Abstract
Introduction:
The abundant expression of leukotrienes (LTs) and their receptors in adenotonsillar tissues of children with obstructive sleep apnea (OSA) suggest that LT antagonists could be useful in treating OSA.
Methods:
The effects of LTD4 and of LT receptor antagonists zileuton, montelukast, and BAY u9773 were examined on mixed cell cultures prepared from dissociated tonsils or adenoids harvested intraoperatively from children with polysomnographically diagnosed OSA. Proliferation was assessed by 3[H]-thymidine incorporation, and inflammatory cytokine production (tumor necrosis factor [TNF]-α, interleukin [IL]-6, IL-8, IL-10, and IL-12) was assessed in supernatants using enzyme-linked immunosorbent assay.
Results:
LTD4 elicited dose-dependent increases in adenotonsillar cell proliferation (p < 0.001; n = 12). All LT antagonists exhibited dose-dependent reductions in adenotonsillar cellular proliferation rates, with montelukast more than BAY u9773 more than zileuton (n = 14/group; p < 0.001). However, BAY u9773 showed partial agonist effects and increased cellular proliferation at higher concentrations (10−4 mmol/L; p < 0.01; n = 12). LTD4 effects were partially blocked by montelukast and BAY u9773 but not by zileuton. All three antagonists reduced TNF-α, IL-6, and IL-12 concentrations, with selective changes in IL-8 and no effects on IL-10 levels.
Conclusions:
LT pathways mediate intrinsic proliferative and inflammatory signaling pathways in adenotonsillar tissues from children with OSA, and targeted pharmacologic disruption of these pathways may provide nonsurgical alternatives for prevention and treatment of this disease.
Obstructive sleep apnea (OSA) is a frequent condition in children characterized by habitual snoring and increased upper airway resistance during sleep, leading to partial or complete intermittent obstructive events of the upper airway, hypoxemia and hypercapnia, and recurrent arousals.1 It has now become clear that although craniofacial, structural, and neuromuscular factors also play a role, hypertrophy of adenotonsillar tissues is by far the predominant etiologic factor involved in pediatric OSA, even if obesity has emerged as another major contributor to pediatric OSA.2,3 As such, the severity of OSA correlates with adenoid and tonsillar size, and surgical excision of these tissues are consequently accompanied by significant clinical improvements.4–7
In the past few years, we and others have shown8,9 evidence of inflammation in both nasal and oropharyngeal mucosa in children with OSA, and we surmised that inflammatory processes may underlie increased adenotonsillar proliferation. Indeed, intranasal corticosteroids have shown favorable outcomes in children with OSA, and their use for periods of 4 to 6 weeks has been associated with improvements in the respiratory disturbance during sleep and partial involution of adenoidal hypertrophy.10–14 Furthermore, increased concentrations of leukotrienes (LTs) in tonsils and upper airway condensate in children with OSA along with a relatively high abundance of LT receptors in these tissues suggested that LT pathways may contribute to the proliferative status of adenotonsillar tissues,15,16 and in fact, improvements in sleep disturbances occurred after treatment in an open-label trial of children with mild OSA.17
We recently developed18 a novel method allowing for in vitro cell culture of tonsils and adenoids derived from children undergoing tonsillectomy and adenoidectomy (T&A). We hypothesized that LT antagonists would lead to dose-dependent reductions in cellular proliferation and increased apoptosis in whole tonsillar and adenoid cell cultures obtained from children with OSA, and that these effects would be associated with a decreased production of proinflammatory cytokines.
Materials and Methods
Subjects
The study was approved by the University of Louisville Human Research Committee, and informed consent was obtained from the legal caregiver of each participant. Assent was also obtained from children > 7 years of age. Consecutive children who underwent tonsillectomy for OSA were identified before surgery and recruited into the study. Overnight polysomnography was performed using standard methods that have been published in detail elsewhere.19 OSA was considered to be present when the obstructive Apnea-Hypopnea Index was ≥ 5 h of total sleep time in the context of habitual snoring in otherwise healthy children without any chronic disorders requiring treatment with medications (including topical or systemic antiinflammatory or antihistaminic medications), or without any known genetic or craniofacial syndromes.
Cell Culture
Surgically removed tonsils and adenoids from children with OSA were immediately placed in ice cold phosphate-buffered saline (PBS) solution plus antibiotics, Sample processing was initiated within 30 min under aseptic conditions. Briefly, tonsils or adenoids were washed thoroughly with PBS solution, manually dissected into Petri dishes, and gently grounded with a syringe plunger through a 70-μ mesh screen to obtain a mixed cell suspension through mechanical dissociation. RBCs were removed by lysis buffer. Cell viability of all specimens was determined by trypan blue exclusion. Specimens with a viability of < 75% were discarded. Cell cultures were established in standard medium RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum plus antibiotics, which included streptomycin, Fungizone, gentamicin, and penicillin to prevent bacterial and fungal contamination. Mixed cell suspensions were transferred onto 96-round bottom-well plates at a concentration of 1 × 106 cells/well. Cells were cultured in a 5% CO2 incubator at 37°C for 72 h. Cells were also cultured using 24-well plates to determine proinflammatory cytokine levels. Cultures were also exposed to LTD4 or to LT antagonists, with control conditions corresponding to the addition of the diluent alone. LTD4 was purchased from Cayman Pharma (catalog No. 20310; Neratovice, Czech Republic). A lipoxygenase inhibitor (zileuton; Sigma-Aldrich; St. Louis, MO), a cysteinyl LT (cysLT) receptor 1 antagonist (montelukast; Sigma-Aldrich), and a dual cysLT receptor 1 and 2 antagonist (BAY-u9773; Biomol Research Laboratories; Plymouth Meeting, PA),20 were added to the medium 24 h after plating to achieve final concentrations ranging from 10−3 to10−8 mmol/L.
Proliferation Assay
Cells were incubated for the final 18 to 20 h of the 72-h culture with 0.0185 MBq (0.5 μCi) 3[H]-thymidine in complete medium (Amersham Biosciences; Little Chalfont, UK). Cells were then harvested onto glass-fiber filters with a cell harvester, and radioactivity was measured in a liquid scintillation counter. All experimental conditions were always performed in triplicate, and 3[H]-thymidine uptake results were expressed as the average of the three wells in counts per minute.
Immunohistochemistry
Coronal sections (40 μm) of tonsils were initially incubated in 1xcitrate buffer (Lab Vision Corporation; Fremont, CA) at 95°C for 45 min, washed several times in PBS solution, and blocked with a PBS/0.4% Triton X-100/0.5% signal amplifier solution (TSA; Perkin Elmer Life Sciences; Boston, MA) blocking reagent/10% normal horse serum for 1 h. Sections were then serially incubated with anti-CD4 antibody (1:300; Santa Cruz Biotechnology; Santa Cruz, CA) or anti-cysLT1 receptor antibody (1:1000; BD Pharmagen; San Jose, CA) at 4°C for 24 h, and then washed in PBS solution six times for 5 min each wash. Sections were incubated at room temperature for 1 h in horse antimouse biotinylated antibody (1:400; Vector Laboratories; Burlingame, CA) in a PBS/0.4% TSA blocking reagent/10% horse serum solution, and then with streptavidin-horseradish peroxidase diluted 1:100 in PBS/0.5% TSA blocking reagent solution. Subsequently, the sections were incubated with TSA fluorescein reagents diluted 1:50 in amplification diluent (Perkin Elmer Life Sciences) for 2 min. Sections were then washed and mounted onto glass slides. Negative controls were prepared by either omitting the primary or the secondary antibody. Sections were prepared from five sets of tonsils from OSA subjects, and they were visualized using a fluorescent microscope by an investigator who was blinded to the sample source.
Cytokine Assays
Concentrations of tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-8, IL-10, and IL-12 were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits from the supernatants of mixed tonsillar cell cultures incubated in 24-well flat-bottom plates in complete RPMI medium supplemented with 10% FBS in the presence or absence of LT antagonists. Supernatants were collected after 48 h and stored at −80°C until assay. Selection of these particular cytokine assays was based on previous experience with some of the proinflammatory mediators,18 and it also aimed to explore other cytokines associated with the activity of the predominant cell types in tonsillar tissues. TNF-α levels were measured according to manufacturer's instructions using a high-sensitivity ELISA assay able to detect concentrations as low as 0.09 pg/mL (BioSource Europe S.A.; Nivelles, Belgium). IL-8 was evaluated using a commercial ELISA kit (R&D Systems; Minneapolis, MN) with a detection range between 0 and 2000 pg/mL. To determine IL-6 concentrations, the IL-6 EASIA assay (BioSource Europe S.A.) was used. Commercial kits were used for IL-10 (OptEIA Kit 555157; BD Biosciences; San Diego, CA) and for IL-12 (R&D Systems; Minneapolis, MN). The concentrations of cytokines in the supernatants were normalized to the number of cells plated and expressed as pg/106 cells. For all assays, calibration curves were performed in duplicate for each experiment.
Statistical Analysis
All data were expressed as mean ± SE, unless stated otherwise. Statistical analyses were performed using SPSS software (version 16.0; SPPS Inc.; Chicago, IL). For normally distributed data, analysis of variance, followed by post hoc tests was used. Cytokine data were not normally distributed and were therefore log-transformed and compared using nonparametric tests (Mann-Whitney). To determine whether significant differences in potency occurred among the various LT modifier compounds, multivariate analyses of variance with Scheffe post hoc tests were used. All p values reported are two-tailed with statistical significance set at < 0.05.
Results
Study Population
A total of 52 children out of 54 suitable candidates with a clinical and polysomnographic diagnosis of OSA undergoing T&A agreed to participate and completed the study. The two children who did not participate were similar in every clinical aspect to those included in the study. Of the 52 collected samples, only 49 samples could be processed because in 3 samples, trypan blue exclusion tests showed excessive cell death (> 25%), which in preliminary experiments has been shown to be associated with unpredictable response patterns.
Table 1 shows the demographic characteristics and major overnight polysomnographic findings for the 49 participants. Of note, the extent of tissue availability constrained the number of experimental conditions that were possible for every patient recruited, such that the number of subjects included in each experiment varied and is indicated as appropriate.
Table 1.
Demographic and Polysomnographic Characteristics in 49 Children With OSA Undergoing T&A*
| Characteristics | OSA (n = 49) |
|---|---|
| Age, yr (range) | 5.7 ± 0.5 (2.1–8.2) |
| Gender, % female | 47 |
| African American, % | 39 |
| BMI z score | 1.31 ± 0.19 |
| AHI (/h TST) | 13.2 ± 1.3 |
| Nadir Sao2, % | 81.5 ± 1.9 |
| Arousal index (/h TST) | 19.1 ± 3.8 |
*AHI = Apnea-Hypopnea Index; BMI = body mass index; Sao2 = arterial oxygen saturation; TST = total sleep time.
Cell Proliferation Assays
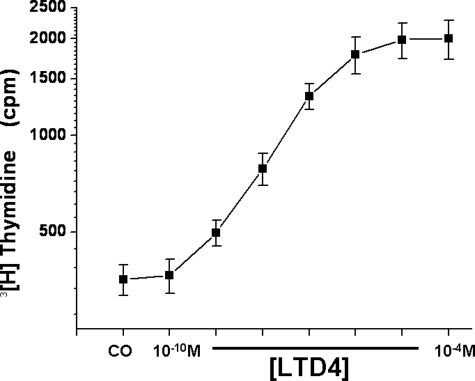
Basal proliferative rates for mixed cell cultures derived from either tonsils or adenoids were similar, and therefore data sets were combined. To assess the effect of LT on basal proliferation, initial experiments were conducted using LTD4. Figure 1 shows the responses to incremental doses of LTD4 on 3[H]-thymidine incorporation, whereby LTD4 increased cellular proliferation rates in this mixed cell culture system and exhibited dose-dependent responses (p < 0.001; n = 12).
Figure 1.
Effects of log increases in LTD4 concentrations on tonsillar cellular proliferation in children with OSA in a mixed cell culture system. The y axis is displayed in a logarithmic scale. CO = control.
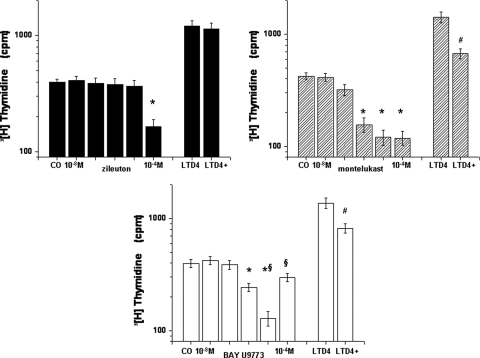
The addition of zileuton, montelukast, or BAY u9773 to the adenotonsillar mixed cell culture system elicited dose-dependent reductions in cell proliferation (Fig 2). However, although these three compounds displayed overall qualitatively similar effects, there were several differences. First, zileuton required higher doses (10−4 mmol/L; n = 14) to induce reductions in proliferative rates compared to either montelukast (10−6 mmol/L; n = 14; p < 0.001) or BAY u9773 (10−6 mmol/L; n = 12; p < 0.001). Second, Bay u9773 had an antagonistic effect at lower concentrations, but at high concentrations it seemed to induce a partial agonist effect as well (p < 0.01 vs dose leading maximal inhibition; n = 12) [Fig 2]. Third, LTD4 was added to the medium at a concentration leading to robust increases in proliferation (ie, 5 × 10−7 mmol/L), zileuton at the effective concentration of 10−4 mmol/L had no effects (difference not significant; n = 8) [Fig 2], whereas both montelukast (10−6mmol/L; n = 10) and BAY u9773 (10−6 mmol/L; n = 7) reduced LTD4 effects (p < 0.01 for both compounds).
Figure 2.
Cellular proliferative responses to increasing log concentrations of zileuton (left top), montelukast (right top), and BAY u9773 (bottom) in a adenotonsillar mixed cell culture system. On the right-hand side of each graph, the proliferative response to 5 × 10−7 mmol/L (M) LTD4 and the effect of one of the three drugs, that is, zileuton (10−4 mmol/L), montelukast (10−6 mmol/L), and BAY u9773 (10−6 mmol/L), on LTD4-induced proliferation changes are also shown. * = vs control (CO); p < 0.001; # = vs LTD4; p < 0.01; § = 10−5 mmol/L vs 10−4 mmol/L BAY u9773; p < 0.01.
Tonsil Immunohistochemistry
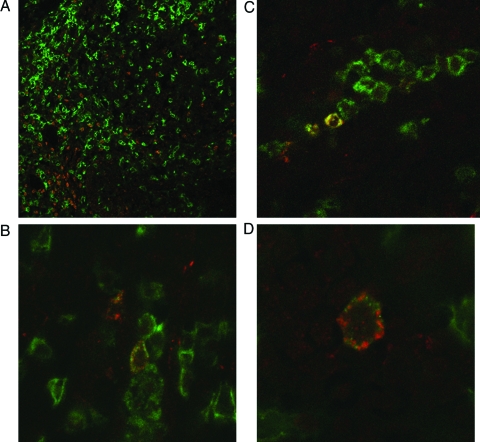
We have previously shown that cysLT receptors are abundantly expressed in tonsils of children with OSA. A recent report16 further suggested that T cells are the predominant cell type harboring cysLT receptors. We therefore conducted double-label immunohistochemical assessment of five tonsils, and assessed whether CD4(+) T cells and cysLT1 receptor co-localize. CD4(+) lymphocytes constitute most of the cells in the tonsillar extrafollicular areas, and the vast majority of cysLT1(+) cells co-localize with CD4(+) lymphocytes (Fig 3).
Figure 3.
Representative overlaid microscope images of CD4 and cysLT1 cellular expression from intact tonsillar tissues in children with OSA. Expression of CD4 (green) and cysLT1 receptor (red) immunoreactivity is abundant, and colocalization occurs frequently (top left, A, ×20 magnification; bottom left, B, and top right, C, ×40 magnification; bottom right, D, ×100 magnification). Similar findings emerged in the tonsils of five OSA subjects.
Cytokine Assays
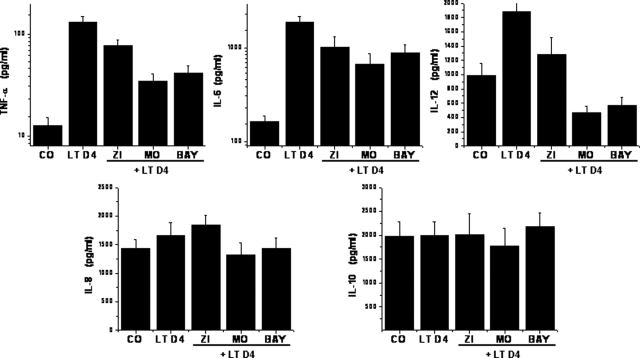
Basal release of TNF-α, IL-8, and IL-6 to the supernatants was increased in tonsillar cultures from children with OSA after addition of LTD4 (Fig 4; n = 8). Addition of LT antagonists induced significant reductions in proinflammatory TNF-α, IL-6, and IL-12 cytokine production (p < 0.001; n = 8) [Fig 4], but it did not affect IL-8 and IL-10 production (n = 8; difference not significant).
Figure 4.
Concentrations of TNF-α, IL-6, IL-8, IL-10, and IL-12 in the supernatants of tonsillar cell cultures in basal conditions (CO [control]), after stimulation with LTD4 (5 × 10−7mmol/L), and after stimulation with LTD4 but in the presence of effective concentrations of zileuton (ZI; 10−4 mmol/L; n = 8), montelukast (MO; 10−6 mmol/L; n = 10), and BAY u9773 (BAY; 10−6 mmol/L; n = 7). LTD4-increased production of TNF-α, IL-6, and IL-12, all LT antagonists, reduced this effect (p < 0.01); only cysLT antagonists attenuated IL-8 release (p < 0.05). The y axis in the upper-panel graphs is displayed in a logarithmic scale.
Discussion
The present study shows that addition of LTD4 to a dissociated mixed cell culture system of adenoids and tonsils derived from children with OSA induces increased proliferative responses and release of proinflammatory cytokines. Furthermore, treatment with LT antagonists not only markedly reduces proliferation in a dose-dependent fashion, but it also reveals striking differences in the potencies of zileuton, montelukast, and the cysLT receptor 1 and 2 antagonist, BAY u9773. Interestingly, the latter also exhibits partial agonist effects at higher concentrations. The effect on proliferative rates by LT antagonists is also accompanied by reductions in the production and release of the proinflammatory cytokines TNF-α, IL-6, and IL-12. Taken together, we would surmise that the reported beneficial effects of anti-LT therapy to reduce the severity of sleep-disordered breathing in children17 may be due to their effects on proliferative and inflammatory pathways within the tonsils and adenoids, and that the in vitro approach reported here may provide new inroads into development of novel nonsurgical therapeutic approaches aiming to resolve OSA in children.
Previous work from our laboratory and others would clearly suggest that tonsillar tissues of children with OSA differ from those of children with other conditions (eg, recurrent tonsillar infections).8,15–18 The global findings would suggest that tonsils from OSA children exhibit increased inflammatory markers, and that these inflammatory mediators may in turn underlie prominent components of the pro-proliferative pathways that ultimately lead to adenotonsillar hypertrophy and OSA. Under such hypothetical framework, increased expression of LT receptors and increased LT concentrations in the adenotonsillar tissues of children with OSA15–17 provided further rationale for the use of anti-LT compounds to reduce the proliferation of upper airway lymphadenoid tissue and to promote the involution of the latter, thereby reducing or resolving the underlying respiratory disturbances during sleep.
Some technical and methodological issues deserve specific comment. First, the cohort included in the present study was not specifically and objectively screened for the presence of allergic rhinitis or asthma, even though subjects were not receiving any topical or systemic medications at the time of the surgical extirpation of adenotonsillar tissues. An increased prevalence of allergic symptoms and asthma has been reported in children with OSA.21–23 However, the mixed tissue cell culture system used in this study should permit future assessment of the effect of allergic conditions, such as allergic rhinitis or asthma, on the differential responses to LT antagonists. In addition, we did not specifically determine the proliferating cell type(s) within the adenoids and tonsils that are affected by alterations in LT bioavailability. Based on the relative abundance of CD4+ T lymphocytes that express cysLT 1 receptors, as evidenced by the immunohistochemical approaches (Fig 3), we would surmise that these cells represent one of the primary targets for LT modifier therapy. However, T cells do not appear to express cysLT1 receptors in human bronchial mucosa,24 even if peripheral T cells do express these receptors.25
The potential application of LT antagonists as a treatment strategy aiming to reduce adenotonsillar size in pediatric OSA was initially advanced after increased expression of cysLT receptors and elevated cysLT concentrations were found in these tissues among children with OSA.15,17 The initial findings from an open-label trial using montelukast in mild OSA were encouraging,17 particularly when considering the risks for potential postoperative complications in the context of surgical treatment. The patients in this open-label trial were selected by design to have very mild OSA, and such an approach would have clearly reduced the potential magnitude of the biological effects of LT modifiers on adenotonsillar tissues, such that a more comprehensive assessment of the magnitude of the effect of LT modifiers remains to be defined.17 However, although a randomized double-blind study is currently under way for pediatric OSA in our program (Clinical Trials.gov identifier: NCT00599534), neither the optimal dosage nor the duration of therapy have been defined, and in addition, there is a pressing need to identify specific OSA subgroups that may either be more or less responsive to treatment with LT modifiers. Finally, it remains unclear whether addition of other antiinflammatory agents, such as topical corticosteroids,26 may further enhance the potential beneficial effects of LT-targeted therapies. All of these questions can be critically examined, at least in preliminary fashion, by employing the in vitro adenotonsillar mixed culture system used in the present study.
The biological potency of the three LT modifiers used in this study differed, with montelukast displaying the greatest potency in our mixed cell system proliferation assay. These findings were somewhat anticipated, particularly when considering the previously reported differences in bioaffinity and activity among these compounds.27–29 Indeed, montelukast has a 20-fold higher relative affinity for the cysLT1 receptor than BAY u9773,27 and the latter has not only cysLT2 receptor antagonistic properties but also partial agonist properties, particularly at higher concentrations.27 The rationale for exploring the potential advantages of zileuton was based on its inhibitor properties of the 5-lipoxygenase pathway, which results in the formation of LTs, including LTB4 and the cysLT, and thus activates all four known LT receptors: BLT1, BLT2, cysLT 1, and cysLT 2. A 2007 reformulation30 of this compound into an effective controlled-release oral agent in the management of asthma further enhances its potential for use in the treatment of pediatric OSA. However, our findings would suggest a relatively lower potency and efficacy for zileuton in the context of pediatric sleep-disordered breathing, particularly when considering not only the higher concentrations required for reducing proliferative rates in adenoids and tonsils but also its lower antiinflammatory effect, as evidenced by the less prominent reductions in proinflammatory cytokines in the supernatants. These findings were somewhat anticipated based on the following considerations: the concentrations of LTs (both B4 and cysLT) were previously found to be increased in tonsillar tissues of children with OSA.17 Therefore, even without addition of LTD4, blocking of the cysLT receptor with montelukast should theoretically reduce the proliferative stimulus conferred by LTs, and in fact these predictions were confirmed by the experimental data. As far as the zileuton effect, this compound would, as already mentioned, block 5-lipoxygenase, and therefore would reduce or block altogether the intrinsic production of LTs, therefore leading to an antiproliferative effect. However, when such effect of zileuton was bypassed by addition of the downstream product (ie, LTD4), then zileuton should not be and indeed was not effective in reducing cellular proliferation. Notwithstanding, although the mechanisms underlying the different responses have yet to be precisely delineated, and the usefulness of any of the three agents remains to be established in the clinical setting, current findings would support the use of montelukast as the first choice in the anti-LT armamentarium.
T-cell lymphocytes appear to be the predominant cell type in tonsils of children with OSA, and these lymphocytes also harbor cysLT receptors 1 and 2 in higher abundance.16 Our study not only confirmed the presence of cysLT receptors among T lymphocytes, but it further indicated that CD4 + T cells in tonsils are the most probable target for the biological effects of LT antagonists. Published evidence would support a role for cysLT in T-cell lymphocyte proliferation and activation,25,31 and furthermore, T-cell proliferation leads to proinflammatory cytokine production in adenotonsillar tissues.32,33 Thus, future studies should explore the specific effects of cysLT 1 and 2 receptor antagonists on the proliferation of T-cell subsets in adenotonsillar cultures in children with OSA.
In summary, tonsils and adenoids obtained from children with OSA undergoing T&A display reductions in cellular proliferative rates and in proinflammatory cytokine production when treated with LT antagonists. Furthermore, the relative potency of the three anti-LT agents used in the study was highest for montelukast and lowest for zileuton. These findings support the use of tonsillar or adenoidal tissue cell cultures as a potentially useful approach for in vitro exploration and identification of the cellular subpopulations associated with increased LT production in pediatric OSA, and for assessment of therapeutic efficacy of anti-LT compounds in eliciting involution of the lymphadenoid hypertrophy that underlies OSA in children.
Abbreviations:
- cysLT
cysteinyl leukotriene
- ELISA
enzyme-linked immunosorbent assay
- LT
leukotriene
- OSA
obstructive sleep apnea
- PBS
phosphate-buffered saline
- T&A
tonsillectomy and adenoidectomy
- TNF
tumor necrosis factor
Footnotes
None of the authors have any conflict of interest to declare.
Dr. Gozal is supported by National Institutes of Health grants Nos. HL-065270, HL-086662, and HL-083075, the Commonwealth of Kentucky Research Challenge for Excellence Trust Fund, and the Children's Foundation Endowment for Sleep Research. Dr. Kheirandish-Gozal is supported by an investigator-initiated grant from the Merck Company. Dr. Bhattacharjee is supported by a fellowship from Jazz Pharmaceuticals.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 3.Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:253–262. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li AM, Wong E, Kew J, et al. Use of tonsil size in the evaluation of obstructive sleep apnea. Arch Dis Child. 2002;87:156–159. doi: 10.1136/adc.87.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam YY, Chan EY, Ng DK, et al. The correlation among obesity, apnea-hypopnea index, and tonsil size in children. Chest. 2006;130:1751–1756. doi: 10.1378/chest.130.6.1751. [DOI] [PubMed] [Google Scholar]
- 6.Brooks LJ, Stephens BM, Bacevice AM. Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr. 1998;132:682–686. doi: 10.1016/s0022-3476(98)70360-9. [DOI] [PubMed] [Google Scholar]
- 7.Schechter MS. Section on Pediatrics Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 8.Goldbart AD, Krishna J, Li RC, et al. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest. 2006;130:143–148. doi: 10.1378/chest.130.1.143. [DOI] [PubMed] [Google Scholar]
- 9.Li AM, Hung E, Tsang T, et al. Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnea. Thorax. 2007;62:75–79. doi: 10.1136/thx.2006.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics. 1995;95:355–364. [PubMed] [Google Scholar]
- 11.Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138:838–844. doi: 10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 12.Alexopoulos EI, Kaditis AG, Kalampouka E, et al. Nasal corticosteroids for children with snoring. Pediatr Pulmonol. 2004;38:161–166. doi: 10.1002/ppul.20079. [DOI] [PubMed] [Google Scholar]
- 13.Berlucchi M, Salsi D, Valetti L, et al. The role of mometasone furoate aqueous nasal spray in the treatment of adenoidal hypertrophy in the pediatric age group: preliminary results of a prospective, randomized study. Pediatrics. 2007;119:e1392–e1397. doi: 10.1542/peds.2006-1769. [DOI] [PubMed] [Google Scholar]
- 14.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122:e149–e155. doi: 10.1542/peds.2007-3398. [DOI] [PubMed] [Google Scholar]
- 15.Goldbart AD, Goldman JL, Li RC, et al. Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infection. Chest. 2004;126:13–18. doi: 10.1378/chest.126.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Kaditis AG, Ioannou MG, Chaidas K, et al. Cysteinyl leukotriene receptors are expressed by tonsillar T cells of children with obstructive sleep apnea. Chest. 2008;134:324–331. doi: 10.1378/chest.07-2746. [DOI] [PubMed] [Google Scholar]
- 17.Goldbart AD, Goldman JL, Veling MC, et al. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–370. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serpero LD, Kheirandish-Gozal L, Dayyat E, et al. A mixed cell culture model for assessment of proliferation in tonsillar tissues from children with obstructive sleep apnea or recurrent tonsillitis. Laryngoscope. 2009 doi: 10.1002/lary.20147. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery-Downs HE, O'Brien LM, Gulliver TE, et al. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 20.Wikström Jonsson E, Rosenqvist U, Dahlén SE. Agonist and antagonist activities of the leukotriene analogue BAY u9773 in guinea pig lung parenchyma. Eur J Pharmacol. 1998;357:203–211. doi: 10.1016/s0014-2999(98)00555-x. [DOI] [PubMed] [Google Scholar]
- 21.McColley SA, Carroll JL, Curtis S, et al. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997;111:170–173. doi: 10.1378/chest.111.1.170. [DOI] [PubMed] [Google Scholar]
- 22.Lu LR, Peat JK, Sullivan CE. Snoring in preschool children: prevalence and association with nocturnal cough and asthma. Chest. 2003;124:587–593. doi: 10.1378/chest.124.2.587. [DOI] [PubMed] [Google Scholar]
- 23.Ng DK, Chan CH, Hwang GY, et al. A review of the roles of allergic rhinitis in childhood obstructive sleep apnea syndrome. Allergy Asthma Proc. 2006;27:240–242. doi: 10.2500/aap.2006.27.2855. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Qiu YS, Figueroa DJ, et al. Localization and upregulation of cysteinyl leukotriene-1 receptor in asthmatic bronchial mucosa. Am J Respir Cell Mol Biol. 2005;33:531–540. doi: 10.1165/rcmb.2005-0124OC. [DOI] [PubMed] [Google Scholar]
- 25.Spinozzi F, Russano AM, Piattoni S, et al. Biological effects of montelukast, a cysteinyl-leukotriene receptor-antagonist, on T lymphocytes. Clin Exp Allergy. 2004;34:1876–1882. doi: 10.1111/j.1365-2222.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- 26.Kheirandish L, Goldbart AD, Gozal D. Intranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing following tonsillectomy and adenoidectomy in children. Pediatrics. 2006;117:E61–E66. doi: 10.1542/peds.2005-0795. [DOI] [PubMed] [Google Scholar]
- 27.Nothacker HP, Wang Z, Zhu Y, et al. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- 28.Ravasi S, Capra V, Panigalli T, et al. Pharmacological differences among CysLT(1) receptor antagonists with respect to LTC(4) and LTD(4) in human lung parenchyma. Biochem Pharmacol. 2002;63:1537–1546. doi: 10.1016/s0006-2952(02)00889-4. [DOI] [PubMed] [Google Scholar]
- 29.Capra V. Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharmacol Res. 2004;50:1–11. doi: 10.1016/j.phrs.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99:178–184. doi: 10.1016/S1081-1206(10)60642-4. [DOI] [PubMed] [Google Scholar]
- 31.Faith A, Fernandez MH, Caulfield J, et al. Role of cysteinyl leukotrienes in human allergen-specific Th2 responses induced by granulocyte macrophage-colony stimulating factor. Allergy. 2008;63:168–175. doi: 10.1111/j.1398-9995.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 32.de Baey A, Mende I, Baretton G, et al. A subset of human dendritic cells in the T cell area of mucosa-associated lymphoid tissue with a high potential to produce TNF-alpha. J Immunol. 2003;170:5089–5094. doi: 10.4049/jimmunol.170.10.5089. [DOI] [PubMed] [Google Scholar]
- 33.Komorowska A, Komorowski J, Banasik M, et al. Cytokines locally produced by lymphocytes removed from the hypertrophic nasopharyngeal and palatine tonsils. Int J Pediatr Otorhinolaryngol. 2005;69:937–941. doi: 10.1016/j.ijporl.2005.01.035. [DOI] [PubMed] [Google Scholar]