Abstract
Physicians previously thought that heart disease was rare in patients with end stage liver disease. However, recent evidence shows that the prevalence of ischemic heart disease and cardiomyopathy is increased in transplant candidates compared to most other surgical candidates. Investigators estimate that up to 26% of all liver transplant candidates have at least one critical coronary artery stenosis and that at least half of these patients will die perioperatively of cardiac complications. Cardiomyopathy also occurs in greater frequency. While all patients with advanced cardiac disease have defects in cardiac performance, a larger than expected number of patients have classical findings of dilated, restrictive and hypertrophic cardiomyopathy. This may explain why up to 56% of patients suffer from hypoxemia due to pulmonary edema following transplant surgery. There is considerable controversy on how to screen transplant candidates for the presence of heart disease. Questions focus upon, which patients should be screened and what tests should be used. This review examines screening strategies for transplant candidates and details the prognostic value of common tests used to identify ischemic heart disease. We also review the physiological consequences of cardiomyopathy in transplant candidates and explore the specific syndrome of “cirrhotic cardiomyopathy”.
Keywords: Coronary artery disease, Coronary atherosclerosis, Echocardiography, Cirrhosis, Cardiomyopathy, Cardiac electrophysiology liver transplantation
INTRODUCTION
Physicians previously thought that heart disease was rare in patients with cirrhosis[1]. Postoperative mortality was previously due to operative complications and poor donor graft function. Better surgical technique and donor organ management have significantly improved patient survival. However, as long term patient survival increased, cardiac complications emerged as a more common cause of early morbidly and mortality. Recent studies report a high incidence of post-transplant cardiovascular complications with arrhythmias and overt congestive heart failure in as many as 25% and 56% of all transplant recipients respectively[2,3].
It is now clear that patients with end stage liver disease are at increased risk of acute coronary occlusion, myocardial failure, arrhythmia and complete cardiovascular collapse following transplantation compared to other major surgical procedures. However, there is no consensus on how to efficiently detect cardiovascular disease in asymptomatic patients prior to transplantation or to determine what risk a transplant candidate with heart disease has of suffering from a serious perioperative adverse event. Without this information it is difficult to determine what type or severity of heart disease should exclude a patient from transplantation.
CARDIOVASCULAR DISEASES IN CANDIDATES FOR LIVER TRANSPLANTATION
The most common cardiovascular diseases in transplant candidates are ischemic coronary artery disease (CAD) and cardiomyopathy. These diseases are independent negative predictors of outcome following transplantation. However, the poor exercise capacity in patients with advanced liver disease makes it difficult to identify cardiac disease. These patients may not experience common symptoms brought on by exercise such as chest pain or shortness of breath. Further, it may be impossible to determine if some symptoms such as shortness of breath are caused by cardiac or liver disease. Thus, screening asymptomatic patients for underlying cardiac disease is an essential step in the evaluation of transplant candidates.
Ischemic heart disease
Patients can have substantial atherosclerosis, but remain asymptomatic. Symptoms only develop once the amount of coronary blood flow is insufficient to meet the oxygen needs of the myocardial tissue. This usually occurs when the degree of occlusion exceeds 50%[4]. However, lesion size alone does not always predict the onset of symptoms. Rather, symptoms are influenced by myocardial tissue demands. Vigorous exercise increases myocardial oxygen demand and may cause symptoms with lesions that are less than 50%. Figure 1 shows the algorithm for the diagnosis of ischemic heart disease.
Figure 1.
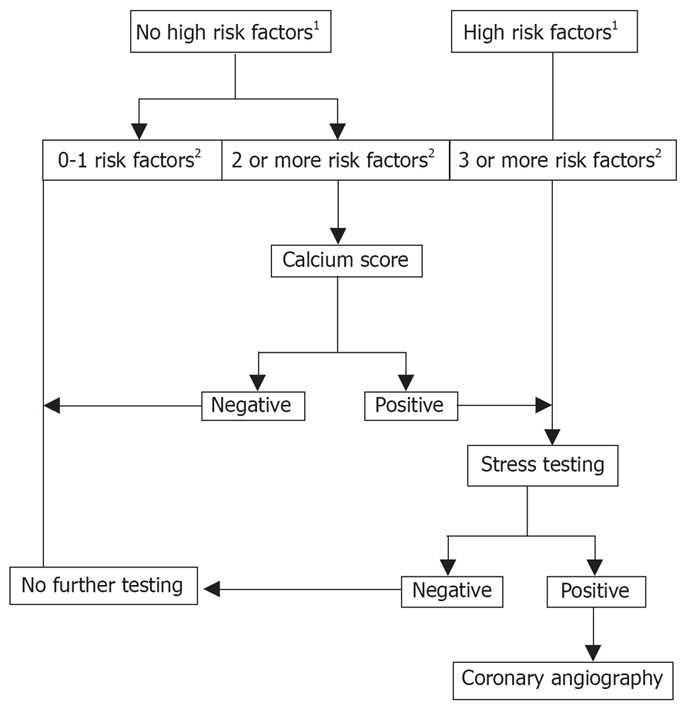
Algorithm for the diagnosis of ischemic heart disease. There is no consensus on how to evaluate asymptomatic liver transplant recipients for the presence of CAD. However, a logical algorithm can be constructed using the current evidence from a limited number of outcome studies. The utility of this algorithm will require outcome testing to determine its sensitivity and specificity. In this approach, asymptomatic liver transplant recipients are divided according to the presence risk factors for CAD. Patients with 0-1 risk factor require no further evaluation. Those with 2 risk factors are first assessed by calcium scoring. If the score is zero, no further testing is required. If the test is not zero patients are referred for stress testing. Patients referred directly for stress testing include those with 3 or more risk factors and those with high risk factors. A positive stress test is an indication for coronary catheterization. 1High Risk Factors for CAD include diabetes, NASH, previous CAD, peripheral vascular disease; 2Risk Factors for CAD include age > 50 yr, hypertension, elevated cholesterol and obesity.
Myocardial infarction can occur in patients who have lesions that occupy less than 50% of the coronary lumen[4,5]. This is caused by plaque rupture, resulting in acute vessel thrombosis. Liver disease may increase the risk of coronary complications in patients with non-occlusive disease. Chronic inflammation coupled with increased blood flow can predispose to plaque rupture. These adverse conditions are worsened by the high metabolic demand that occurs during liver transplantation.
Evidence suggests that CAD is more prevalent in transplant candidates. Studies show that at least one critical coronary artery lesion occurs in 5% to 26% of all liver transplant candidates who are asymptomatic[6–9]. Up to 50% of patients with significant CAD will die perioperatively from cardiac complications[7]. This is substantially greater than the one year mortality rate (10%) for all liver transplant recipients[10], or the mortality rate from cardiac complications for other study populations[11,12]. A previous history or classic symptoms of CAD places patients in well-tested protocols that direct further evaluation[13,14]. However, the challenge remains in identifying those patients who are asymptomatic, but have significant CAD.
RISK FACTORS FOR CAD IN CIRRHOSIS
Specific patient attributes are correlated with CAD[15]. The actual demographic profile of a patient with CAD is remarkably similar throughout the world[16,17]. High blood pressure, elevated cholesterol, diabetes and obesity are the most prevalent attributes. Risk factors for CAD are also prevalent in liver transplant candidates[9]. Age > 50 years, male gender, diabetes and obesity are common. In addition, altered lipid metabolism and other unknown factors may make cirrhosis an independent risk factor. Cirrhotic patients with 0-1 risk factors have a low likelihood for CAD. However, the presence of two or more factors (other than age) places patients at moderate to severe risk of CAD[9]. A diagnosis of non-alcoholic steatohepatitis (NASH) independently increases the risk of CAD[18]. Critical CAD occurs in approximately 23% of patient with NASH[18]. Even though NASH patients have additional risk factors including obesity and diabetes, the prevalence of CAD is greater than the sum of associated risk factors[19,20]. Overall transplant candidates with diabetes, peripheral vascular disease including carotid atherosclerosis, abdominal aneurysm, chronic renal failure or high risk-Framington Score are likely to have CAD and need further evaluation[14].
SCREENING TESTS FOR CAD IN CIRRHOSIS
The prognostic value of any screening test is related to the prevalence of the disease in the population under study[14]. Some investigators argue that the prevalence of CAD in transplant candidates is insufficient to warrant testing all asymptomatic patients greater than 50 years of age[21]. However, this opinion does not take into account the high perioperative mortality associated with CAD in these patients. Therefore, it is reasonable to err on the side of caution by optimizing sensitivity at the expense of lowering specificity. While few patients with only one risk factor have positive findings, 2 risk factors significantly increases the chance of critical CAD in this population[6,21]. However, not all risk factors confer an equal chance of CAD. Diabetes and NASH are more predictive of CAD than other attributes such as age or gender[21,22]. Therefore, the decision to proceed with noninvasive testing must be tempered by the relative predictive strength of each risk factor rather than just the number of factors present.
Calcium scores
Calcium crystals are commonly deposited in coronary atherosclerotic lesions and the degree of calcification correlates with the severity of occlusive coronary atherosclerosis[23]. Computerized tomography is a non-invasive and rapid way to measure calcium deposits within the coronary vasculature. The amount of cardiac calcium is summated into scores (CACS) that are reported as a percentile according to age and gender. Higher scores suggest a greater degree of coronary artery stenosis[24]. Calcium scores are, therefore, used to detect and grade the severity of CAD[25]. However, the CACS has limited predictive value as a single screening study for CAD. The CACS is relatively insensitive with a weighted average of only 40% for detecting critical lesions[25]. A comparison of CACS with echocardiography also suggests a lack of specificity[26–30]. Despite these limitations, CACS does provide incremental prognostic information to assess cardiac risk with respect to cardiac death, myocardial revascularization or myocardial infarction[31]. Patients with a CACS > 100 are 5 times more likely to have ischemic coronary events compared to those with a CACS < 100 and significant coronary disease is rare with a score of zero.
Non-calcified coronary plaque can also cause myocardial infarction and death. However, non-calcified lesions are uncommon in patients with a CACS of zero[32]. When present, stenosis is usually less than 50% of the luminal diameter. This finding reinforces the strong predictive value of a CACS of zero. However, non-calcified lesions and their degree of stenosis increase significantly as the CACS increases. Current evidence suggest that CACS as a single test has limited value except to separate patients with minimal risk of CAD (CACS = zero) from those who have more advanced disease.
Stress testing
Stress testing is used to increase myocardial oxygen demand in order to identify critically obstructed coronary vessels. Graded exercise and the intravenous administration of drugs that increase oxygen consumption are the two most efficient ways to test for reversible ischemia. Ischemic changes are captured using common imaging techniques such as echocardiographic wall motion abnormalities and radionuclide myocardial perfusion defects. A stress test cannot identify disease caused by atherosclerotic plaques that are too small to limit coronary blood flow, even if these lesions are at risk of rupture.
More severe disease (more vessels involved and/or more severe stenosis) improves the predictive power of the test. In general, graded exercise stress tests coupled with echocardiography or myocardial perfusion imaging have similar sensitivity and specificity[33] but, the predictive values of the tests diverge when pharmacologic agents are used. Echocardiography is less sensitive but more specific than myocardial perfusion with dobutamine[34] and dobutamine is better than vasodilators such as adenosine or dipyridamole for inducing ischemia with either technique[35].
Dobutamine myocardial perfusion imaging is more sensitive than dobutamine echocardiography for detecting myocardial ischemia in patients with liver disease[36]. In transplant candidates these tests have a better negative rather than positive predictive value[37]. Thus, a negative test confers a minimal chance of CAD. There is a greater chance that no lesion will be identified angiographically if the test is positive. However, the unique physiological stress associated with liver transplantation places patients at greater risk of plaque rupture, even if less than 50% of the vessel is occluded. There are more false positive stress tests in transplant candidates. However, there are also more postoperative deaths in transplant recipients who had abnormal myocardial perfusion studies but no angiographic lesions[36]. These patients die of perioperative cardiovascular complications, sepsis and donor graft failure[38]. Investigators suggest that myocardial perfusion defects with a normal angiogram are not necessarily benign findings and could be caused by reduced microvascular coronary blood flow[38].
Questions remain as to what degree of lesion requires intervention and what type of treatment is best. Most subcritical coronary stenoses in surgical patients can be medically managed. However, investigators report a perioperative death rate of more than 50% in transplant recipients with CAD who were managed medically[7]. Further myocardial infarction during transplant surgery has occurred with as little as 30% vessel occlusion[36]. To date there is no consensus on what degree of stenosis needs treatment in liver transplant candidates because there are very few reports on the outcomes of coronary interventions in transplant candidates.
CARDIOMYOPATHY
The prevalence of cardiomyopathy is greater in patients with end stage liver disease than the general popu-lation[39–41]. Conditions such as hepatitis C can cause immune-mediated myocarditis and fibrosis resulting in restrictive cardiomyopathy[41]. Hemochromatosis and amyloidosis also cause restrictive cardiomyopathy due to the infiltration of iron and protein respectively. Findings similar to dilated cardiomyopathy are also commonly reported from routine echocardiographic screening of transplant candidates[40]. Further, an increased reporting of hypertrophic cardiomyopathy in transplant candidates suggests that this disorder may also be more prevalent in cirrhotic patients[42,43].
Symptoms of liver disease are often similar to those of heart failure. Therefore, it is difficult to determine if fatigue, shortness of breath and adventitious heart sounds are due to liver disease alone or if patients have cardiac failure. Routine screening of transplant candidates with echocardiography is an effective way to identify comorbid cardiac disease. Even though cardiac disease can be readily identified, there is no agreement on how disease influences transplant candidacy. Currently, there is no outcome data using cardiomyopathy as a primary study variable. However, investigators have reported that patients with dilated cardiomyopathy seem to improve after successful transplantation[40]. In contrast, the prognosis for infiltrative processes such as Hepatitis C, amyloidosis and hemochromatosis does not seem as good and cardiac disease is reported to progress to overt heart failure despite successful transplantation[41,44].
Cirrhotic cardiomyopathy
Some cirrhotic patients have obvious features of cardiomyopathy, however most have more subtle defects in myocardial function that are not apparent on cursory examination. Early cardiac decompensation is often missed because the cardiac workload is reduced by peripheral vasodilation caused by liver failure[45]. These patients are dismissed as having normal heart function. However, when these patients are subject to physiological or pharmacological stress, they develop clinical signs of suboptimal perfusion including renal failure and acidosis. Investigators have concluded that exercise uncovers an intrinsic defect in myocardial function that predisposes to heart failure[46,47]. This condition is called “cirrhotic cardiomyopathy” and although the clinical presentation can be variable, all patients have four common features. These are: (1) Baseline increased cardiac output; (2) Attenuated systolic contraction and diastolic relaxation; (3) Electrophysiological abnormalities including repolarization change; and (4) A reduced response of the heart to direct beta stimulation (β-incompetence)[48]. These changes occur in the absence of overt congestive failure.
Myocardial function in cirrhotic cardiomyopathy
Indices of left ventricular contractility such as the stroke index, mean systolic ejection rate, left ventricular stroke-work and left ventricular stroke-power are greater than expected in the cirrhotic patient at rest. The only clue early that the heart may not be normal is a blunted response to exercise[49]. The standing position and physical or mental stress have minimal impact on cardiac output in cirrhotic patients. But, the absolute and relative increase in cardiac output in response to exercise is reduced compared to controls[49]. Thus, aerobic exercise capacity and maximal heart rate are lower than expected[49–51].
Early histological changes include myocardial hypertrophy, interstitial and cellular edema and signs of cellular injury[52]. This causes thickening of the left ventricle with the septum affected more than the free wall[49]. Overall, these effects are more pronounced in patient with ascites compared to those without[46]. As wall thickness increases so does the degree of diastolic dysfunction. Impaired diastolic relaxation prolongs isovolumetric relaxation and the ventricular pressure is greater than normal for any given end diastolic volume. The left atrium dilates in response to the higher impedance to left ventricular filling. When these patients experience circulatory changes that rapidly increase filling pressure, congestive heart failure develops. This likely explains why patients experience an increased incidence of congestive heart failure after procedures such as transjugular intrahepatic shunts and liver transplantation[53]. In fact, diastolic disturbance is such a consistent feature of cirrhotic cardiomyopathy that many investigators suggest that some degree of diastolic dysfunction is present in all patients with liver disease[52].
At rest, systolic function appears to be normal in most patients with liver disease. However, the mechanics of systolic contraction are commonly disturbed. This is shown by examination of the systolic time interval. The length of systole remains constant, but left ventricular ejection period takes up a larger percentage of the time interval. This in turn shortens the pre-ejection period[54]. In contrast to changes in diastole, systolic dysfunction usually only becomes evident during exercise. Ejection fraction does not increase as expected under conditions of stress. Further, an increase in filling pressures does not increase ejection fraction and the Frank Starling curve flattens[54]. As liver disease progresses systolic function can be insufficient to meet the resting tissue oxygen demands[55]. The impaired systolic response to stress is etiologic in the increased incidence of pulmonary edema and congestive heart failure following procedures that abruptly increase blood flow to the heart[54].
Electromechanical disorders in cirrhotic cardiomyopathy
The events of electrical depolarization and mechanical systole are normally tightly linked in time and exhibit little variability. Thus, the events are considered “coupled”. The time interval needed for ventricular repolarization is limited and also has little variability. This is to prevent the next depolarizing current from advancing into a partially depolarized conducting system and causing re-entry arrhythmias. Patients with liver disease exhibit three common cardiac electrophysiological disturbances in cardiac function. These include: (1) Electromechanical dissociation; (2) Prolongation of ventricular repolarization (the QT interval); and (3) Chronotropic incompetence[52].
The time period between electrical and mechanical systole is longer in patients with cirrhosis[56]. If the conducting system is still partially depolarized when the next action potential arrives, electrical depolarization cannot capture all the mechanical activity of the myocardium. Thus investigators think that defects in electromechanical coupling may contribute to impaired systolic performance in cirrhosis by failing to recruit all available myocardium for the next ventricular contraction[57]. Once electromechanical dissociation becomes severe, it prolongs the time required for repolarization (QT interval). Prolongation and variability in the QT interval can affect cardiac rhythm and cause serious disturbances including ventricular fibrillation. The severity of electromechanical dissociation is clearly related to the severity of liver disease. This is shown by the fact that the length of the QT interval correlates directly with the Child-Pugh score[54]. Further, the QT interval in cirrhotics varies in different regions of the heart[58]. This dispersion of the QT interval is distinctly abnormal and the degree of abnormality is also related to the severity of liver disease. While patients with congenital prolonged QT appear to have defects in the sodium receptors that regulate electrical gating, the potassium gates of the conducting system are primarily affected in patients with liver disease[59].
Normally, stimulation of β-adrenergic cardiac receptors in healthy subjects causes an increase in both the rate and force of cardiac contractions. However, cirrhotic patients exhibit a blunted response to both physiological and pharmacological β stimulation[55]. A suboptimal response of heart rate to β stimulation, termed chronotropic incompetence is found in patients with heart failure in addition those with cirrhosis. It is a proven poor prognostic indicator in all types of heart failure[60]. Chronotropic incompetence is likely also a predictor of mortality in patients with liver disease[47].
Studies of experimental animals show that the progressive failure of β-agonists to elicit a positive chronotropic and inotropic response is caused by an acquired defect in the mechanism of β-receptor signaling. There is both a reduction in the number of β-adrenoceptors in the cell membrane and multiple defects in the pathway that link receptor stimulation to contractility[61]. These findings occur to some degree in all patients with liver disease[48]. The response to β-stimulation is relatively preserved early in the course of liver disease but becomes increasingly “incompetent” as liver disease progresses. In end stage liver disease, no significant increase in heart rate occurs in response to Valsalva maneuver or tilting. Further, the dose of isoproterenol required to raise heart rate is much larger than in healthy subjects[62]. Investigators think that failure of β-adrenergic receptors to appropriately stimulate all potential myocardial activity may also contribute to systolic dysfunction in cirrhotic cardiomyopathy[48]. This helps to explain why signs of cardiomyopathy are only observed under conditions of physical or pharmacological stress.
CONCLUSION
Cardiac disease appears more frequently in patients with end stage liver disease. The two most common conditions are ischemic heart disease and cardiomyopathy. Some question whether it is cost effective to screen all transplant candidates for CAD. The unusually high perioperative mortality in transplant patients who do have CAD warrants a systematic evaluation in every patient which presumes a greater risk of atherosclerotic coronary disease. No single test has 100% predictive value. Therefore diagnostic protocols must account for the variation in prevalence that occurs in subsets of transplant candidates and the limitation of each type of test.
In contrast to ischemic heart disease, most patients with advanced liver disease have myocardial defects that cause systolic and diastolic impairment that is not always evident at rest. There are also underlying electrophysiological defects that cause an uncoupling of mechanical and electrical activity. Diagnosis of “cirrhotic cardiomyopathy” is difficult since the findings can be subtle as some patients will develop frank heart failure when exposed to pharmacological or physiological stress such as liver transplantation.
Peer reviewer: Yasuhiko Sugawara, MD, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine University of Tokyo, Tokyo, Japan
S- Editor Li DL L- Editor Lalor PF E- Editor Yin DH
References
- 1.Turner TB, Bennett VL, Hernandez H. The beneficial side of moderate alcohol use. Johns Hopkins Med J. 1981;148:53–63. [PubMed] [Google Scholar]
- 2.Snowden CP, Hughes T, Rose J, Roberts DR. Pulmonary edema in patients after liver transplantation. Liver Transpl. 2000;6:466–470. doi: 10.1053/jlts.2000.7580. [DOI] [PubMed] [Google Scholar]
- 3.Donovan CL, Marcovitz PA, Punch JD, Bach DS, Brown KA, Lucey MR, Armstrong WF. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61:1180–1188. doi: 10.1097/00007890-199604270-00011. [DOI] [PubMed] [Google Scholar]
- 4.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999;82:265–268. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagoshi T, Koiwaya Y, Doi H, Eto T. Angiographic coronary morphology in patients with ischemic heart disease. J Cardiol. 2000;36:91–102. [PubMed] [Google Scholar]
- 6.Carey WD, Dumot JA, Pimentel RR, Barnes DS, Hobbs RE, Henderson JM, Vogt DP, Mayes JT, Westveer MK, Easley KA. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59:859–864. [PubMed] [Google Scholar]
- 7.Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg. 1996;2:426–430. doi: 10.1002/lt.500020604. [DOI] [PubMed] [Google Scholar]
- 8.Morris JJ, Hellman CL, Gawey BJ, Ramsay MA, Valek TR, Gunning TC, Swygert TH, Shore-Lesserson L, Lalehzarian F, Brayman KL. Case 3-1995. Three patients requiring both coronary artery bypass surgery and orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 1995;9:322–332. doi: 10.1016/s1053-0770(05)80330-1. [DOI] [PubMed] [Google Scholar]
- 9.Tiukinhoy-Laing SD, Rossi JS, Bayram M, De Luca L, Gafoor S, Blei A, Flamm S, Davidson CJ, Gheorghiade M. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178–181. doi: 10.1016/j.amjcard.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 10.United Network for Organ Sharing Organ Procurement: Available at The U.S. Transplant Network/Scientific Registry of Transplant Recipients 2006 Annual report: Transplant data. Available from: URL: http://www.ustransplant.org/annual_reports/current/survival_rates.htm. [Google Scholar]
- 11.Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–990. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173:627–634. doi: 10.1503/cmaj.050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–1788. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 14.Eagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, Fleisher LA, Froehlich JB, Gusberg RJ, Leppo JA, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) J Am Coll Cardiol. 2002;39:542–553. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 15.Welten GM, Schouten O, van Domburg RT, Feringa HH, Hoeks SE, Dunkelgrun M, van Gestel YR, Goei D, Bax JJ, Poldermans D. The influence of aging on the prognostic value of the revised cardiac risk index for postoperative cardiac complications in vascular surgery patients. Eur J Vasc Endovasc Surg. 2007;34:632–638. doi: 10.1016/j.ejvs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Boersma E, Poldermans D, Bax JJ, Steyerberg EW, Thomson IR, Banga JD, van De Ven LL, van Urk H, Roelandt JR. Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA. 2001;285:1865–1873. doi: 10.1001/jama.285.14.1865. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 18.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 20.London RM, George J. Pathogenesis of NASH: animal models. Clin Liver Dis. 2007;11:55–74, viii. doi: 10.1016/j.cld.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Kryzhanovski VA, Beller GA. Usefulness of preoperative noninvasive radionuclide testing for detecting coronary artery disease in candidates for liver transplantation. Am J Cardiol. 1997;79:986–988. doi: 10.1016/s0002-9149(97)00030-1. [DOI] [PubMed] [Google Scholar]
- 22.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–1545. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- 23.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 24.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, Pohost GM, Shaw LJ, Weintraub WS, Winters WL Jr. American College of Cardiology/American Heart Association Expert Consensus Document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol. 2000;36:326–340. doi: 10.1016/s0735-1097(00)00831-7. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishna G, Breen JF, Mulvagh SL, McCully RB, Pellikka PA. Relationship between coronary artery calcification detected by electron-beam computed tomography and abnormal stress echocardiography: association and prognostic implications. J Am Coll Cardiol. 2006;48:2125–2131. doi: 10.1016/j.jacc.2006.04.105. [DOI] [PubMed] [Google Scholar]
- 27.Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, Friedman JD, Kang X, Polk D, Hachamovitch R, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–930. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Anand DV, Lim E, Raval U, Lipkin D, Lahiri A. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. J Nucl Cardiol. 2004;11:450–457. doi: 10.1016/j.nuclcard.2004.06.125. [DOI] [PubMed] [Google Scholar]
- 29.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 30.Nasir K, Shaw LJ, Liu ST, Weinstein SR, Mosler TR, Flores PR, Flores FR, Raggi P, Berman DS, Blumenthal RS, et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50:953–960. doi: 10.1016/j.jacc.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 31.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 32.Cheng VY, Lepor NE, Madyoon H, Eshaghian S, Naraghi AL, Shah PK. Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–1186. doi: 10.1016/j.amjcard.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Loong CY, Anagnostopoulos C. Diagnosis of coronary artery disease by radionuclide myocardial perfusion imaging. Heart. 2004;90 Suppl 5:v2–v9. doi: 10.1136/hrt.2003.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geleijnse ML, Krenning BJ, Nemes A, Soliman OI, Galema TW, ten Cate FJ. Diagnostic value of dobutamine stress echocardiography in patients with normal wall motion at rest. Echocardiography. 2007;24:553–557. doi: 10.1111/j.1540-8175.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- 35.Paetsch I, Jahnke C, Wahl A, Gebker R, Neuss M, Fleck E, Nagel E. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110:835–842. doi: 10.1161/01.CIR.0000138927.00357.FB. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsui JM, Mukherjee S, Elhendy A, Xie F, Lyden ER, O'Leary E, McGrain AC, Porter TR. Value of dobutamine stress myocardial contrast perfusion echocardiography in patients with advanced liver disease. Liver Transpl. 2006;12:592–599. doi: 10.1002/lt.20651. [DOI] [PubMed] [Google Scholar]
- 37.Zoghbi GJ, Patel AD, Ershadi RE, Heo J, Bynon JS, Iskandrian AE. Usefulness of preoperative stress perfusion imaging in predicting prognosis after liver transplantation. Am J Cardiol. 2003;92:1066–1071. doi: 10.1016/j.amjcard.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Guckelberger O, Byram A, Klupp J, Neumann UP, Glanemann M, Stockmann M, Neuhaus R, Neuhaus P. Coronary event rates in liver transplant recipients reflect the increased prevalence of cardiovascular risk-factors. Transpl Int. 2005;18:967–974. doi: 10.1111/j.1432-2277.2005.00174.x. [DOI] [PubMed] [Google Scholar]
- 39.Nagarakanti R, Whellan D, Rubin S, Mather PJ. Reversible cardiomyopathies. Cardiol Rev. 2007;15:178–183. doi: 10.1097/CRD.0b013e31804c98b1. [DOI] [PubMed] [Google Scholar]
- 40.Torregrosa M, Aguade S, Dos L, Segura R, Gonzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68–74. doi: 10.1016/j.jhep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144–147. doi: 10.1161/01.RES.0000156077.54903.67. [DOI] [PubMed] [Google Scholar]
- 42.Harley ID, Jones EF, Liu G, McCall PR, McNicol PL. Orthotopic liver transplantation in two patients with hypertrophic obstructive cardiomyopathy. Br J Anaesth. 1996;77:675–677. doi: 10.1093/bja/77.5.675. [DOI] [PubMed] [Google Scholar]
- 43.Paramesh AS, Fairchild RB, Quinn TM, Leya F, George M, Van Thiel DH. Amelioration of hypertrophic cardiomyopathy using nonsurgical septal ablation in a cirrhotic patient prior to liver transplantation. Liver Transpl. 2005;11:236–238. doi: 10.1002/lt.20327. [DOI] [PubMed] [Google Scholar]
- 44.Goss JA, Stribling R, Martin P. Adult liver transplantation for metabolic liver disease. Clin Liver Dis. 1998;2:187–210. doi: 10.1016/s1089-3261(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 45.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 46.Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro S, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 47.Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451–459. doi: 10.1002/hep.510240226. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol. 2002;26:842–847. [PubMed] [Google Scholar]
- 49.Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268–275. doi: 10.1136/gut.49.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epstein SK, Ciubotaru RL, Zilberberg MD, Kaplan LM, Jacoby C, Freeman R, Kaplan MM. Analysis of impaired exercise capacity in patients with cirrhosis. Dig Dis Sci. 1998;43:1701–1707. doi: 10.1023/a:1018867232562. [DOI] [PubMed] [Google Scholar]
- 51.Campillo B, Fouet P, Bonnet JC, Atlan G. Submaximal oxygen consumption in liver cirrhosis. Evidence of severe functional aerobic impairment. J Hepatol. 1990;10:163–167. doi: 10.1016/0168-8278(90)90046-t. [DOI] [PubMed] [Google Scholar]
- 52.Milani A, Zaccaria R, Bombardieri G, Gasbarrini A, Pola P. Cirrhotic cardiomyopathy. Dig Liver Dis. 2007;39:507–515. doi: 10.1016/j.dld.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz JM, Beymer C, Althaus SJ, Larson AM, Zaman A, Glickerman DJ, Kowdley KV. Cardiopulmonary consequences of transjugular intrahepatic portosystemic shunts: role of increased pulmonary artery pressure. J Clin Gastroenterol. 2004;38:590–594. doi: 10.1097/00004836-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Bernardi M, Rubboli A, Trevisani F, Cancellieri C, Ligabue A, Baraldini M, Gasbarrini G. Reduced cardiovascular responsiveness to exercise-induced sympathoadrenergic stimulation in patients with cirrhosis. J Hepatol. 1991;12:207–216. doi: 10.1016/0168-8278(91)90940-d. [DOI] [PubMed] [Google Scholar]
- 55.Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, Hayes PC. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326–332. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- 56.Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Moller S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol. 2002;36:513–520. doi: 10.1016/s0168-8278(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 57.Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23:243–248. doi: 10.1034/j.1600-0676.2003.00833.x. [DOI] [PubMed] [Google Scholar]
- 58.Hansen S, Moller S, Bendtsen F, Jensen G, Henriksen JH. Diurnal variation and dispersion in QT interval in cirrhosis: relation to haemodynamic changes. J Hepatol. 2007;47:373–380. doi: 10.1016/j.jhep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Roden DM, Viswanathan PC. Genetics of acquired long QT syndrome. J Clin Invest. 2005;115:2025–2032. doi: 10.1172/JCI25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brubaker PH, Kitzman DW. Prevalence and management of chronotropic incompetence in heart failure. Curr Cardiol Rep. 2007;9:229–235. doi: 10.1007/BF02938355. [DOI] [PubMed] [Google Scholar]
- 61.Laffi G, Lagi A, Cipriani M, Barletta G, Bernardi L, Fattorini L, Melani L, Riccardi D, Bandinelli G, Mannelli M, et al. Impaired cardiovascular autonomic response to passive tilting in cirrhosis with ascites. Hepatology. 1996;24:1063–1067. doi: 10.1053/jhep.1996.v24.pm0008903376. [DOI] [PubMed] [Google Scholar]
- 62.Ma Z, Meddings JB, Lee SS. Membrane physical properties determine cardiac beta-adrenergic receptor function in cirrhotic rats. Am J Physiol. 1994;267:G87–G93. doi: 10.1152/ajpgi.1994.267.1.G87. [DOI] [PubMed] [Google Scholar]


