data are expressed as means ± standard error of the mean (n = 10). *, P < 0.05, compared with control group.
Increased cytokine production in mouse lung tissue after fish oil feeding and OVA challenge
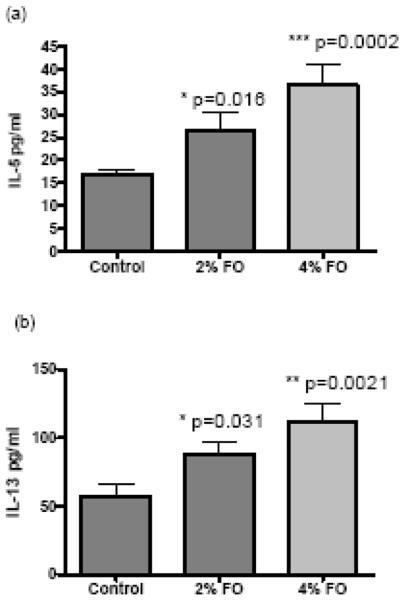
Inhibition of COX resulted in an augmented Type-2 immune response and allergen-induced AHR. This amplified Type-2 immune response is characterized by an increase in lung IL-5 and IL-13 production in OVA model [22]. IL-5 is a critical cytokine for eosinophil growth, differentiation, and survival whereas IL-13 has also been reported to be a factor that stimulate mucus production in the airway [54]. In our experiments, we assessed the levels of IL-5 and IL-13 as markers of lung inflammation to study the effect of fish oil supplementation. The IL-5 and IL-13 levels in the mouse lung tissue of 2% fish oil feeding group and 4% fish oil feeding group were significant greater than that of control group (Figure 5). Thus, fish oil feeding resulted in an elevated inflammatory response in mouse lung as indicated by higher levels of cytokines IL-5 and IL-13. However, we did not observe a difference in the number of BAL inflammatory cells including macrophages, eosinophils, neutrophils, and leukocytes among these three groups (data not shown).
Figure 5.
Levels of IL-5 (A) and IL-13 (B) in mice lung tissue after fish oil feeding and OVA challenge. Control, control mice; 2% FO, 2% fish oil treated mice; 4% FO, 4% fish oil treated mice. The data are expressed as means ± standard error of the mean (n = 10). *, P<0.05, compared with control mice.
Discussion
Lipid mediators derived from free radical oxidation, such as IsoPs, and COX, such PGs, are key regulators of allergic lung inflammation. Levels of these lipid-derived regulators can be modulated by fish oil supplementation, which may have profound impact on allergic lung inflammation. Epidemiological evidence has suggested a protective role of ω-3 fatty acids against asthma, however, clinical data on the effect of fish oil supplement in asthma has been equivocal [1, 28]. We reported herein, for the first time, that fish oil supplementation leads to enhanced lung inflammation as measured by elevated levels of IL-5 and IL-13 in mouse model of OVA sensitization and challenge, and is associated with suppression of PGE2 production in the lung.
Lipid mediators derived from PUFAs play an important role in allergic lung inflammation [20]. COX activities lead to the production of downstream lipid products including PGD2, PGE2, PGF2α, PGI2 and Thromboxane A2 (TXA2). COX-1 is constitutively expression in most cell types whereas COX-2 in general is induced by inflammatory stimuli. Current in vivo animal studies suggest that PGD2 and TXA2 increase the allergic inflammation whereas PGE2 and PGI2 suppress the inflammatory response in the lung. Furthermore, inhibition of COX by non-selective or selective COX inhibitors increases allergic airway inflammation through augmented production of IL-5 and IL-13 in OVA mouse model [21-23]. These studies suggested that the overall effect of PGs during the allergen sensitization and challenge process is to restrain allergic inflammation. PGE2 is one of the well studied PGs in lung inflammation and increasing evidence suggests that PGE2 exerts anti-inflammatory and bronchoprotective mechanism in asthma. PGE2 inhibits eosinophil trafficking through EP-2 receptor and EP-3 receptor appears to be an important negative regulator of allergic inflammation [25, 26]. Depending on the levels of dietary intake, ω-3 fatty acids compete with and displace ω-6 fatty acids for the acylation sites in the cellular membranes [55-60]. In our study, ω-3 fatty acids EPA and DHA can be dose-dependently incorporated into mouse lung tissue and reach steady state after four weeks of feeding of fish oil diet. It is noteworthy that the same trends were also observed in other mouse tissues such as liver, brain, kidney and plasma (data not shown).
Dietary fat intake may change the membrane composition of fatty acids and modulate the types of eicosanoids produced in the pathway, thus influencing the inflammatory response of the cells. It has been postulated that EPA and DHA competitively inhibit enzymatic oxidation of AA thus reducing the generation of pro-inflammatory lipid mediators, such as four-series of LTs and 2-series PGs [28, 37, 61]. Because significantly increased consumption of EPA and DHA results in a decrease in the amount of AA in the cell membranes, there will be less substrate available for synthesis of eicosanoids from AA. Recent studies showed that the ability of ω-3 PUFAs to influence production of eicosanoids may extend beyond simply decreasing substrate availability [37, 61, 62]. EPA competitively inhibits the oxygenation of AA by cyclooxygenases [62] and can act as a substrate for both COX and 5-lipoxygenase (5-LOX), giving rise to derivatives that differ in structure from those produced from AA (i.e., 3-series PG and TX, and 5-series LT) [37, 61]. Thus, the EPA-induced suppression in the production of AA-derived eicosanoids may be accompanied by an elevation in the production of EPA-derived eicosanoids [56, 60]. Furthermore, the eicosanoids produced from EPA are generally considered to be less biologically potent than the analogs synthesized from AA, although the full range of biological activities of these compounds has not been investigated [61]. In our experiment, we observed the decreased production of PGE2 in the mouse lung; but the level of urinary PGE-M was elevated. The data are intriguing in that total body production of PGE2 is elevated even with the decreased level of AA after fish oil supplementation. In addition, DHA derived mediators termed D-series resolvins, docosatrienes and neuroprotectins, also produced by COX-2, have been identified and appear to be anti-inflammatory [63-65]. Recent studies showed that protectin D1 was generated in human asthma and it dampened airway inflammation and AHR in OVA model [39]. The similar effect was also observed for resolvin E1 [38]. It should be noted that in these experiments the pure compounds were administered to the animals and reductions of PGE2 in these experiments were not expected. Elevated levels of resolvins and protectins are anticipated after fish oil supplementation but the overall effect of fish oil supplementation may be dictated by the reduction of anti-inflammatory PGs such as PGE2.
Free radical oxidation products of PUFAs and oxidative stress have also been associated with asthma. PUFAs are major constituents of the membrane phospholipids of cells and free radical attack on phospholipids generates an array of oxidation products including IsoPs [66]. Elevated levels of F2-IsoPs, the best biomarker for oxidative stress in vivo, have been observed in asthma animal models and asthmatic patients [67-69]. Levels of F2-IsoPs in urine, plasma, and breath condensate of asthma patients correlate well with the severity of asthma [15-17]. Furthermore, some of the IsoPs have been found to cause potent vasoconstriction in human and guinea pig airways, elicit AHR in isolated mouse lungs, and cause airway obstruction and airway plasma exudation in guinea pigs in vivo [10, 18, 19]. These findings may lead to the hypothesis that reduction of F2-IsoPs may have anti-inflammatory effects in allergic lung inflammation. Furthermore, little is known about the roles of oxidation products derived from ω-3 PUFAs, such as F3-IsoPs and F4-IsoPs, in the OVA model and asthma pathogenesis. Even though fish oil supplementation reduced the production of F2-isoPs at basal condition as well as post OVA challenge, the overall effect of fish oil still showed enhanced inflammatory response in mouse lung. These results suggest that suppression of pro-inflammatory F2-IsoPs alone is not enough to dampen the inflammatory response in the lung.
Recent report showed that COX inhibition during allergic sensitization has a strong stimulatory effect on the primary and memory immune response in a STAT6-independent manner [24]. In contrast, COX inhibition during the allergic challenge phase did not affect the OVA-induced Th2 response. In our experiments, fish oil supplementation before (protocol a) or after the sensitization (protocol b) did not have much impact on the inflammatory outcome. In a typical OVA protocol, the mice were challenged for four days. It is extremely difficult to study the effects of fish oil supplementation on the inflammatory response only at the challenge phase because our experiments showed that it took at least two weeks to replace the arachidonic acid by ω-3 fatty acids.
In summary, we have studied the effects of fish oil supplementation in a murine model of allergic lung inflammation. Our studies showed that EPA and DHA can be dose and time-dependently incorporated into mouse lung tissue. OVA challenge caused a significant increase of arachidonate-derived F2-IsoPs, suggesting an elevated level of oxidative stress. Fish oil supplementation suppressed the level of F2-IsoPs in mouse lung. Surprisingly, however, fish oil supplementation also resulted in enhanced pro-inflammatory cytokine IL-5 and IL-13 production. We attribute the overall effects of dietary fish oil supplementation on allergic lung inflammation to the suppression of PGE2 production in mouse lung. These dichotomous results bring into question the role of fish oil supplementation in the treatment of asthma.
Acknowledgment
This work is supported by a pilot grant from Center in Molecular Toxicology, Vanderbilt University (P30 ES00267), and NIH grants of DK48831, ES13125, and GM15431. We thank Drs. David Hachey, Wade Calcutt, and Mrs. Dawn Overstreet of the Mass Spectrometry Research Center of Vanderbilt University for their assistance with the MS analysis. The PGE-M analysis was carried out with the help of the Eicosanoid Core at Vanderbilt University. We gratefully acknowledge the discussion with Drs. Weisong Zhou and Ryszard Dworski at Vanderbilt University. This manuscript is dedicated to the memory of Dr. Jason Morrow.
Abbreviations
- AA
arachidonic acid
- AHR
airway hyperresponsiveness
- BHT
butylated hydroxyl toluene
- BSTFA
N,O-bis(trimethylsilyl)-trifluoroacetamide
- CID
collision induced dissociation
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- ESI
electrospray ionization
- GC
gas chromatography
- IL
interleukin
- IsoPs
isoprostanes
- LA
linoleic acid (C18:2, ω-9)
- LC
liquid chromatography
- 5-LOX
5-lipoxygenase
- LTs
leukotrienes
- MS
mass spectrometry
- OVA
ovalbumin
- PFB
pentafluorobenzyl
- PGs
prostaglandins
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SRM
selective reaction monitoring
- TX
thromboxanes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Wong KW. Clinical efficacy of n-3 fatty acid supplementation in patients with asthma. J. Am. Diet. Assoc. 2005;105:98–105. doi: 10.1016/j.jada.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [2].Barnes PJ. Reactive oxygen species and airway inflammation. Free Radical Biol. Med. 1990;9:235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- [3].Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003;21:177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- [4].Fujisawa T. Role of oxygen radicals on bronchial asthma. Curr. Drug Targets Inflamm. Allergy. 2005;4:505–509. doi: 10.2174/1568010054526304. [DOI] [PubMed] [Google Scholar]
- [5].Talati M, Meyrick B, Peebles JRS, Davies SS, Dworski R, Mernaugh R, Mitchell D, Boothby M, Roberts Ii LJ, Sheller JR. Oxidant stress modulates murine allergic airway responses. Free Radical Biology and Medicine. 2006;40:1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- [6].Yin H, Porter NA. New Insights Regarding the Autoxidation of Polyunsaturated Fatty Acids. Antioxid. Redox Signal. 2005;7:170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- [7].Morrow JD, Hill E, Burk RF, Nammour TM, Badr KF, Roberts LJ., Jr. A Series of Prostaglandin-F2-Like Compounds Are Produced in vivo in Humans by a Noncyclooxygenase, Free radical-Catalyzed Mechanism. Proc. Natl. Acad. Sci. USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morrow JD, Roberts LJ. The isoprostanes: Unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- [9].Morrow JD, Roberts I, Roberts L. Jackson Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. In: Packer L, editor. Methods in Enzymology. Academic Press; San Diego, CA: 1999. pp. 3–12. [DOI] [PubMed] [Google Scholar]
- [10].Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003;21:177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- [11].Montuschi P, Barnes PJ, Roberts LJ., II Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- [12].Zanconato S, Carraro S, Corradi M, Alinovi R, Pasquale MF, Piacentini G, Zacchello F, Baraldi E. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. Journal of Allergy and Clinical Immunology. 2004;113:257–263. doi: 10.1016/j.jaci.2003.10.046. [DOI] [PubMed] [Google Scholar]
- [13].Dworski R, Jackson Roberts Ii L, Murray JJ, Morrow JD, Hartert TV, Sheller JR. Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2) Clinical & Experimental Allergy. 2001;31:387–390. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- [14].Dworski R, Murray JJ, Jackson RL, II, Oates JA, Morrow JD, Fisher L, Sheller JR. Allergen-induced Synthesis of F2-Isoprostanes in Atopic Asthmatics. Evidence for Oxidant Stress. Am. J. Respir. Crit. Care Med. 1999;160:1947–1951. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]
- [15].Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-Isoprostane, a Marker of Oxidative Stress, in Exhaled Condensate of Asthma Patients. Am. J. Respir. Crit. Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- [16].Baraldi E, Ghiro L, Piovan V, Carraro S, Ciabattoni G, Barnes PJ, Montuschi P. Increased exhaled 8-isoprostane in childhood asthma. Chest. 2003;124:25–31. doi: 10.1378/chest.124.1.25. [DOI] [PubMed] [Google Scholar]
- [17].Baraldi E, Carraro S, Alinovi R, Pesci A, Ghiro L, Bodini A, Piacentini G, Zacchello F, Zanconato S. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58:505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tazzeo T, Miller J, Janssen LJ. Vasoconstrictor responses, and underlying mechanisms, to isoprostanes in human and porcine bronchial arterial smooth muscle. 2003;140:759–763. doi: 10.1038/sj.bjp.0705482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Catalli A, Janssen LJ. Augmentation of bovine airway smooth muscle responsiveness to carbachol, KCl, and histamine by the isoprostane 8-iso-PGE2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1035–1041. doi: 10.1152/ajplung.00138.2004. [DOI] [PubMed] [Google Scholar]
- [20].Moore ML, Peebles RS., Jr Update on the role of prostaglandins in allergic lung inflammation: Separating friends from foes, harder than you might think. Journal of Allergy and Clinical Immunology. 2006;117:1036–1039. doi: 10.1016/j.jaci.2005.12.1314. [DOI] [PubMed] [Google Scholar]
- [21].Peebles RS, Jr., Dworski R, Collins RD, Jarzecka K, Mitchell DB, Graham BS, Sheller JR. Cyclooxygenase Inhibition Increases Interleukin 5 and Interleukin 13 Production and Airway Hyperresponsiveness in Allergic Mice. Am. J. Respir. Crit. Care Med. 2000;162:676–681. doi: 10.1164/ajrccm.162.2.9911063. [DOI] [PubMed] [Google Scholar]
- [22].Peebles RS, Jr., Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, Christman JW, Kang K-H, Jarzecka K, Furlong J, Mitchell DB, Talati M, Graham BS, Sheller JR. Selective Cyclooxygenase-1 and -2 Inhibitors Each Increase Allergic Inflammation and Airway Hyperresponsiveness in Mice. Am. J. Respir. Crit. Care Med. 2002;165:1154–1160. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- [23].Peebles RS, Jr, Hashimoto K, Sheller JR, Moore ML, Morrow JD, Ji S, Elias JA, Goleniewska K, O'Neal J, Mitchell DB, Graham BS, Zhou W. Allergen-Induced Airway Hyperresponsiveness Mediated by Cyclooxygenase Inhibition Is Not Dependent on 5-Lipoxygenase or IL-5, but Is IL-13 Dependent. J Immunol. 2005;175:8253–8259. doi: 10.4049/jimmunol.175.12.8253. [DOI] [PubMed] [Google Scholar]
- [24].Zhou W, Newcomb DC, Moore ML, Goleniewska K, O'Neal JF, Peebles RS., Jr. Cyclooxygenase Inhibition during Allergic Sensitization Increases STAT6-Independent Primary and Memory Th2 Responses. J Immunol. 2008;181:5360–5367. doi: 10.4049/jimmunol.181.8.5360. [DOI] [PubMed] [Google Scholar]
- [25].Sturm EM, Schratl P, Schuligoi R, Konya V, Sturm GJ, Lippe IT, Peskar BA, Heinemann A. Prostaglandin E2 Inhibits Eosinophil Trafficking through E-Prostanoid 2 Receptors. J Immunol. 2008;181:7273–7283. doi: 10.4049/jimmunol.181.10.7273. [DOI] [PubMed] [Google Scholar]
- [26].Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, Tanaka H, Nagai H, Ichikawa A, Narumiya S. Suppression of allergic inflammation by the prostaglandin E receptor subtype EP3. Nat. Immunol. 2005;6:524–531. doi: 10.1038/ni1188. [DOI] [PubMed] [Google Scholar]
- [27].Chung KF. Evaluation of Selective Prostaglandin E2 (PGE2) Receptor Agonists as Therapeutic Agents for the Treatment of Asthma. Sci. STKE. 2005;2005:pe47. doi: 10.1126/stke.3032005pe47. [DOI] [PubMed] [Google Scholar]
- [28].Mickleborough TD, Rundell KW. Dietary polyunsaturated fatty acids in asthma- and exercise-induced bronchoconstriction. Eur. J. Clin. Nutr. 2005;59:1335–1346. doi: 10.1038/sj.ejcn.1602250. [DOI] [PubMed] [Google Scholar]
- [29].Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr. Allergy Immunol. 2004;15:517–522. doi: 10.1111/j.1399-3038.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- [30].Prescott SL, Calder PC. N-3 polyunsaturated fatty acids and allergic disease. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:123–129. doi: 10.1097/00075197-200403000-00004. [DOI] [PubMed] [Google Scholar]
- [31].Peat JK, Mihrshahi S, Kemp AS, Marks GB, Tovey ER, Webb K, Mellis CM, Leeder SR. Three-year outcomes of dietary fatty acid modification and house dust mite reduction in the Childhood Asthma Prevention Study. J. Allergy Clin. Immunol. 2004;114:807–813. doi: 10.1016/j.jaci.2004.06.057. [DOI] [PubMed] [Google Scholar]
- [32].Almqvist C, Garden F, Xuan W, Mihrshahi S, Leeder SR, Oddy W, Webb K, Marks GB. Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. Journal of Allergy and Clinical Immunology. 2007;119:1438–1444. doi: 10.1016/j.jaci.2007.01.046. [DOI] [PubMed] [Google Scholar]
- [33].Mickleborough TD, Ionescu AA, Rundell KW. Omega-3 fatty acids and airway hyperresponsiveness in asthma. J. Altern. Complement. Med. 2004;10:1067–1075. doi: 10.1089/acm.2004.10.1067. [DOI] [PubMed] [Google Scholar]
- [34].Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- [35].Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur. Respir. J. 2000;16:861–865. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- [36].Prescott S, Dunstan J. Prenatal fatty acid status and immune development: The pathways and the evidence. Lipids. 2007;42:801–810. doi: 10.1007/s11745-007-3030-z. [DOI] [PubMed] [Google Scholar]
- [37].Calder PC. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:S1505–1519. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- [38].Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochemical and Biophysical Research Communications. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- [39].Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 Is Generated in Asthma and Dampens Airway Inflammation and Hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yin H, Porter NA. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid. Redox Signal. 2005;7:170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- [41].Yin H, Havrilla CM, Gao L, Morrow JD, Porter NA. Mechanisms for the formation of isoprostane endoperoxides from arachidonic acid. "Dioxetane" intermediate versus β-fragmentation of peroxyl radicals. J. Biol. Chem. 2003;278:16720–16725. doi: 10.1074/jbc.M300604200. [DOI] [PubMed] [Google Scholar]
- [42].Yin H, Porter NA, Morrow JD. Separation and identification of F2-isoprostane regioisomers and diastereomers by novel liquid chromatographic/mass spectrometric methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;827:157–164. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- [43].Porter N, Caldwell S, Mills K. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- [44].Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- [45].Yin H, Musiek ES, Gao L, Porter NA, Morrow JD. Regiochemistry of neuroprostanes generated from the peroxidation of docosahexaenoic acid in vitro and in vivo. J. Biol. Chem. 2005;280:26600–26611. doi: 10.1074/jbc.M503088200. [DOI] [PubMed] [Google Scholar]
- [46].Musiek ES, Cha JK, Yin H, Zackert WE, Terry ES, Porter NA, Montine TJ, Morrow JD. Quantification of F-ring isoprostane-like compounds (F4-neuroprostanes) derived from docosahexaenoic acid in vivo in humans by a stable isotope dilution mass spectrometric assay. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;799:95–102. doi: 10.1016/j.jchromb.2003.10.036. [DOI] [PubMed] [Google Scholar]
- [47].Quan LG, Cha JK. Preparation of isoprostanes and neuroprostanes. J. Am. Chem. Soc. 2002;124:12424–12425. doi: 10.1021/ja027451z. [DOI] [PubMed] [Google Scholar]
- [48].Gao L, Yin H, Milne GL, Porter NA, Morrow JD. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J. Biol. Chem. 2006;281:14092–14099. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- [49].Morrow JD, Roberts LJ., Jr. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- [50].Morrow J, Minton T, Mukundan C, Campbell M, Zackert W, Daniel V, Badr K, Blair I, Roberts L., 2nd Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1994;269:4317–4326. [PubMed] [Google Scholar]
- [51].Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal. Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- [52].Hashimoto K, Sheller JR, Morrow JD, Collins RD, Goleniewska K, O'Neal J, Zhou W, Ji S, Mitchell DB, Graham BS, Peebles RS., Jr. Cyclooxygenase Inhibition Augments Allergic Inflammation through CD4-Dependent, STAT6-Independent Mechanisms. J Immunol. 2005;174:525–532. doi: 10.4049/jimmunol.174.1.525. [DOI] [PubMed] [Google Scholar]
- [53].Cao H, Xiao L, Park G, Wang X, Azim AC, Christman JW, van Breemen RB. An improved LC-MS/MS method for the quantification of prostaglandins E2 and D2 production in biological fluids. Analytical Biochemistry. 2008;372:41–51. doi: 10.1016/j.ab.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in α-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur. J. Clin. Invest. 2000;30:260–274. doi: 10.1046/j.1365-2362.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- [56].Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary ω-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J. Clin. Investig. 1993;91:651–660. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gibney MJ, Hunter B. The effects of short- and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur. J. Clin. Nutr. 1993;47:255–259. [PubMed] [Google Scholar]
- [58].Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor a and interleukin 1ß production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996;63 doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- [59].Healy DA, Wallace FA, Miles EA, Calder PC, Newsholme P. The effect of low to moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35:763–768. doi: 10.1007/s11745-000-0583-1. [DOI] [PubMed] [Google Scholar]
- [60].Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J, Spur BW, Robinson DR, Corey EJ, Lewis RA, Austen KF. Effects of dietary enrichment with eicosapentaenoic acid and docosahexaenoic acid on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Engl. J. Med. 1985;312:1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- [61].Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Obata T, Nagakura T, Masaki T, Maekawa K, Yamashita K. Eicosapentaenoic acid inhibits prostaglandin D2 generation by inhibiting cyclooxygenase-2 in cultured human mast cells. Clin. Exp. Allergy. 1999;29:1129–1135. doi: 10.1046/j.1365-2222.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- [63].Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- [64].Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- [65].Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Milne GL, Yin H, Morrow JD. Human Biochemistry of the Isoprostane Pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zanconato S, Carraro S, Corradi M, Alinovi R, Pasquale MF, Piacentini G, Zacchello F, Baraldi E. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J. Allergy Clin. Immunol. 2004;113:257–263. doi: 10.1016/j.jaci.2003.10.046. [DOI] [PubMed] [Google Scholar]
- [68].Dworski R, Roberts LJ, Jr., Murray JJ, Morrow JD, Hartert TV, Sheller JR. Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2) Clin. Exp. Allergy. 2001;31:387–390. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- [69].Dworski R, Murray JJ, Jacksonroberts L, II, Oates JA, Morrow JD, Fisher L, Sheller JR. Allergen-induced synthesis of F2-isoprostanes in atopic asthmatics. Evidence for oxidant stress. Am. J. Respir. Crit. Care Med. 1999;160:1947–1951. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]