Abstract
We have applied fluorescence imaging of two-photon linear dichroism to measure the subresolution organization of the cell membrane during formation of the activating (cytolytic) natural killer (NK) cell immune synapse (IS). This approach revealed that the NK cell plasma membrane is convoluted into ruffles at the periphery, but not in the center of a mature cytolytic NK cell IS. Time-lapse imaging showed that the membrane ruffles formed at the initial point of contact between NK cells and target cells and then spread radialy across the intercellular contact as the size of the IS increased, becoming absent from the center of the mature synapse. Understanding the role of such extensive membrane ruffling in the assembly of cytolytic synapses is an intriguing new goal.
Natural killer (NK) cells selectively kill virus infected or tumor cells based on a balance between activating and inhibitory signals generated by a repertoire of receptors and ligands. Fluorescence imaging studies published a decade ago revealed that proteins can segregate into micrometer-scale clusters at the intercellular contact or immune synapse (IS) between NK or T cells and their respective target cells (1–3). Many functions have been suggested for the IS, including facilitating directed secretion. Here we apply a new imaging technique, fluorescence detected two-photon linear dichroism (LD) (4), to study membrane morphology at the NK cell IS.
We used the NK cell line YTS and the EBV-transformed B cell line 721.221 (herafter called 221) as a model system for activating, cytolytic immune synapses (5). NK cells transfected to express the membrane bound glycosylphosphatidylinositol (GPI)-linked green fluorescent protein (GFP, YTS/GPI-GFP) were imaged together with target cells by time-lapse confocal microscopy (Fig. 1). During maturation of the IS, the area of intercellular contact grows and forms a structure characterized by a ring of higher GPI-GFP fluorescence in an enface view of the synapse. In parallel with membrane spreading, the Golgi and secretory apparatus commonly translocate toward the intercellular contact (6). To better examine the NK IS morphology, we analyzed confocal planes acquired in the midsection of several NK:target conjugates (Fig. 1, D and E). The average circumference of the NK cells was measured to be ∼66 μm and on average ∼26% of the plasma membrane was engaged in the synapse (Fig. 1 D). Furthermore, the IS was often found to be concave with an average “depth” of ∼3 μm (Fig. 1 E). Thus, IS formation involves dynamic spreading of the NK cell membrane across the target cell into a cup-like structure. By analogy with previous studies of the interaction between the endothelium and leukocytes, we here refer to this morphology as the “docking structure” (7).
Figure 1.
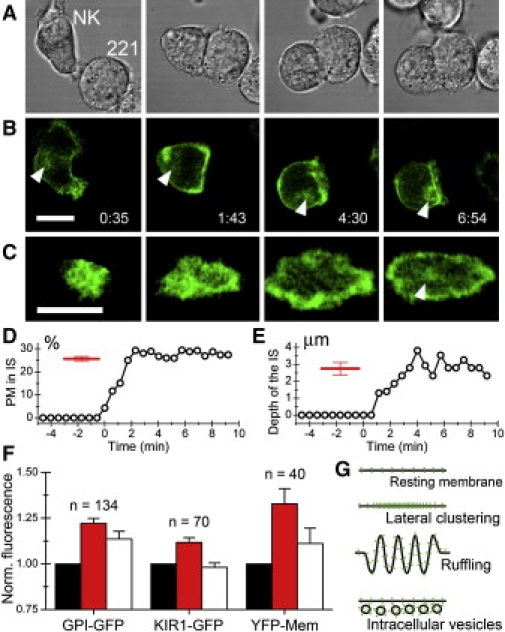
NK cell docking structure. Time-lapse imaging of a NK cell (YTS/GPI-GFP) forming a contact with a target cell (221). Bright-field (A), 3D rendered fluorescence of GPI-GFP (B) and enface view of the IS (C). White arrowhead follows the translocation of a pool of internal membranes to the intercellular contact. Scale bars = 10 μm. The fraction of the total length of the plasma membrane (PM) at the IS (D) and the depth of the IS (E) was assesed from single optical sections in the middle of the NK:target conjugate for each time-point in the time sequence shown in (A). Red bars represent average and confidence levels (95%) from snapshots of several conjugates (n=62). The fluorescence intensity of GPI-GFP, KIR2DL1-GFP and YFP-Mem was assessed in the plasma membrane outside the IS (black bars), at the periphery of the IS (red bars), and in the center of the IS (white bars) (F). Error bars represent confidence levels of 95%. Elevated fluorescence intensity could be due to lateral clustering of fluorescent molecules or accumulation of membrane in ruffles or vesicles (G).
Next, we set out to analyze the organization of the plasma membrane within the docking structure. The distributions of GPI-GFP, anchored to the outer leaflet of the plasma membrane, yellow fluorescent protein (YFP)-Mem (i.e., palmitoylated YFP), anchored to the inner leaflet of the plasma membrane and the transmembrane inhibitory NK receptor KIR2DL1 tagged with GFP (KIR1-GFP) were assessed. The target cells do not express cognate receptors for these proteins so their distributions should not be directly affected by molecular interactions across the intercellular contact. By analyzing single confocal planes acquired in the midsection of NK:target cell conjugates, the average fluorescence intensities of these proteins outside, within the center and at the periphery of the IS were compared (Fig. 1 F). All three membrane proteins accumulated at the periphery of the IS compared to both the center and outside of the IS. For GPI-GFP and YFP-Mem, we also observed elevated fluorescence intensity in the center compared to outside the IS. This could be explained by association of these proteins to microdomains (e.g., lipid rafts) that are enriched in the IS. Together, these results suggest that accumulation of plasma membrane proteins to the periphery of the IS is an inherent property of the NK cell docking structure.
Elevated fluorescence intensity of the proteins studied could in principle be due to an increase in the average number of molecules per unit membrane area (lateral clustering) or due to an accumulation of membrane, through, i.e., membrane ruffling or intracellular vesicles (Fig. 1 G). Due to fundamental limits in resolution, conventional confocal microscopy cannot resolve the basis for such protein clustering, although methods using point spread function reconstruction or polarized light have been described for studies of membrane topology (8,9).
Here, we set out to probe the membrane organization at the NK cell IS using fluorescence detected LD. This technique compares the fluorescence intensity generated by excitation light of orthogonal polarizations, and is thus sensitive to the orientation of fluorescent molecules (see the Supporting Material). To get a net LD signal, it is essential that the population of fluorescent molecules within the observation volume has a preferred orientation generated by an ordered scaffold, i.e., the plasma membrane. Thereby, LD could be used to study subresolution membrane disorder. The measured LD for a membrane bound probe is a combination of its microscopic orientation in the membrane, determined by molecular interactions with, for example, neighboring lipids and proteins, and the macroscopic orientation, i.e., the topology of the membrane. The macroscopic orientation can be divided into two different components, one that is possible to resolve by optical microscopy, i.e., the contour of the membrane in a high resolution image, and one that describes the subresolution topology of the membrane (<0.5 μm, in our case). Importantly, when the LD is normalized to the total fluorescence intensity (then denoted LDr), it is insensitive to molecular density. The lipid probes DiO and DiI bind to cell membranes with the transition dipoles oriented parallel to the membrane surface (Fig. 2 A), making them suitable probes for measuring subresolution topology changes of the plasma membrane with polarized microscopy (4,8).
Figure 2.
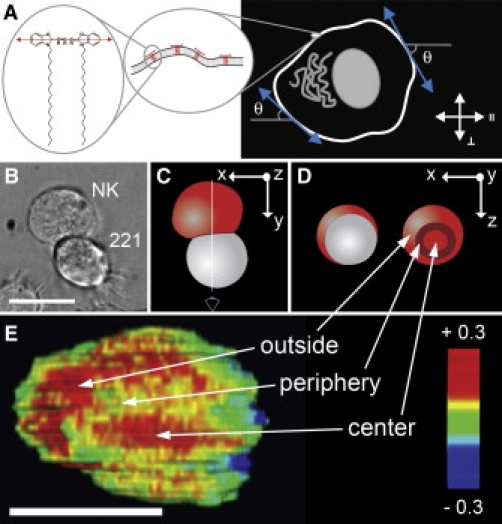
Plasma membrane is convoluted into ruffles at the periphery of the NK cell docking structure. (A) Chemical structure of DiO and schematic representation of its association to the plasma membrane. The measured LDr value is a function of the microscopic orientation of the molecule in the plasma membrane, the subresolution ruffling of the plasma membrane and the angle between the plasma membrane and the polarization of the excitation light (θ). (B) Bright-field image of a conjugate between a NK (YTS, DiO labeled) and B cell (221, unlabeled). Scale bar = 15 μm. (C and D) Schematic representation of the NK:target (red:white) conjugate in (B). The dark red outer ring represents the periphery of the docking structure. (E) Several false colored two-photon LDr optical sections of the conjugate shown in (B) were rendered in 3D and rotated to get an enface view of the synapse. The view angle is shown as an eye and white line in (C) and the rotation of the conjugate can be understood from the coordinate systems shown in (C) and (D). The highly polarized area (red) is interrupted by membrane ruffles in the periphery of the docking structure (yellow arc). Scale bar = 10 μm.
DiO labeled NK cells in conjugates with 221 target cells were analyzed by fluorescence detected LD imaging. A 3D rendered stack of false color LDr images, shown as a reconstruction of the en face synapse, revealed a region of lower LDr values at the synapse periphery (Fig. 2 E). Thus, these data show that the periphery of the NK cell IS is defined by a region where the membrane is disordered. To quantify the relative degree of membrane disorder we measured the DiO LDr at several points across the plasma membrane in optical sections acquired in the middle of NK:target conjugates. This data is visualized by plotting LDr values as a function of the plasma membrane orientation, as determined by visual inspection of high resolution images (θ in Fig. 2 A). A large variation of LDr values with θ are indicative of high dipole orientation of DiO equivalent to flat membrane regions. The orientation of the plasma membrane of NK cells was found to be similar in the center and outside the IS, but significantly lower at the periphery (Fig. 3 A). The vesicular proteins lysosomal-associated membrane protein 1 or transferrin receptor do not accumulate at the periphery of the IS (data not shown). Furthermore, calculations showed that ∼2 layers of vesicles would have to be stacked beneath the plasma membrane in the periphery of the IS to account for the LDr difference observed (Supporting Material). This inconsistency makes us conclude that the NK cell membrane is convoluted into <0.5 μm ruffles at the periphery of the docking structure. In contrast the plasma membrane of the 221 target cell was found to have similar LD values across the IS and hence does not follow the membrane ruffles of the NK cell (Fig. 3 B). For comparison, the relative degree of membrane ruffling across the different regions of the NK cell docking structure can be represented by a sinusoid of specific amplitude (Fig. 3 C).
Figure 3.
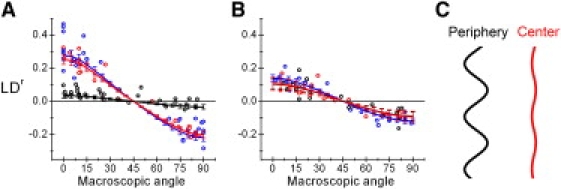
Quantification of the relative membrane ruffling in the docking structure. LD was measured in DiO stained NK cells (n = 18) or target cells (n = 16) during IS formation. Point measurements from the outside (blue), periphery (black) and center (red) of the docking structure are plotted versus θ for NK (A) or target cells (B). Lines and bars represent best fits and 95% confidence levels to the LD model derived for DiO (4). Sinusoid showing the relative degree of ruffling in the periphery and center of the NK cell IS, assuming that the plasma membrane outside the docking structure is flat (C).
We here present what we believe is a novel technique to reveal membrane disorder and we apply this to show that the NK cell plasma membrane is extensively ruffled at the periphery, but not in the center, of the mature cytolytic NK cell IS. Putative functions for the observed membrane ruffling could be to allow receptors and ligands, expressed by the same cell, to bind across the convoluted sheets of plasma membrane (cis interaction) (10), or the ruffles could form a “gasket” preventing leakage of lytic molecules from the synaptic cleft (11).
Supporting Material
Materials and methods are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(08)00049-0.
Supporting Material
Acknowledgments
The authors thank members of our labs for helpful comments on the manuscript.
We are grateful for financial support from The Wenner-Gren Foundation, Swedish Research Council, the Swedish Foundation for Strategic Research, Biotechnology and Biological Sciences Research Council, Department of Trade and Industry, Medical Research Council, a Lister Institute Research Prize (to D.M.D.), Wolfson Royal Society Research Merit Awards (to D.M.D. and P.M.W.F) and a CASE studentship supported by the Engineering and Physical Sciences Research Council, and Kentech Instruments (for R.K.P.B.).
References and Footnotes
- 1.Monks C.R., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 2.Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 3.Davis D.M., Chiu I., Fassett M., Cohen G.B., Mandelboim O. The human natural killer cell immune synapse. Proc. Natl. Acad. Sci. USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benninger R.K.P., Önfelt B., Neil M.A.A., Davis D.M., French P.M.W. Fluorescence Imaging of Two-Photon Linear Dichroism: Cholesterol Depletion Disrupts Molecular Orientation in Cell Membranes. Biophys. J. 2005;88:609–622. doi: 10.1529/biophysj.104.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Allan D.S., Krzewski K., Ge B., Kopcow H. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc. Natl. Acad. Sci. USA. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupfer A., Dennert G., Singer S.J. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc. Natl. Acad. Sci. USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreiro O., Yanez-Mo M., Serrador J.M., Montoya M.C., Vicente-Manzanares M. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sund S.E., Swanson J.A., Axelrod D. Cell membrane orientation visualized by polarized total internal reflection fluorescence. Biophys. J. 1999;77:2266–2283. doi: 10.1016/S0006-3495(99)77066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rheenen J., Jalink K. Agonist-induced PIP(2) hydrolysis inhibits cortical actin dynamics: regulation at a global but not at a micrometer scale. Mol. Biol. Cell. 2002;13:3257–3267. doi: 10.1091/mbc.E02-04-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Held W., Mariuzza R.A. cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat. Rev. Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thauland T.J., Koguchi Y., Wetzel S.A., Dustin M.L., Parker D.C. Th1 and Th2 cells form morphologically distinct immunological synapses. J. Immunol. 2008;181:393–399. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


