Abstract
AIM: To conduct a multicentre retrospective review of virological response rates in Asians infected with genotype 1 chronic hepatitis C (CHC) treated with combination interferon and ribavirin and then to compare their responses to that among Caucasians.
METHODS: Asian patients infected with genotype 1 CHC treated at 4 Australian centres between 2001 to 2005 were identified through hospital databases. Baseline demographic characteristics, biochemical, virological and histological data and details of treatment were collected. Sustained virological responses (SVR) in this cohort were then compared to that in Caucasian subjects, matched by genotype, age, gender and the stage of hepatic fibrosis.
RESULTS: A total of 108 Asians with genotype 1 CHC were identified. The end of treatment response (ETR) for the cohort was 79% while the SVR was 67%. Due to the relatively advanced age of the Asian cohort, only sixty-four subjects could be matched with Caucasians. The ETR among matched Asians and Caucasians was 81% and 56% respectively (P = 0.003), while the SVR rates were 73% and 36% (P < 0.001) respectively. This difference remained significant after adjusting for other predictive variables.
CONCLUSION: Genotype 1 CHC in Asian subjects is associated with higher rates of virological response compared to that in Caucasians.
Keywords: Hepatitis C, Treatment, Asians, Retro-spective studies, Comparative study, Interferon, Ribavirin, Statistical data analysis
INTRODUCTION
Chronic hepatitis C (CHC) virus infection is the leading cause of chronic liver disease worldwide. The prevalence of hepatitis C virus (HCV) infection in western countries including Australia and the United States approximates 1%[1,2], while it is more common in most Asian countries[3,4]. Combination therapy with pegylated interferon and ribavirin given for 24 wk or 48 wk remains the most effective antiviral treatment, achieving sustained virological response (SVR) rates ranging from 50% to 80%[5–9].
Various factors have been identified that influence response rates, including HCV genotype, body mass index and co-existent liver disease. Ethnicity was recently noted to impact on treatment responses. Studies conducted in African Americans suggest that these individuals have lower SVR rates when compared to Caucasians, even after adjusting for confounders that could potentially influence treatment response rates[10–13]. More recently, comparative studies between Asians and Caucasians have suggested a higher SVR to antiviral therapy among Asians[14–16].
In Asians, HCV prevalence rates are approximately 6%[3]. Often subjects acquire the infection at a younger age and are therefore at increased risk of developing advanced liver disease and hepatocellular carcinoma. Despite this, Asians seem to be under-represented in clinical trials evaluating SVRs, with the largest available study comprising only 52 individuals[16]. In addition, there has been no head-to-head comparative study evaluating responses to antiviral therapy between Asians and Caucasians. The aims of the present study were therefore to: (1) assess the overall SVR rates in Asians infected with genotype 1 CHC receiving combination antiviral therapy; and (2) to undertake a case-control study comparing SVR rates in Asians compared to that in Caucasians matched by infecting virus genotype, age, gender and the extent of hepatic fibrosis.
MATERIALS AND METHODS
Clinical databases of HCV infected patients who received combination interferon and ribavirin therapy between 2001 to 2005 at four Australian centres were reviewed and all Asian patients identified. Individual patient files were retrieved and their clinical status confirmed. Asian and Caucasian patients over the age of 18 years who received antiviral therapy for genotype 1 CHC were subsequently identified.
Exclusion criteria included patients who were HBsAg-positive, co-infection with HIV, liver transplant recipients or those receiving dialysis for chronic renal failure. Patient demographic characteristics, baseline biochemical, virological and histological data prior to commencing anti-viral therapy were recorded. Those with bridging fibrosis or cirrhosis were considered to have advanced liver disease. While baseline viral loads of individual patients were recorded, multiple different assays and units were used at the various study centres over time, making this data not reportable, or comparable. For alcohol intake, we defined significant intake as either documented daily consumption of more than 30 grams, or medical record documentation that alcoholism was an issue. Details of antiviral therapy were recorded. These included a history of previous anti-viral treatment, treatment regimen (interferon and ribavirin or pegylated interferon and ribavirin), adverse effects on therapy and any treatment dose reductions or interruptions due to either adverse effects or non-compliance. As a standard of care, all patients with HCV genotype 1 were scheduled for 48 wk of treatment.
The primary end point of this study was the proportion of patients achieving an SVR, defined as a documented non-detectable HCV RNA at least 24 wk after treatment. The end of treatment response (ETR) was defined as non-detectable HCV RNA at the end of treatment. Patients who received at least one dose of interferon but did not complete 48 wk of treatment for any reason were defined as non-responders. Asians and Caucasians infected with HCV genotype 1 were then matched by three criteria: age (within 5 years), gender and the extent of hepatic fibrosis, and their ETR and SVR rates were compared.
All statistical analyses were performed by SAS software v11 (SAS Institute Inc., Cary, NC). Continuous variables are reported as median (range). Comparison of baseline demographics was performed by the paired Student t-test, Mann-Whitney test or χ2 test as appropriate. Univariate analysis was performed with SVR as the dependent variable.
RESULTS
Baseline features of the Asian cohort
A total of 108 HCV genotype 1 infected Asian patients were identified. Their demographic characteristics, treatment details and treatment outcomes are shown in Table 1. The majority of patients were born in Vietnam, China or Cambodia; 69% were male. The cohort had a median age of 51 (22-76) and a median body weight of 64 kg (33-104 kg). The baseline alanine aminotransaminase (ALT) was 94 IU/mL (11-558 IU/mL). 21% of those who had liver biopsies had histological evidence of bridging fibrosis or cirrhosis. Eighty-nine percent received pegylated interferon and ribavirin while the remainder received standard interferon and ribavirin. Dose reduction was required in 33%, while 11% required dose interruption, for either adverse effects or non-compliance.
Table 1.
Demographic characteristics, treatment details and treatment responses of 108 HCV genotype 1-infected Asian patients
| Variables | Frequency |
| Countries of origin | |
| Vietnam | 43 |
| China | 34 |
| Cambodia | 19 |
| Korea | 5 |
| Burma | 3 |
| Others | 4 |
| Gender (%) | |
| Male | 69% |
| Age (yr, range) | 51 (22–76) |
| Median weight (kg, range) | 64 (33-104) |
| Median ALT (IU/mL, range) | 94 (11-558) |
| Extend of fibrosis (%) | |
| Bridging fibrosis or cirrhosis | 21% |
| Treatment regimen (%) | |
| Pegylated interferon + Ribavirin | 89% |
| Interferon + Ribavirin | 11% |
| Dose reduction of either drug | 33% |
| Dose interruption of either drug | 11% |
| Treatment responses (%) | |
| End of treatment virological response | 77% |
| SVR | 67% |
End of treatment and SVR rates in Asians
An ETR occurred in 77% of the Asian cohort while 67% achieved an SVR. Factors influencing SVR rates including treatment regimen, fibrosis stage, age, gender and weight were examined by univariate analysis (Table 2). None of these factors were found to be predictive of an SVR in the Asian cohort. In particular, we did not observe a difference in ETR or SVR between those who received pegylated interferon versus standard interferon.
Table 2.
Univariate analysis of 108 HCV genotype 1-infected Asian patients: Predictors of SVR
| Variables | OR (95% CI) | P-value |
| Gender (male) | 0.77 (0.32-1.85) | 0.55 |
| Age (yr) | 1.02 (0.99-1.06) | 0.20 |
| Weight (kg) | 0.98 (0.95-1.06) | 0.41 |
| Pegylated interferon vs standard interferon | 1.00 (0.28-3.57) | 0.99 |
| Treatment naïve | 0.49 (0.22-1.12) | 0.09 |
| Dose reduction not required | 1.45 (0.65-3.34) | 0.39 |
| Dose interruption not required | 1.50 (0.44-5.10) | 0.52 |
| Absence of bridging fibrosis or cirrhosis | 1.57 (0.61-4.07) | 0.35 |
Baseline features of the matched cohort
Due to the more advanced age and extent of hepatic fibrosis among Asians, we were only able to match 64 Asian subjects with Caucasians by the set criteria. Comparison of the baseline demographics between the two groups is shown in Table 3. Only 18% of Asian patients weighed more than 75 kg while 58% of their Caucasian counterparts weighed more than 75 kg (P < 0.01). Alcohol intake was less in Asian subjects with 14% of Asians and 29% of Caucasians reporting a significant alcohol intake history (P = 0.12). There was no difference between the two groups in terms of previous therapy, type of treatment received or in the extent of dose reduction or interruptions from treatment adverse effects or non-compliance.
Table 3.
Comparison of the demographic characteristics and treatment details of the matched Asian and Caucasian patients
| Variables | Asians | Caucasians | P-value |
| Age (yr, range) | 47 (28-64) | 46 (30-61) | Matched |
| Gender | 70% male | 70% male | Matched |
| Weight (kg) | |||
| < 75 | 82% | 42% | < 0.01 |
| > 75 | 18% | 58% | |
| Alcohol intake | |||
| Minimal intake | 86% | 71% | 0.12 |
| Significant intake | 14% | 29% | |
| Liver fibrosis | |||
| Minimal injury | 48 (75%) | 48 (75%) | Matched |
| Bridging fibrosis/Cirrhosis | 16 (25%) | 16 (25%) | |
| Treatment naïve | 80% | 91% | NS |
| Peginterferon + Ribavirin | 83% | 88% | NS |
| Dose modification | 30% | 28% | NS |
| Dose interruption | 8% | 10% | NS |
End of treatment and SVR rates in the matched cohort
Comparison of the ETR and SVR between the 64 Asian patients and the matched Caucasian cohort is illustrated in Figure 1. Compared to Caucasians, Asian subjects had a significantly better ETR, 81% vs 56% (P = 0.003) and SVR, 73% vs 36% (P < 0.001). On univariate analysis (Table 4), Asian ethnicity was the most predictive factor for a SVR. Other factors that were associated with an SVR in this cohort were body weight < 75 kg, and minimal alcohol intake. Due to the large number of variables and the limited number of patients, we were unable to perform a multiple logistic regression analysis with SVR as the dependent variable. Hence the effect of ethnicity on SVR was only adjusted for individual variables that were not matched. In this analysis (Table 5), Asian ethnicity remained a significant predictor of SVR after allowing for other variables that were not matched.
Figure 1.
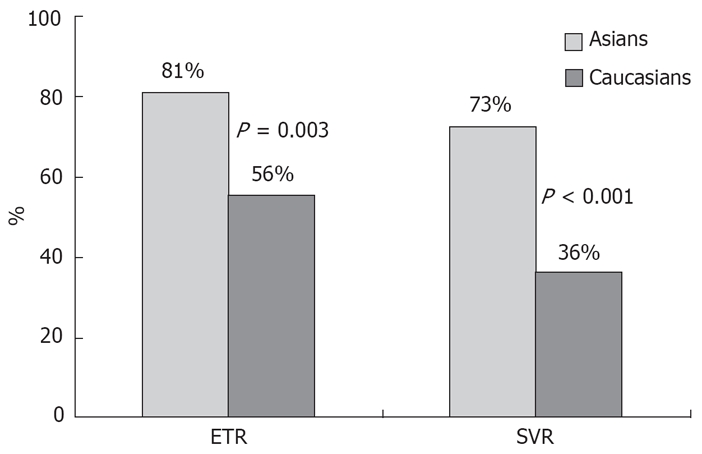
Comparison of ETR and SVR rates between HCV genotype 1-infected Asians and Caucasians matched for age, gender and fibrosis stage.
Table 4.
Univariate analysis of predictors of SVR on matched HCV genotype 1-infected Asians and Caucasians
| Variables | OR (95% CI) | P-value |
| Ethnicity (Asians) | 4.92 (2.32-10.50) | < 0.001 |
| Gender (male) | 0.83 (0.39-1.79) | 0.64 |
| Age (per year) | 1.04 (1.00-1.96) | 0.061 |
| Weight (< 75 kg) | 2.59 (1.20-5.61) | 0.016 |
| Minimal alcohol intake | 3.20 (1.17-8.73) | 0.023 |
| Peginterferon vs standard interferon | 1.10 (0.42-2.92) | 0.84 |
| Treatment naïve | 0.77 (0.29-2.04) | 0.60 |
| Dose reduction not required | 1.04 (0.48-2.23) | 0.93 |
| Dose interruption not required | 2.31 (0.64-8.33) | 0.20 |
| Absence of bridging fibrosis or cirrhosis | 1.80 (0.80-4.04) | 0.15 |
Table 5.
Effects of ethnicity on SVR after adjusting for individual unmatched variables
| Adjusted OR (95% CI) | P-value | |
| Weight (< 75 kg) | 4.64 (1.97-10.94) | < 0.001 |
| Minimal alcohol intake | 2.74 (0.97-7.75) | 0.057 |
| Pegylated interferon vs standard interferon | 5.01 (2.35-10.71) | < 0.001 |
| Treatment naïve | 5.01 (2.32-10.81) | < 0.001 |
| Dose reduction not required | 4.94 (2.32-10.50) | < 0.001 |
| Dose interruption not required | 4.84 (2.26-10.38) | < 0.001 |
DISCUSSION
In this retrospective study, we confirmed that in CHC, ethnicity is an important variable influencing response to antiviral therapy. Our study of Asians infected with HCV genotype 1 has permitted several important observations to be made. Firstly, the overall SVR among Asians approached 70%. Secondly, Asians with genotype 1 CHC were more likely to respond favourably to antiviral therapy compared to matched Caucasians. Finally, excess alcohol intake and increased body weight adversely affected treatment outcomes.
The observation of a favourable response rate among Asians is in accordance with other reports[14–16]. Importantly, the effect of ethnicity on SVR rates remained significant after adjusting for other confounders including age, gender, treatment regimen and the extent of hepatic fibrosis. The present study also observed that lower body weight and minimal alcohol intake were predictive of an SVR.
Body mass index[17,18], insulin resistance[19–21] and hepatic steatosis[22–24] are now known to play a major role in the pathogenesis of HCV infection. Insulin resistance in genotype 1 CHC is most likely related to host factors, in particular obesity, rather than virological factors, and is associated with reduced treatment response rates[25]. It was therefore interesting to observe in our study that the matched Asian cohort overall had lower body weights than Caucasians. Similar findings were reported in the studies by Hepburn et al[15] and Missiha et al[16]. Although in these previous studies, as well as in the current report, the effect of ethnicity remained significant after allowing for body weight, central adiposity or underlying insulin resistance were not measured. This is a limitation of the retrospective nature of this study. There are no published data on the impact of hepatic steatosis on CHC infection among Asians. It is therefore important to examine these factors in future studies as they might potentially explain the reasons why Asians having better treatment responses.
Not surprisingly, we observed that Asian subjects consumed less alcohol, possibly a reflection of cultural influences. Our study was the first to adjust for this variable. We noted that the effect of ethnicity on SVR was modified after allowing for alcohol consumption and did not reach statistical significance (P = 0.057). Although the difference in SVR was not significant (probably related to patient numbers), this observation highlights the importance of taking alcohol intake into account in studies comparing response rates stratified for ethnicity[26,27].
The biological basis for the difference in SVR rates between Asians and Caucasians has not been examined previously. Studies into the effects of ethnicity on CHC treatment has to date focussed entirely on African Americans. It was found that African Americans had different class II human-leukocyte antigen alleles from Caucasians[28], which could have accounted for their worse SVR. Further, viral kinetics studies have shown that African Americans exhibit significantly lower interferon effectiveness and achieve a lower reduction in HCV RNA in the first 24 h of treatment[29]. It was also noted that African Americans had different pre-treatment cytokine profiles[30]. In addition, while they mounted a more robust HCV-specific CD4 Th1 proliferative response, it did not translate into a higher rate of IFN-gamma production, potentially secondary to their dysfunctional nature, which was associated with a failure of interferon therapy[31,32]. The significance of these studies is that the impact of ethnicity, on treatment response is more likely to be related to host factors, particularly to genetic differences in immune regulation rather than environmental factors.
Our study suffered the usual limitations of retrospective observational reports. In particular, we now know that a proportion of Asians who were initially genotyped by INNO-LiPA as 1b, were in fact genotypes 7, 8 or 9 if direct sequencing of the core region is performed[14]. Direct core sequencing was not performed in our study. It is arguable, however, if inclusion of genotypes 7, 8 or 9 would have altered the better SVR rates achieved by the Asian cohort since the original article noted that SVR rates were identical among Asians infected with genotypes 7, 8 or 9 and those infected with genotype 1 HCV infection[14]. Similar findings were noted in another study where it was shown that there was no difference in response rates between genotype 1b infected Asians and the rest of the genotype 1 infected Asians[15]. We therefore believe that the difference in SVR rates noted in our study was more likely related to ethnicity than a bias from a potentially small group of patients who might have been mistyped. It is clear however that prospective trials on larger patient cohorts including all genotypes are needed to clarify this issue.
The other limitation of this type of retrospective comparative study, as pointed out by both Hepburn et al[15] and Missiha et al[16], is that it failed to recognise the wide genetic heterogeneity and different environmental factors that might exist within the same ethnic group. While we defined Asians as those who migrated from East Asia including China, Japan, Korea and South East Asia, and of parents of those origin, we do not, and could not, know if they essentially represent the same group of patients genetically.
In conclusion, Asians infected with HCV genotype 1 achieved a higher SVR rate when compared to a cohort of matched Caucasians. Future studies should focus on confirming our observations in large prospective cohorts and on characterizing the immunogenetic basis for these observations.
COMMENTS
Background
Treatment with interferon and ribavirin combination therapy remains currently the most effective treatment for chronic hepatitis C (CHC) virus infection, but treatment response can only be achieved in 50% to 80% of patients. Various factors including genotype, viral load, extent of liver injury on liver biopsy, age and gender of patients were known to impact on treatment responses. Identification of these factors aids selection of patients for treatment and determination of duration of therapy.
Research frontiers
Recent studies on African Americans suggested that ethnicity might also impact on treatment responses. Data from studies on one ethnic group may not be extrapolated to other ethnic groups, and different ethnic groups may require different treatment regimens.
Innovations and breakthroughs
This study found that Asian patients infected with hepatitis C genotype 1 had better treatment responses than Caucasian patients, even after adjusting for other predictive factors.
Applications
The implication of the results of this study is twofold. Firstly, it prompts further basic scientific research into difference in immune response to CHC among different ethnic groups to better understand the pathogenesis of CHC. Secondly, it suggests that clinically, different treatment regimen should be studied and compared among different ethnic groups.
Peer review
The results provide sufficient evidence, which allow authors to make firm conclusion that ethnicity is an important factor variable influencing response to antiviral therapy in patients with CHC. The further studies to confirm the results of this observational study for patients from different ethnic backgrounds are needed. The references are appropriate, relevant and updated.
Peer reviewers: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., Moscow 107031, Russia; Heitor Rosa, Professor, Department of Gastroenterology and Hepatology, Federal University School of Medicine, Rua 126 n.21, Goiania-GO 74093-080, Brazil
S- Editor Li DL L- Editor Negro F E- Editor Ma WH
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Law MG, Dore GJ, Bath N, Thompson S, Crofts N, Dolan K, Giles W, Gow P, Kaldor J, Loveday S, et al. Modelling hepatitis C virus incidence, prevalence and long-term sequelae in Australia, 2001. Int J Epidemiol. 2003;32:717–724. doi: 10.1093/ije/dyg101. [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Fung KT, Fung J, Lai CL, Yuen MF. Etiologies of chronic liver diseases in Hong Kong. Eur J Gastroenterol Hepatol. 2007;19:659–664. doi: 10.1097/MEG.0b013e3281ace0b7. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 7.Marcellin P, Heathcote EJ, Craxi A. Which patients with genotype 1 chronic hepatitis C can benefit from prolonged treatment with the 'accordion' regimen? J Hepatol. 2007;47:580–587. doi: 10.1016/j.jhep.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis c genotype 1-infected slow responders. Hepatology. 2007;46:1688–1694. doi: 10.1002/hep.21919. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, Shafran SD, Barange K, Lin A, Soman A, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 10.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, Belle SH, Hoofnagle JH, Kleiner DE, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702–1708. doi: 10.1002/hep.20212. [DOI] [PubMed] [Google Scholar]
- 12.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson IM, Brown RS Jr, McCone J, Black M, Albert C, Dragutsky MS, Siddiqui FA, Hargrave T, Kwo PY, Lambiase L, et al. Impact of weight-based ribavirin with peginterferon alfa-2b in African Americans with hepatitis C virus genotype 1. Hepatology. 2007;46:982–990. doi: 10.1002/hep.21670. [DOI] [PubMed] [Google Scholar]
- 14.Dev AT, McCaw R, Sundararajan V, Bowden S, Sievert W. Southeast Asian patients with chronic hepatitis C: the impact of novel genotypes and race on treatment outcome. Hepatology. 2002;36:1259–1265. doi: 10.1053/jhep.2002.36781. [DOI] [PubMed] [Google Scholar]
- 15.Hepburn MJ, Hepburn LM, Cantu NS, Lapeer MG, Lawitz EJ. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med. 2004;117:163–168. doi: 10.1016/j.amjmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Missiha S, Heathcote J, Arenovich T, Khan K. Impact of asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol. 2007;102:2181–2188. doi: 10.1111/j.1572-0241.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 17.Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639–644. doi: 10.1053/jhep.2003.50350. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509–1515. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]
- 20.Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28–34. doi: 10.1016/j.jhep.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, George J. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44:1648–1655. doi: 10.1002/hep.21429. [DOI] [PubMed] [Google Scholar]
- 22.Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology. 2006;43:1177–1186. doi: 10.1002/hep.21239. [DOI] [PubMed] [Google Scholar]
- 23.Narita R, Abe S, Tabaru A, Otsuki M. Impact of steatosis on insulin secretion in chronic hepatitis C patients. Am J Gastroenterol. 2007;102:2173–2180. doi: 10.1111/j.1572-0241.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 24.Bedossa P, Moucari R, Chelbi E, Asselah T, Paradis V, Vidaud M, Cazals-Hatem D, Boyer N, Valla D, Marcellin P. Evidence for a role of nonalcoholic steatohepatitis in hepatitis C: a prospective study. Hepatology. 2007;46:380–387. doi: 10.1002/hep.21711. [DOI] [PubMed] [Google Scholar]
- 25.Zekry A, McHutchison JG, Diehl AM. Insulin resistance and steatosis in hepatitis C virus infection. Gut. 2005;54:903–906. doi: 10.1136/gut.2004.059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boccato S, Pistis R, Noventa F, Guido M, Benvegnu L, Alberti A. Fibrosis progression in initially mild chronic hepatitis C. J Viral Hepat. 2006;13:297–302. doi: 10.1111/j.1365-2893.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 27.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 28.Thio CL, Thomas DL, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O'Brien SJ, Karacki P, Marti D, Astemborski J, et al. Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis. 2001;184:16–21. doi: 10.1086/321005. [DOI] [PubMed] [Google Scholar]
- 29.Layden-Almer JE, Ribeiro RM, Wiley T, Perelson AS, Layden TJ. Viral dynamics and response differences in HCV-infected African American and white patients treated with IFN and ribavirin. Hepatology. 2003;37:1343–1350. doi: 10.1053/jhep.2003.50217. [DOI] [PubMed] [Google Scholar]
- 30.Kimball P, Elswick RK, Shiffman M. Ethnicity and cytokine production gauge response of patients with hepatitis C to interferon-alpha therapy. J Med Virol. 2001;65:510–516. [PubMed] [Google Scholar]
- 31.Sugimoto K, Stadanlick J, Ikeda F, Brensinger C, Furth EE, Alter HJ, Chang KM. Influence of ethnicity in the outcome of hepatitis C virus infection and cellular immune response. Hepatology. 2003;37:590–599. doi: 10.1053/jhep.2003.50103. [DOI] [PubMed] [Google Scholar]
- 32.Rosen HR, Weston SJ, Im K, Yang H, Burton JR Jr, Erlich H, Klarquist J, Belle SH. Selective decrease in hepatitis C virus-specific immunity among African Americans and outcome of antiviral therapy. Hepatology. 2007;46:350–358. doi: 10.1002/hep.21714. [DOI] [PubMed] [Google Scholar]


