Abstract
We show that high quantum efficiency fluorophores can exhibit reversible photobleaching. This observation provides the basis for an imaging technique we call reversible photobleaching microscopy. We demonstrate applicability of this technique using antibody labeled biological samples in standard aqueous (or glycerol based) media to produce far-field images at ∼30 nm resolution. Our novel method relies on intense illumination to reversibly induce a very long-lived (>10 s) dark state from which single fluorochromes slowly return stochastically. As in other localization microscopy methods, reversible photobleaching microscopy localizes single fluorochromes, but has the advantage that specialized photoactivatible and photoswitchable molecules or special immersion/embedding media are not required.
It is well known that the diffraction of light imposes a limit on the resolution of any conventional far-field optical system (1) and commercial optical microscopes routinely reach the limit of ∼250 nm set by the practical limit of microscope numerical aperture and visible light. However, the diffraction limit can be surpassed by using fluorescence imaging with nonlinear structured illumination (2) as well as stimulated emission depletion and related microscopies (3, 4). An alternative approach to further increase resolution arises from localization microscopy, in which several modalities have been developed such as PALM (5), fPALM (6), STORM (7) or PALMIRA (8). The key to the latter methods resides in the control of the number of fluorescent molecules active at any one time so that the position of single molecules may be accurately derived from the centroid of their normal diffraction pattern. These approaches have led to a near order of magnitude improvement in resolution to ∼30 nm.
Although the optical apparatus for localization microscopy is simpler than for stimulated emission depletion or structured illumination, the need for photoactivatable (5, 6) or photoswitchable (7, 9) fluorochromes is a limiting factor. This need arises from the requirement to limit the number of actively fluorescing molecules in the field of view to permit identification and accurate determination of position. Recently, a study showed that inclusion of special embedding media, such as polyvinyl alcohol and glucose oxidase, can allow the use of conventional fluorochromes in the absence of oxygen by inducing millisecond duration dark states (10). Here we show that intense illumination can cause a slowly reversible photobleaching of common fluorochromes in standard mounting media. The rate of return of molecules from the photobleached (or dark) state is sufficiently low to allow the construction of high-resolution images. In our approach, only an excitation laser of moderate power is needed and the sample can be mounted in standard aqueous or glycerol based media. During the initial exposure of the sample a widefield image is obtained which is then followed by the images in which single molecules can be observed (as the fluorochromes return from the dark state).
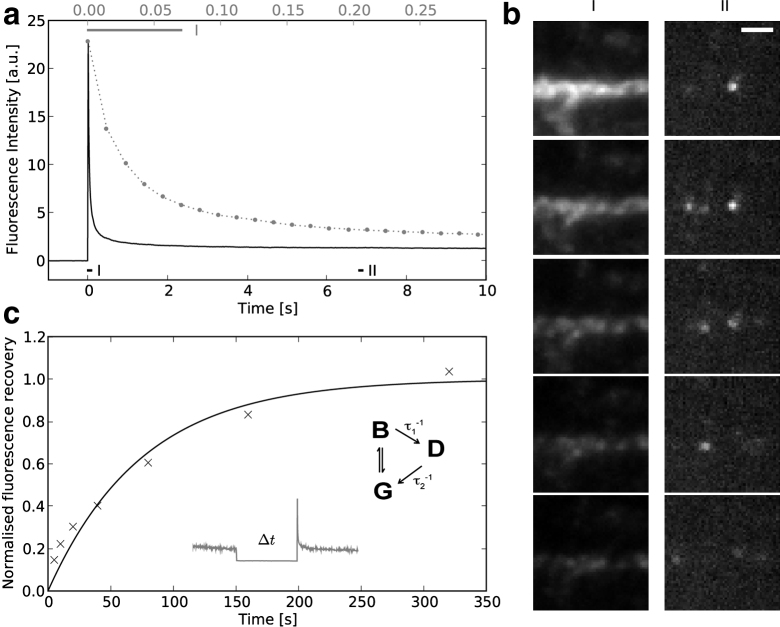
Reversible photobleaching is illustrated in Fig. 1, which shows the time course of fluorescence emission of Alexa 488-labeled structures in fixed cells embedded in a mixture of phosphate-buffered saline and glycerol. When illuminated with intense 488 nm light from an argon laser (∼105 W/cm2) and viewed with a modified Nikon total internal reflection fluorescence (TIRF) microscope (Supporting Material) fluorescence rapidly decays, as might be expected from a photobleaching effect. However, fluorescence does not decay exponentially to baseline as expected for simple photobleaching. Instead, fluorescence stabilized at a level <6% of the peak fluorescence until the excitation light was switched off. When the same level of illumination is switched on again after a period of several seconds, there is a substantial recovery of fluorescence, showing that the apparent photobleaching observed during the first period of illumination was reversible and not associated with photo-destruction of the fluorochromes. The recovery of fluorescence was dependent on the interval between illumination periods consistent with the idea that the intense illumination actually drove a large fraction of the fluorochrome molecules into a dark state from which they gradually returned. The time course of recovery of the peak fluorescence shown in Fig. 1c was approximately exponential with a half time of ∼60 s, considerably longer than would be expected from an electronic state such as the triplet. During the plateau phase, the fluorescence gradually decays at a much reduced rate (Fig. 1a) reflecting the development of genuine photodamage.
Figure 1.
Reversible photobleaching of Alexa 488. (a) Upon exposure to intense light a rapid decline in fluorescence of Alexa 488 is observed, followed by a plateau phase. The dotted line shows the initial decay on a magnified timescale (top axis). (b) Consecutive frames taken during initial bleaching (I), show the whole stained structure, whereas those taken during the plateau phase (II) show the stochastic reappearance of single fluorophores as they revert from the dark state. (c) After a period of darkness a significant recovery in the Alexa 488 fluorescence is observed with an approximately logarithmic dependence on the length of the dark period, implying a half time of ∼1 min. A simple scheme illustrates the dark state behavior, see text. Scale bar, 1 μm.
In addition to Alexa 488, we have observed reversible photobleaching in a number of common high quantum efficiency fluorophores including Alexa 568, tetramethyl rhodamine iso-thiocyanate and fluorescein (Fig. S1 in the Supporting Material) suggesting the long-lived light-induced dark state may not be an unusual phenomenon. The recovery kinetics were solvent dependent with recovery slowest in mounting media that had a higher viscosity (e.g., glycerol, Fig. S2) but were largely unaffected by the inclusion of the triplet state quencher mercaptoethanol (not shown). Reversible photobleaching was also observed in dry samples in air which excluded the possibility that the observed fluorescence recovery merely represented the diffusion of fresh fluorophore into the bleached region (Fig. S1).
These basic observations are compatible with a simple model involving a ground state G, a bright excited state B and a long-lived dark state D (Fig. 1c). If the transitions between G and B are fast as compared to the rates at which molecules move into (τ1−1) and out of (τ2−1) D, then the asymptotically remaining fluorescence fraction F/F0 =τ1/(τ2f), where f is the fraction of molecules that are in the bright state immediately after onset of illumination. Assuming that the illumination initially propels 10% of the molecules into the bright state and with transition rates compatible with our data (τ1 ≈60 ms, τ2 ≈ 60s) F/F0 is on the order of 1%, comparable with the asymptotic values seen. However we note that the actual photophysics are probably more complex than this simple model since the photobleaching time course is multiexponential.
This slowly recovering reversible photobleaching forms the basis of a what we believe is new, simple, localization microscopy method that we call reversible photobleaching microscopy (RPM). As shown, high-intensity illumination rapidly pushes most molecules into a dark state so that only a small fraction of molecules fluoresce at any one time. Under constant illumination, molecules stochastically return from the dark state and fluoresce briefly before either bleaching irreversibly or returning to the reversible dark state.
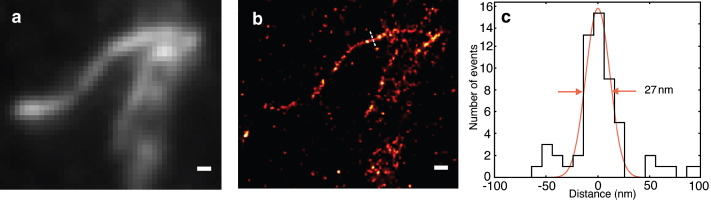
Fig. 2 shows the results of using RPM to image F-actin in HEK293 cells labeled with Alexa 488 conjugated phalloidin imaged under conditions as in Fig. 1. Images were acquired with an electron-multiplying charge coupled device using a frame integration time of 14 ms (Supporting Material). A diffraction-limited image, Fig. 2a, was obtained by averaging the first 100 frames, taken while the fluorescence intensity was decreasing during the initial stage of development of reversible photobleaching. After ∼1 s of high intensity illumination, the sample brightness had decreased to the point where single fluorochromes (Fig. S3) could be identified and localized. By analyzing all frames, a new localization image is obtained showing the positions of all detected fluorochromes. As shown in Fig. 2c, the width of the filament in the upper left was measured to be ∼27 nm, which is thus an upper bound on the resolution that was achieved and far higher than that seen in the initial widefield image.
Figure 2.
Imaging of Alexa 488 phalloidin labeled F-actin. (a) Diffraction limited image obtained by averaging the first 100 frames. (b) Image reconstructed from single fluorophore localization events, showing considerably better resolution. (c) Line profile along dotted line in b with a fitted Gaussian width of 27 nm. Scale bars, 200 nm.
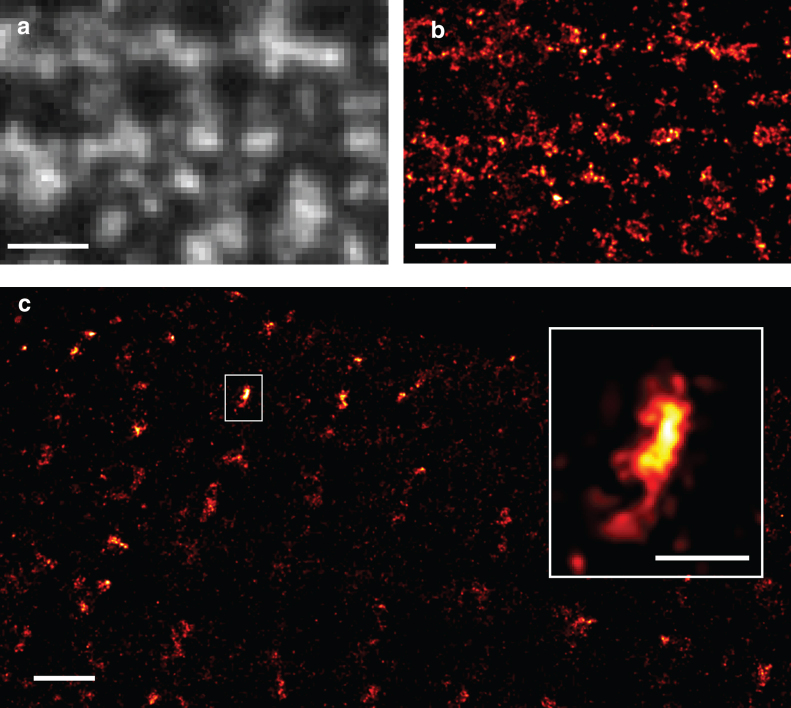
Fig. 3 further illustrates the utility of RPM with images of the distribution of the membrane protein caveolin-3 (Fig. 3, a and b) and the intracellular ryanodine receptor (Fig. 3c) in rat ventricular myocytes. In each case we used labeling protocols developed for epifluorescence imaging with commercial fluorescent antibodies and embedding media. The ability to see the labeled structures with conventional widefield imaging (in the first stage of photobleaching) simplified the selection of areas for detailed localization imaging. This is not possible with photoactivation localization microscopies as the sample is essentially invisible before localization imaging.
Figure 3.
RPM images of cardiac caveolin and RyRs. (a) TIRF image of caveolin-3 in the surface membrane of a rat ventricular myocyte (gray), (b) a RPM image of the same region—some of the smaller clusters are likely associated with single caveolae. (c) Clusters of RyRs at the membrane. The magnified cluster (inset) has a size of ∼60 × 180 nm corresponding to ∼12 of the large, ∼30 nm diameter receptors. Scale bars: a, 1 μm; b, 200 nm; and c, 1 μm; inset, 200 nm.
It is remarkable that such high resolution may be achieved by using an illumination regimen that was previously thought to be counterproductive for fluorescence although some earlier epifluorescence measurements had noted partial fluorescence recovery (11, 12) between periods of illumination. While preparing this manuscript, Fölling et al. (10) suggested a similar method based on triplet state production, but there are several fundamental differences to our method: i), RPM does not require any special embedding media (such as polyvinyl alcohol, mercaptoethanol or glucose oxidase). This has the important benefits of making RPM easier to implement, allows the use of low refractive index media for TIRF imaging, and is compatible with live cell experiments. ii), We do not believe that triplet state production is a prerequisite for RPM as inclusion of mercaptoethanol did not affect reversible photobleaching (RP) significantly. iii), The RP that we describe occurs over a much longer timescale than the dark states of organic fluorochromes reported by Fölling et al. (10). The solvent dependence and the slow time course of recovery of RP are compatible with a mechanism that involves reversible chemical transitions. It is possible that the presence of oxygen in our samples could induce dark states associated with transient radical formation.
RPM provides a simple adjunct to conventional fluorescence microscopy since one obtains both a widefield and a high-resolution image with the only requirement being a bright illumination source and a high sensitivity CCD camera. The method may be extended beyond the TIRF basis used here into 3D imaging as described for photoactivatible probes (13). The observation of reversible photobleaching is also relevant for mobility studies since it could be mistakenly attributed to a slowly mobile species in photobleaching recovery experiments. Future studies will be needed to clarify the spectroscopic and molecular underpinning of RPM, but we anticipate that it may provide a much needed tool for nanobiophysics which seeks to reveal the complexity of protein-protein interactions.
Acknowledgments
We thank Emma Kay for providing cultured HEK cells, and the Health Research Council of New Zealand and the Auckland University Research Committee for financial support.
Editor: Michael Edidin.
Footnotes
Methods, materials, three figures, and references are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(08)00076-3.
Supporting Material
References and Footnotes
- 1.Abbe E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch. f. mikroskop. Anat. 1873;9:413–420. [Google Scholar]
- 2.Gustafsson M.G. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA. 2005;102:13081–13086. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyba M., Jakobs S., Hell S.W. Immunofluorescence stimulated emission depletion microscopy. Nat. Biotechnol. 2003;21:1303–1304. doi: 10.1038/nbt897. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann M., Eggeling C., Jakobs S., Hell S.W. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. USA. 2005;102:17565–17569. doi: 10.1073/pnas.0506010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betzig E., Patterson G.H., Sougrat R., Lindwasser O.W., Olenych S., et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 6.Hess S.T., Girirajan T.P., Mason M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006;91:4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rust M.J., Bates M., Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat. Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egner A., Geisler C., von Middendorff C., Bock H., Wenzel D., et al. Fluorescence nanoscopy in whole cells by asnychronous localization of photoswitching emitters. Biophys. J. 2007;93:3285–3290. doi: 10.1529/biophysj.107.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemmer P., Gunkel M., Baddeley D., Kaufmann R., Urich A., et al. SPDM: light microscopy with single-molecule resolution at the nanoscale. Appl. Phys. B. 2008 doi: 10.1007/s00340–008–3152-x. [DOI] [Google Scholar]
- 10.Fölling J., Bossi M., Bock H., Medda R., Wurm C.A., et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods. 2008 doi: 10.1038/NMETH.1257. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman G.I., Nester J.F., Wasserman D.E. An experimental study of lasers as excitation sources for automated fluorescent antibody instrumentation. J. Histochem. Cytochem. 1971;19:469–476. doi: 10.1177/19.8.469. [DOI] [PubMed] [Google Scholar]
- 12.Rundquist I., Enerbäck L. Millisecond fading and recovery phenomena in fluorescent biological objects. Histochemistry. 1976;47:79–87. doi: 10.1007/BF00492556. [DOI] [PubMed] [Google Scholar]
- 13.Juette M.F., Gould T.J., Lessard M.D., Mlodzianoski M.J., Nagpure B.S., et al. Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat. Methods. 2008;5:527–529. doi: 10.1038/nmeth.1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.