Abstract
A fully synthetic anticancer vaccine 2 has been prepared via bio-conjugation of unimolecular pentavalent construct 1 – containing five prostate and breast cancer associated carbohydrate antigens, Globo-H, GM2, STn, TF and Tn – to maleimide-modified carrier protein KLH. An improved conjugation protocol has been developed, which allowed us to obtain a higher epitope ratio of the unimolecular pentavalent glycopeptide antigen to the carrier protein (505/1 versus 228/1 for the previous version). KLH conjugate 2 has been subsequently submitted to preclinical immunogenic evaluation in mice in the presence of QS-21 as an adjuvant. Through standard ELISA assay, this vaccine candidate showed high promise in inducing IgG and IgM antibodies against each of the five individual carbohydrate antigens. In addition, FACS analysis indicated that these antibodies were able to react with MCF-7 breast cancer cell lines expressing these five carbohydrate antigens.
Introduction
It is well-known that malignantly transformed cells often display aberrant levels and types of surface glycosylation, a feature which serves to characteristically differentiate tumor cells from normal, healthy cells. This abnormal glycosylation pattern on the tumor cell surface provides a potential opportunity for tumor immunologists to develop carbohydrate-based anticancer vaccines for cancer therapeutic treatment. Hypothetically, proper exposure of vaccine constructs containing tumor-associated carbohydrate antigens to the immune system would stimulate the formation of corresponding antibodies. These antibodies, in turn, would selectively bind and help eradicate tumor cells overexpressing those carbohydrate epitopes.
Toward this end, synthetic chemists and cancer immunologists have been striving to develop effective carbohydrate-based anticancer vaccines for cancer immunotherapy. In recent years, important advances in this field have been reported by Boons,1 Kunz,2 Schmidt3 and their associates, as well as by our group.4
Our own extensive research in this area recently culminated in the synthesis of a superior first-generation unimolecular pentavalent construct, targeting prostate and breast cancer. In this construct, which was conjugated to the KLH carrier protein, five different prostate and breast cancer associated carbohydrate antigens – Globo-H, Ley, STn, TF and Tn – were incorporated on a single peptide backbone.5 This KLH conjugate was then evaluated in mice in conjunction with a suitable adjuvant (QS-21) and its immunogenicity was compared with that of the corresponding pooled monovalent vaccines. Experimental results indicated that this KLH conjugate was optimal for inducing antibodies against all of the carbohydrate antigens, with the exception of Ley. The disappointing immunogenicity observed with the Ley antigen most likely arises from the fact that it is endogenously expressed at a relatively high level.
Fluorescent Activated Cell Sorter (FACS) assay analysis indicated that the antibodies induced by this first-generation unimolecular pentavalent vaccine reacted significantly with the three cell lines evaluated, which each express high levels of two or more of the corresponding antigens. These cumulative data thus suggest that the immunological properties of the individual antigens are preserved in the context of these highly elaborate vaccines.
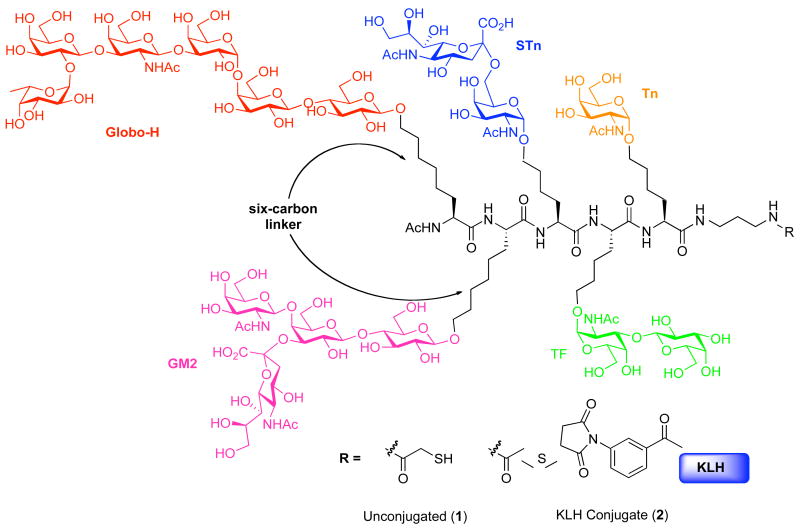
The results of biological studies with this unimolecular pentavalent vaccine led us to pursue the synthesis of a second-generation unimolecular pentavalent construct 1 against prostate and breast cancer, as shown in Figure 1.5 In this particular construct, the previously used pentasaccharide Ley antigen was replaced with the prostate and breast cancer-associated tetrasaccharide antigen GM2.6 The GM2 antigen was selected for inclusion on the basis of reports which indicate that GM2-induced antibodies are active against human GM2-positive cells. Moreover, human clinical trials conducted with GM2 alone have demonstrated a correlation between enhanced GM2 antibody levels and survival.6
Figure 1.
A unimolecular pentavalent vaccine construct containing Globo-H, GM2, STn, TF and Tn.
Synthetic Studies
Unimolecular pentavalent construct KLH conjugate (UPC-KLH, 2)
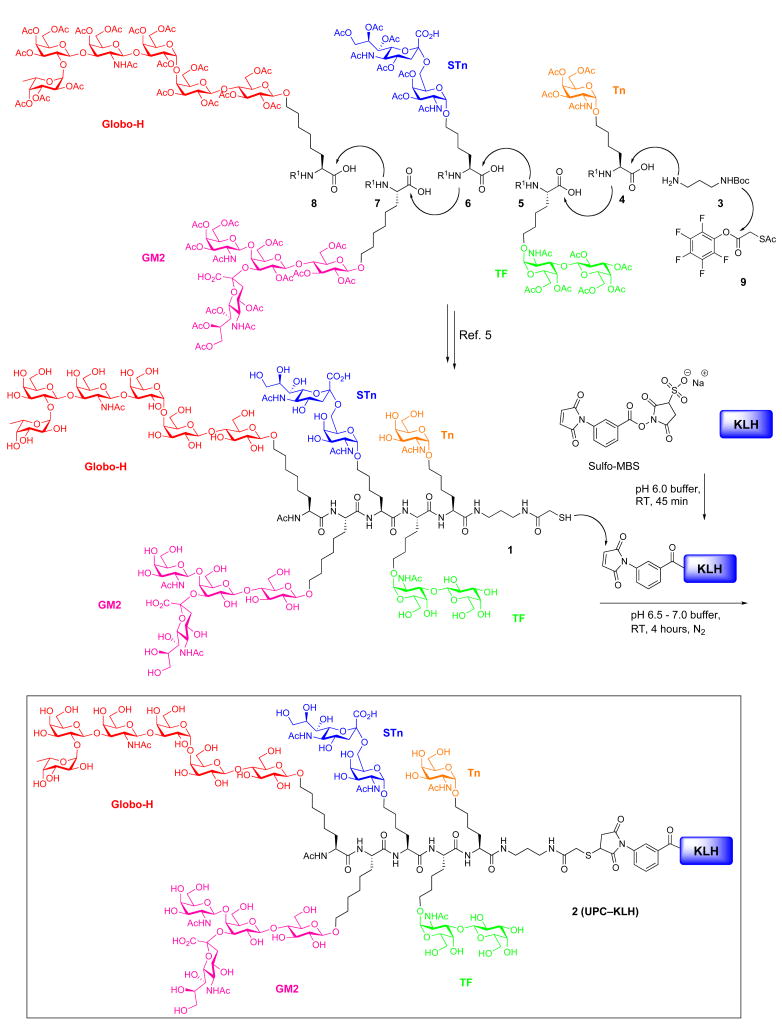
We previously reported the synthesis of the pentavalent glycopeptide construct, 1, through the assembly of a pool of glycosylamino acids presenting the Globo-H, GM2, STn, TF and Tn carbohydrate antigens (4–8, Scheme 1). The synthesis proceeded through initial coupling of Tn glycosylamino acid (4) with tert-butyl N-(3-aminopropyl)carbamate (3), followed by a series of iterative Fmoc deprotections and glycosylamino acid couplings. Following completion of this cycle, the construct was subjected to removal of the Boc carbamate functionality. Subsequent amidation with activated S-acetylthioglycolic acid pentafluorophenyl ester (SAMA-OPfp, 9) and final global deprotection afforded the unimolecular pentavalent glycopeptide 1.
Scheme 1.
Synthesis of UPC–KLH conjugate 2 containing Globo-H, GM2, STn, TF and Tn
With unimolecular pentavalent construct 1 in hand, we turned our efforts toward the preparation of the UPC-KLH conjugate (2) to provide the fully synthetic vaccine.7 In earlier studies, a structurally related first-generation unimolecular pentavalent construct had been conjugated to KLH carrier protein with a conjugation efficiency of 228 glycopeptides per KLH.5 In this study, we sought to obtain a higher epitope/KLH ratio for the UPC–KLH conjugate (2), in the hopes of generating a more robust immune response. We thus performed the conjugation under the slightly modified experimental procedures detailed below.
In the event, carrier protein KLH was first incubated with sulfo-MBS (m-maleimidobenzoyl-N-hydroxysuccinimide) in pH 6.0 phosphate buffer for one hour. Next, the unconjugated Sulfo-MBS was eliminated by passage over a G25 Sephadex column and maleimide-activated KLH was then obtained. Glycopeptide construct 1 (freshly prepared, passed through TCEP gel immediately prior to use) was mixed with freshly prepared maleimide-activated KLH in pH 6.5–7.0 phosphate buffer and stirred at room temperature for 4 hours. Following incubation, unreacted glycopeptide was removed using a 30,000 molecular weight cut off Pellicon XL filter (Millipore). Finally the corresponding KLH conjugate 2 (UPC-KLH) was obtained as a phosphate buffer solution.
The number of copies of glycopeptide construct incorporated in the KLH conjugate was determined to be 505 per KLH by hydrolytic carbohydrate analysis8 and standard protein analysis (Bio-Rad dye-binding method). The conjugation yield was 50% with respect to the unimolecular pentavalent glycopeptide construct, and the recovery yield of KLH was ca. 98%. This modified procedure had thus provided a much more efficient conjugation loading than the previous one (228/1). The key to achieving such a high epitope ratio is the use of freshly prepared glycopeptide construct 1, which contains a highly oxidizable mercapto group. One of the known possible oxidations of the sensitive mercapto group results in formation of its putative dimer, via disulfide formation. It is therefore necessary to pass this construct through TCEP gel immediately prior to use, in order to reduce the dimer and allow recovery of the glycopeptide construct 1. Preservation of the intact mercapto group is vital for the subsequent bio-conjugation reaction, which presumably proceeds via Michael addition to the maleimide. With the UPC-KLH conjugate 2 in hand, we were now ready to commence immunological studies in preclinical mouse settings. The promising results of these studies are described below.
Biological Studies
Animal immunizations
Group of five mice (female; C57BL/6J) were immunized subcutaneously at one site with UPC-KLH vaccine (2), containing 10 μg of unimolecular pentavalent construct (UPC) plus 20 μg of QS-21 adjuvant in 200 μL PBS, at 0, 1, 2 and 5 weeks. “Pre-treatment” serum was taken one week before the first vaccination. “Post” serum was taken one week after the third vaccination. “Boost” serum was taken one week after the fourth vaccination.
Serum ELISA Assay
Enzyme-linked immunosorbent assays (ELISA) were performed, as described previously,9 to determine the IgM and IgG serum antibody titers achieved associated with each of the individual carbohydrate antigens (Globo-H, GM2, STn, TF and Tn). In particular, Globo-H ceramide, GM2 ceramide, ovine submaxillary mucin (OSM, expressing sTn), desialylated porcine submaxillary mucin (dPSM expressing TF), and desialylated ovine submaxillary mucin (dOSM, expressing Tn), were each coated on ELISA plates at an antigen dose of 0.1 μg/well, and were incubated overnight at 4 °C. Nonspecific sites were blocked with 3% human serum albumin (HSA) for 2 h, and serially diluted antiserum was added to each well. After 1 h of incubation, the plates were washed, and alkaline phosphatase labeled goat anti-mouse IgM or IgG was added at 1:200 dilution (Southern Biotechnology Associates Inc., Birmingham, AL). The antibody titer was defined as the highest dilution with absorbance of 0.1 or greater over that of normal control mouse sera.
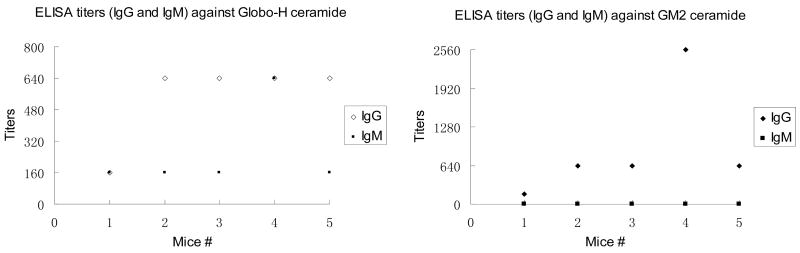
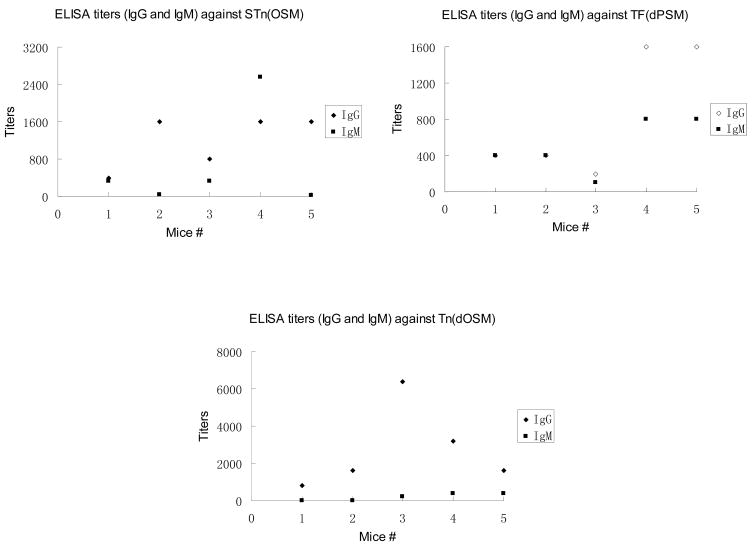
As shown in Table 1 and Figure 2, pre-vaccination sera from none of the five mice showed reactivity against the five antigens in the vaccine. Post-immunization sera from mice immunized with the UPC-KLH 2 vaccine plus QS-21 adjuvant produced substantial titers of antibodies corresponding to each of the five carbohydrate antigens: Globo-H ceramide, GM2 ceramide, STn (OSM), TF (dPSM) and Tn (dOSM). Unlike our previous first-generation unimolecular pentavalent construct KLH conjugate, which did not successfully induce antibodies against one of the antigens (Lewisy), this second-generation construct, UPC-KLH 2 did produce excellent IgG and IgM antibody titers against all five antigens, including the GM2 antigen, which replaced Lewisy in this construct. Since GM2 is an important epitope that is over-expressed on prostate and breast cancer cell lines,10 its incorporation into the construct is considered to greatly enhance the immunogenicity of the UPC-KLH conjugate, 2. All of the data indicate that the immunological properties of the individual carbohydrate antigens are preserved in the context of these highly complex vaccine constructs.
Table 1.
Antibody titers by Enzyme-Linked ImmunoSorbent Assay (ELISA)a
| Mouse # | Globo-H Ceramide | GM2 Ceramide | OSM for sTn | dPSM for TF | dOSM for Tn | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | |
| Pre-Serum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 160 | 0 | 0 |
|
| ||||||||||
| 1 | 160 | 160 | 160 | 0 | 400 | 320 | 400 | 400 | 800 | 0 |
| 2 | 640 | 160 | 640 | 0 | 1600 | 40 | 400 | 400 | 1600 | 0 |
| 3 | 640 | 160 | 640 | 0 | 800 | 320 | 200 | 100 | 6400 | 200 |
| 4 | 640 | 640 | 2560 | 0 | 1600 | 2560 | 1600 | 800 | 3200 | 400 |
| 5 | 640 | 160 | 640 | 0 | 1600 | 20 | 1600 | 800 | 1600 | 400 |
|
| ||||||||||
| Median | 640 | 160 | 640 | 0 | 1600 | 320 | 800 | 400 | 1600 | 200 |
IgM or IgG antibody reciprocal titers of pre- and post-vaccination sera tested for the five antigens in the pentavalent vaccines. ELISA assays were performed to determine IgM and IgG serum antibody titers as previously described.9 In brief, Globo-H ceramide, GM2 ceramide, ovine submaxillary mucin (OSM expressing sTn), desialylated ovine submaxillary mucin (dOSM expressing Tn), or desialylated porcine submaxillary mucin (dPSM expressing TF) was coated on ELISA plates at an antigen dose of 0.1 μg/well and incubated overnight at 4 °C. Nonspecific sites were blocked with 1% human serum albumin (HSA) for 2 h, and serially diluted antiserum was added to each well. After 1 h of incubation, the plates were washed, and alkaline phosphatase labeled goat anti-mouse IgM or IgG was added at 1:400 dilution (Southern Biotechnology Associates Inc., Birmingham, AL). The antibody titer was defined as the highest dilution with absorbance of 0.1 or greater over that of normal control mouse sera.
Figure 2.
Mice immunized with this UPC-KLH 2 produced substantial titers of antibodies corresponding all of the carbohydrate antigens including Globo-H ceramide, GM2 ceramide, STn (OSM), TF (dPSM) and Tn (dOSM).
Flow Cytometry
The cell surface reactivity of the antibodies induced by the UPC-KLH 2 vaccine was determined by FACS assay analysis with MCF-7 breast cancer cells, as described previously.11 Single-cell suspensions of 5 × 105 cells/tube were washed in phosphate-buffered saline with 3% fetal calf serum, and then incubated with 20 μL of 1/200 diluted antisera for 30 min on ice. A total of 20 μL of 1/15 goat anti-mouse IgG or IgM labeled with FITC was added, and percent positive cells and mean fluorescent intensity (MFI) of stained cells were analyzed using a FACScan (Becton Dickinson, San Jose, CA).
The post- and boost- vaccination sera flow cytometry results for the five mice receiving vaccine 2 are described in Table 2. Serologic responses to these vaccinations were almost exclusively IgM. As shown, the median percentage of positive cells in the post-vaccination serum increased from 10% to 36%, and the mean fluorescent intensity (MFI) increased from 85 to 302. The boost-vaccination serum showed a further increase in percentage of positive cells to 70%, with an MFI increase to 486. These experimental results suggest that UPC-KLH 2 may indeed be a very effective and clinically useful vaccine candidate, especially in conjunction with boost-injection.
Table 2.
Antibody Binding Studies by Fluorescence-Activated Cell Sorting (FACS)a
| Mouse # | Post-Vaccination Serum MCF-7 Cells (Breast Cancer) | Boost-Vaccination Serum MCF-7 Cells (Breast Cancer) | ||||||
|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgM | IgG | |||||
| % Pos | MFI | % Pos | MFI | % Pos | MFI | % Pos | MFI | |
| Pre-Serum | 10% | 85 | 10% | 64 | 10% | 85 | 10% | 64 |
|
| ||||||||
| 1 | 53% | 302 | 14% | 87 | 70% | 561 | 8% | 55 |
| 2 | 33% | 203 | 8% | 78 | 72% | 486 | 1% | 33 |
| 3 | 36% | 313 | 16% | 245 | 72% | 897 | 3% | 41 |
| 4 | 49% | 321 | 8% | 52 | 51% | 424 | 3% | 20 |
| 5 | 34% | 195 | 10% | 55 | 56% | 450 | 10% | 68 |
|
| ||||||||
| Median | 36% | 302 | 10% | 78 | 70% | 486 | 3% | 41 |
IgM and IgG FACS profiles for the five mice immunized with 5 μg of unimolecular pentavalent-KLH vaccine plus QS-21 tested on MCF-7 breast cancer cell lines. Pre-, post- and boost-vaccination results shown, % positive cells (MFI). FACS analysis (v(a)): MCF-7 human breast cancer cells expressing all five antigens (but especially globo H). Single-cell suspensions of 5 × 107 cells/tube were washed in phosphate-buffered saline with 3% fetal calf serum and incubated with 20 μL of 1/200 diluted antisera for 30 min on ice. A total of 20 μL of 1/15 goat anti-mouse IgG or IgM labeled with FITC was added, and percent positive cells and mean fluorescent intensity (MFI) of stained cells were analyzed using a FACScan (Becton Dickinson, San Jose, CA). Pre-, post- and boost-vaccination sera were analyzed together, and the pretreatment percent positive cells gaited at 10%. Results were considered positive when percent positive cells was 3-fold the negative controls (>30% positive cells) and the MFI was 150% or more of the negative control MFI.
Conclusion
In summary, we have described herein the preparation and biological evaluation in mice of a fully synthetic second-generation unimolecular pentavalent construct KLH conjugate, 2. In particular, an improved bio-conjugation protocol has been developed, which enabled a significant increase in epitope ratio of the KLH conjugate (2). In the presence of QS-21 as an adjuvant, this unimolecular pentavalent KLH conjugate demonstrated high promise in inducing antibodies against each of the carbohydrate antigens, as indicated by ELISA assay. In addition, the FACS profiles of the UPC-KLH conjugate 2 confirms that the antibodies in the resulting serum are highly reactive to cancer cells overexpressing these antigens, such as MCF-7 breast cancer cell line. We suspect that the resultant high epitope ratio may correlate to the enhanced immunogenicity of this KLH conjugate. Moreover, the efficient production of the corresponding antibodies in sera may greatly help to eradicate tumor cells expressing the corresponding carbohydrate antigens. Based on these promising results, this vaccine is scheduled to enter phase I clinical trials in the near future. It is our hope that these research efforts will lead to the discovery of a clinically useful vaccine for prostate and breast cancer treatment.
Acknowledgments
Support for this work was provided by the National Institutes of Health (CA28824 to S.J.D. and PO1CA052477 to P.O.L.) and by the Breast Cancer Research Foundation. Special thanks go to Rebecca Wilson for editorial consultation and Dana Ryan for her assistance with the preparation of the manuscript. We thank Dr. George Sukenick, Ms. Hui Fang, and Ms. Sylvi Rusli of the Sloan-Kettering Institute’s NMR core facility for mass spectral and NMR spectroscopic analysis.
References
- 1.(a) Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Nat Chem Biol. 2007;3:663. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buskas T, Ingale S, Boons GJ. Angew Chem, Int Ed. 2005;44:5985. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kunz H, Dziadek S, Wittrock S, Becker T. ACS Symposium Series. 2008;989:293. Carbohydrate-Based Vaccines. [Google Scholar]; (b) Westerlind U, Hobel A, Gaidzik N, Schmitt E, Kunz H. Angew Chem, Int Ed. 2008;47:7551. doi: 10.1002/anie.200802102. [DOI] [PubMed] [Google Scholar]; (c) Wittrock S, Becker T, Kunz H. Angew Chem, Int Ed. 2007;46:5226–5230. doi: 10.1002/anie.200700964. [DOI] [PubMed] [Google Scholar]; (d) Dziadek S, Brocke C, Kunz H. Chem Eur J. 2004;10:4150. doi: 10.1002/chem.200400228. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris Adrian L, Old L, Cerundolo V. J Immunol. 2003;171:5140. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]; (b) Schmidt RR, Castro-Palomino JC, Retz O. Pure Appl Chem. 1999;71:729. [Google Scholar]
- 4.(a) Danishefsky SJ, Bilodeau MT. Angew Chem Int Ed Engl. 1996;35:1380. [Google Scholar]; (b) Danishefsky SJ, Allen JR. Angew Chem Int Ed Engl. 2000;39:836. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]; (c) Keding SJ, Danishefsky SJ. Carbohydrate-Based Drug Discovery. 2003;1:381. [Google Scholar]; (d) Warren JD, Geng X, Danishefsky SJ. Top Curr Chem. 2007;267:109. [Google Scholar]
- 5.(a) Keding SJ, Danishefsky SJ. Proc Natl Acad Sci USA. 2004;101:11937. doi: 10.1073/pnas.0401894101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ragupathi G, Koide F, Livingston PO, Cho YS, Atsushi E, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. J Am Chem Soc. 2006;128:2715. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 6.(a) Livingston PO, Natoli EJ, Calves MJ, Stockert E, Oettgen HF, Old LJ. Proc Natl Acad Sci USA. 1987;84:2911. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G. J Clin Oncol. 1994;12:1036. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 7.The extension of these findings to other conjugates and their applications in other animal systems have been demonstrated in search of optimal phase I vaccines. These results will be disclosed in due course.
- 8.(a) Lloyd KO, Savage A. Glycoconjugate J. 1991;8:439. doi: 10.1007/BF00769849. [DOI] [PubMed] [Google Scholar]; (b) Hardy MR, Townsend RR. Proc Natl Acad Sci USA. 1988;85:3289. doi: 10.1073/pnas.85.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Ragupathi G, Cappello S, Yi SS, Canter D, Spassova M, Bornmann WG, Danishefsky SJ, Livingston PO. Vaccine. 2002;20:1030. doi: 10.1016/s0264-410x(01)00451-0. [DOI] [PubMed] [Google Scholar]; (b) Ragupathi G, Koide F, Sathyan N, Kagan E, Spassova M, Bornmann W, Gregor P, Reis CA, Clausen H, Danishefsky SJ, Livingston PO. Cancer Immunol Immunother. 2003;52:608. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Livingston PO, Natoli EJ, Calves MJ, Stockert E, Oettgen HF, Old LJ. Proc Natl Acad Sci USA. 1987;84:2911. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G. J Clin Oncol. 1994;12:1036. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 11.Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, Spassova M, Zatorski A, Spriggs D, Aghajanian C, Soignet S, Peyton M, O’Flaherty C, Curtin J, Lloyd KO. Int J Cancer. 2000;87:79. [PubMed] [Google Scholar]