Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide with an annual occurrence of one million new cases. An etiologic association between HBV infection and the development of HCC has been established with a relative risk 200-fold greater than in non-infected individuals. Hepatitis C virus is also proving an important predisposing factor for this malignancy with an incidence rate of 7% at 5 years and 14% at 10 years. The prognosis depends on tumor stage and degree of liver function, which affect the tolerance to invasive treatments. Although surgical resection is generally accepted as the treatment of choice for HCC, new treatment strategies, such as local ablative therapies, transarterial embolization and liver transplantation, have been developed nowadays. With increasing detection of small HCCs from screening programs for cirrhotic patients, it is foreseen that locoregional therapy will play an important role in the near future.
Keywords: Hepatocellular carcinoma, Hepatoma, Resection, Ablation, Transplantation, ICG clearance, Remnant liver volume, Milan criteria, MELD score
INTRODUCTION
Surgery, including liver transplantation (OLT) re-mains the most efficient treatment of patients with hepatocellular carcinoma (HCC)[1,2]. However, less than 30% of patients are eligible for liver resection (LR) due to HCC multifocality on a background of chronic liver disease[1,2]. Over the past 10 years, there has been considerable progress in both diagnosis and surgical outcome of HCC patients[1,2]. Selective preoperative morphological assessment, preoperative use of portal vein embolization[3] and the improvement of surgical techniques[4,5] are factors that improve the safety of LR. In addition, better selection of the candidates for LR has been accomplished by both imaging advancements and preoperative accurate evaluation of liver functional reserve. We review herein the available data regarding selection criteria for LR in the setting of HCC and underlying liver disease.
CLINICAL CLASSIFICATION
Cancer classification aims to establish prognosis and select the adequate treatment for the best candidates. The Barcelona-Clinic Liver Cancer (BCLC) classification has emerged as the standard classification for clinical management of HCC[2,6]. This system links tumor stage with treatment strategy and has been externally validated. Three stages of HCC have been reported according to BCLC group: Early stages, Intermediate-advanced HCC and End-stage HCC. At the early stages tumor status is defined by size of the main nodule and multicentricity (single < 2 cm, single 2-5 cm, 3 nodules < 3 cm). Variables related to liver function are relevant in patients not suitable for transplantation as portal hypertension and normal bilirubin in patients undergoing resection. The limitations of one-dimensional systems, such as the Okuda staging and the Child-Pugh classification have been overcome. Several proposals reported recently and sub-classify patients at advanced stages such as the CUPI and the CLIP score[7,8]. The new TNM in accordance with the AJCC is based on series of patients undergoing resection. Pathological information is needed, thus representing a limitation for preoperative clinical use. Finally, the Japan Integrated Staging (JIS) includes two previous classifications: the TNM and the Japanese version of the Child-Pugh classification and offers advantages compared to CLIP score[9].
MILAN CRITERIA FOR LIVER TRANSPLANTATION
In the past 10 years, results of OLT have improved steadily because of careful patient selection pioneered by the introduction of the Conventional Milan Criteria (CMC)[10]. The aim of these criteria was to achieve a good outcome in patients who fulfilled the criteria and avoid a poor prognosis in patients who exceed them. These are patients with single HCC < 5 cm or up to three nodules < 3 cm who in major units achieve 70% survival at 5 years with a recurrence below 15%. The major drawback of OLT is the scarcity of donors. The increase of waiting time has led to 20% of the candidates to drop out due to progression of disease jeopardizing the outcome according to intention-to-treat analysis.
Pre-transplantation neoadjuvant treatments
Several attempts have been made to prevent tumor progression during waiting time by applying adjuvant therapies, mainly percutaneous ablation and transcatheter arterial chemoembolization (TACE). These therapies have been tested only in the setting of observational studies, case series and cohort studies that provide limited information because of heterogeneity of patient and tumor characteristics, variable waiting times, use of different treatment modalities, variable evaluation of response and lack of consensus about criteria of drop-out[1,2,9]. Among the case series and cohort studies, some investigators suggest a favorable impact of treatment in decreasing the dropout rate. Mazzaferro reported no dropouts in 50 patients within Milan criteria treated with RFA[11]. Some studies reported no dropouts in patients within Milan treated by TACE and short waiting time (178 d) while others documented a probability of dropout of 15% at 6 mo and 25% at 12 mo[9]. Cumulative results show that RFA achieves the highest rates of complete necrosis (12%-55%) compared with TACE (22%-29%). Complete necrosis is best achieved with percutaneous ablation in tumors < 3 cm in diameter. It is estimated that drop-out rates will increase with the expansion of selection criteria as demonstrated by Roayaie[12]. Recurrence rates are majorly related to tumor stage than to neo-adjuvant therapies. It is recognized that recurrence rates are low when applying the Milan criteria, compared to a wide selection of candidates.
Milan versus expanded criteria for OLT
The growing experience and success of OLT for HCC have fuelled controversies related to expansion of Milan criteria. Among the proposed expanded criteria the UCSF criteria (single tumor nodule up to 6.5 cm; or three or fewer tumors, the largest of which is ≤ 4.5 cm with the sum of the tumor diameters ≤ 8 cm) reflect a modest expansion of tumor size limits[13]. However, there are limitations in applicability of the UCSF criteria in the pre-transplant setting, considering that most of the patients adhering to the UCSF were also within the Milan criteria. More to the point, the overlapping population of patients adhering to the UCSF, but not the Milan criteria is often negligible and estimated to be less than 10% of the total transplanted population[1,2,9]. In addition, the limitations of pre-transplant imaging studies, exemplified by tumor under-staging in 20% of patients, have been a major concern for liberalizing the existing criteria for OLT.
Down-staging refers to a change as a result of treatment so that disease will reach the Milan criteria, as assessed by imaging techniques. In the seminal study from Majno et al[14], TACE induced down-staging of HCC > 3 cm and resulted in good survival rates after OLT, but the benefit was not confirmed in other investigations that studied TACE or RFA. The UCSF group reported a cohort of 30 patients treated heterogeneously, so that disease would reach the Milan criteria. Half the patients with down-staged tumors were effectively transplanted, although some bias exists and control studies required. Therefore, nowadays down-staging should be assessed in the setting of clinical research.
LIVER RESECTION
Although surgery remains the only treatment for HCC in patients with or without cirrhosis, most individuals with HCC are ineligible for surgical intervention. In eligible patients, the methods of surgical therapy are partial hepatectomy and liver transplantation. In addition to resection and liver transplantation, percutaneous ablation is considered as a treatment option that offers a high rate of complete response and thus a potential for cure. In selected patients, a 5-year survival rate of 60% to 75% can be achieved after surgery[1,2,9]. However, in those with advanced HCC, the consequent improvement in long-term survival is poor because of the high rate of recurrence or the development of intra-hepatic metastases that disseminate via the portal vein or spread to other parts of the liver. Nevertheless, the management of HCC has undergone major changes over the last few decades. Earlier detection enabled by screening methods that use ultrasonographic evaluation and AFP analysis in high-risk populations, more accurate patient assessment, advances in imaging, improved surgical techniques, and the availability of local treatment options have improved outcomes.
HCC in patients with a non-cirrhotic liver
Only 5% of the cases of HCC in Western countries (as opposed to 40% in Asia) develop in a non-cirrhotic liver[1]. When HCC occurs in a non-cirrhotic liver, solitary tumor nodes that are limited to one liver lobe and lack satellite foci are frequently present. Without predisposing cirrhosis, HCC is often not diagnosed, until the tumor causes symptoms because of its size. Sometimes HCC is an incidental finding revealed by ultrasonographic studies[1].
The treatment approach for patients with HCC without cirrhosis should be based on factors such as extra-hepatic tumor manifestation, tumor size and the number and distribution of nodules. In such patients, curative resection should be considered whenever possible.
Major hepatectomy defined as resection of more than three liver segments is feasible if the remnant liver volume is adequate. The evolution of transection devises and postoperative care had a major impact in both morbidity and mortality after LR. Most centers documented a less than 5% mortality rate recently[1,2,4,5] compared to a higher incidence reported 10 years ago. Blood transfusion requirements have also been restricted from 80% to 20% in major reference centres. This was accomplished by bloodless techniques with intermittent inflow occlusion and better selection of candidates with single lesion and absence of portal hypertension[1,2,4,5,15,16].
Pre-treatment imaging studies such as high-resolution triple-phase computed tomography (CT) and nuclear magnetic resonance imaging (MRI), either with or without angiography, can be used to match patients and their most appropriate treatment. Positron emission tomography (PET) is also useful in the identification of extra-hepatic metastases that considerably influence clinical decision-making. Knowledge about the relation of the tumor to regional anatomic structures such as large vessels is crucial because it provides valuable information about resectability. Furthermore, volumetric studies can be used to define the residual parenchyma exactly. If there is any suspicion of lymph node metastasis or peritoneal dissemination, diagnostic laparoscopy with intra-operative ultrasonography is useful, and if multiple metastases are confirmed, explorative laparotomy can be prevented.
The determination of hepatic reserve is also signi-ficant when resection is considered. The healthy liver has a great capability for regeneration and adjusts to the metabolic requirements of the host after LR due to hypertrophy of the residual liver. Therefore, even in patients with a large tumor, extensive resection is possible. In an otherwise healthy liver, up to 75% of the parenchyma can be resected.
Patients with a localized unilobar tumor in a non-cirrhotic liver or Child class A cirrhosis with adequate remnant liver parenchyma may be considered for partial hepatectomy (lobectomy). Partial hepatectomy usually ensures a safety margin of at least 1 cm and is associated with an operative mortality rate of less than 5%. From an oncologic perspective, anatomic resection that may include satellite lesions is more effective than limited resection without a surrounding margin. For patients with inadequate or borderline remnant parenchyma, hypertrophy of the prospective liver remnant can be induced by preoperative portal vein embolization (PVE). In certain circumstances, an unfavorable location of the tumor and involvement of the confluence of the three hepatic veins and either the caval vein or the retro-hepatic caval vein can render resection by conventional techniques impossible. In these rare cases, special techniques such as in situ or ante situm resection can be used.
The overall long-term results after resection are favourable. However, only 20% to 30% of patients with HCC are eligible for resection because of advanced or multifocal disease or inadequate functional hepatic reserve. In patients with solitary lesions of less than 5 cm, no vascular invasion, and a negative surgical margin of at least 1 cm, the 5-year survival rate after resection is reported to be greater than 70%[17]. Despite earlier detection, safer surgical procedures, and more aggressive treatment of HCC, recurrence (because of multicentric carcinogenesis or intrahepatic metastases from the primary tumor) is likely. In selected patients, repeated resection provides good long-term benefits and is an option for those with solitary peripheral tumors that can be treated with segmental or atypical resection.
HCC in patients with cirrhosis
HCC in patients with cirrhosis is a challenge due to both pre-existing liver damage and possible tumor multifocality. Portal hypertension and reduced functional capacity of the cirrhotic liver significantly increase the peri-operative risk. These facts influence two significant decisions regarding surgery: patient selection and the choice of the surgical therapeutic method.
The resection margin of HCC in cirrhotic patients does not represent a significant predictive factor for recurrence, unless residual tumor directly invades the raw surface of the liver[1]. In most HCC patients, tumor recurrence results from disseminated tumor, and in the remaining patients, recurrence is caused by metachronous tumors that arise in the oncogenic cirrhotic liver, as is typical in the cirrhosis that develops after hepatitis C infection[1]. Because of the difficulty to prevent recurrence by resection with an adequate safety margin, resection (preferably segmentectomy or subsegmentectomy rather than wedge resection) should be as limited as possible. Because of the threat of insufficient liver function coupled with a greater risk of mortality, the decision to perform major resection should be considered with caution.
The reduced functional reserve capacity in patients with cirrhosis of the liver limits the choice of surgical therapy. Various tests have been developed to quantify liver function.
Refined selection criteria and technical advances, including a broader knowledge of segmental anatomy, vascular occlusion techniques, and the use of intra-operative ultrasonography, have facilitated resection and improved outcome. Operative mortality rates have decreased to less than 5%[2,9]. A considerable decrease in intra-operative blood loss has been achieved by means of numerous technical improvements such as the use of ultrasonographic dissectors and bipolar and argon beamer coagulation. In individual cases, hilar occlusion (the Pringle manoeuvre) has become either unnecessary or the occlusion time can be shortened, both of which result in reduced ischemia-reperfusion damage. Despite a decrease in the operative mortality rate and improved results after resection, overall survival after the resection of HCC has changed little due to absence of effective adjuvant treatment to eliminate postoperative recurrence.
Liver function assessment: The clinical assessment of hepatic function by the use of “Child” system, developed to understand the significance of cirrhotic liver injury and portal hypertension as they related to patient survival after portal-systemic shunt surgery was used mainly in the past in its original version. In general, Child class A or Child class B patients may tolerate a resection of up to 50% and 25% of liver parenchyma, respectively. However, evaluating hepatic reserve by means of the CTP classification may lead to an inconsistent predictive value, because as Child class A patients may already have significant functional impairment and may demonstrate an increase in the bilirubin level as well as portal hypertension and fluid retention. These features indicate advanced liver disease and preclude resection. Limited discriminatory ability, subjective interpretation of parameters, and variability in the measurement of laboratory parameters are further limitations of CPT. Makuuchi et al[18] was first to described three parameters in patients with cirrhosis associated with morbidity after hepatectomy. Ascites, abnormal serum bilirubin and ICG clearance were defined as independent factors affected postoperative morbidity. They noted that a cut-off ICG clearance level below 20% is adequate for safe hepatectomy. Other groups use a cut-off level of 14% to discriminate high-risk candidates[19]. If that level is greater than 40% postoperative liver failure is likely, even with minimal resection. However, ICG clearance criteria are not absolute and every effort for further extension depends on the liver remnant size and severity of cirrhosis. In addition ICG retention measurement is cumbersome requiring accurate sampling. Furthermore, the dynamic tests (ICG, Galactose elimination capacity etc) cannot take into account all the complexities of liver function and, therefore, they have limitations. ICG clearance is not a true index of parenchymal function because there is also a substantial influence of hepatic blood flow. Clearance is considered to be impaired when 15% or more of the dye remains within the plasma 15 min following the injection of 0.5 mg/kg ICG. Thus, patients with CP scores of 5 or 6 (Child A) and ICG15 of greater than 14% are the “high risk” CP-A patients with limited functional reserve.
Nuclear imaging has been used recently to evaluate liver function. A functional imaging with great promise involves receptor targeting with radio-labelled synthetic asialoglycoproteins (99 m-Tc-GSA). GSA provides volumetric receptor data and kinetic distribution curves. However, GSA is still preliminary and although is correlated with CP and ICG clearance its potential role in evaluating resection must demonstrate that they are improvements over CP stratification.
In Europe and North America, the selection of optimal candidates for LR is usually based on the degree of portal hypertension and an elevated bilirubin level. Portocaval pressure gradient > 10 mmHg or the presence of oesophageal varices (grade 2, 3) are good indicators of portal hypertension (PH). A low platelet count < 100 000/mL and splenomegaly is also used as a surrogate marker of PH. A bilirubin concentration that is within normal limits and a hepatic vein pressure gradient of less than 10 mmHg (measured by hepatic vein catheterization) are the best predictors of excellent outcome after resection and are associated with almost no risk of postoperative liver failure[2]. In the setting of Child A cirrhosis with none of these factors presented, a 70% 5-year survival was documented despite the fact that portal hypertension and abnormal bilirubin decrease long-term survival by half. Measurement of the liver remnant volume is helpful in selecting patients for major hepatic resection, however, preoperative evaluation of the severity of cirrhosis may be mandatory by biopsy of non- tumorous liver for histological grading.
The Model for End-Stage Liver Disease (MELD) score has gained widespread acceptance to prioritize candidates for liver transplantation. Few studies[20–23] including the one performed in our department[24], demonstrated a strong correlation of postoperative morbidity after hepatectomy and MELD value. More precisely a cut-off value of 8 was associated with higher morbidity and a cut-off value of 11 with high mortality rate. In this particular situation, our group suggests liver transplantation rather than resection.
Apart from the factors mentioned above the presence of co-morbid illnesses such as cardiovascular disease has been shown to increase the risk of hepatectomy. While the presence of severe co-morbid illnesses such as congestive heart failure and chronic renal failure should be considered a contraindication for hepatectomy, HCC patients with less severe co-morbid illnesses, such as diabetes, may still benefit from hepatic resection provided with meticulous peri-operative care. However, the importance of optimum peri-operative control of the blood glucose level and vigilant postoperative care in such cases needs to be emphasized. Finally, the Memorial Sloan Kettering data reported by Jarnagin[25–28] suggests that experience may play a critical, positive role in patient selection. The avoidance of greater than four segment resections in “bad-risk” Child-Pugh Class A patients is a clear-cut goal, unless the option of portal vein occlusion is to be pursued.
Portal vein embolization: Portal vein embolization (PVE) has been applied in the setting of inadequate liver remnant volume to induce hypertrophy. Although the concept of contra-lateral liver hypertrophy after PVE has been challenged by some due to the impaired cirrhotic liver regenerative capacity, a better selection of those patients not amenable to major resection is feasible. Proposed guidelines for PVE application include less than 40% remnant liver volume in the non-cirrhotic group of patients underwent major hepatectomy or less than 60% in the group of cirrhotic individuals with ICG15 < 20%.
The volume of functional liver mass left after resection is an important factor in the development of postoperative complications and subsequent mortality[29,30]. Liver volume measurement for adult living donor liver transplantation is standardized to achieve a graft weight to recipient weight of at least 1% because of the clear link between adequate functional hepatic mass of the donor graft to recipient weight and postoperative complications.
In the non-transplant LR setting, the minimum acceptable liver volume remaining post-resection has not been well assessed but is generally thought to be about 25% of the normal liver volume. In cases where there is liver dysfunction such as cirrhosis or cholestasis, 40% of the normal volume is acceptable[31]. The advent of PVE allows optimization of the remnant liver volume in cases where it is projected to be less than ideal. However, no randomized trials examining the effectiveness of portal vein embolization on liver regeneration or its impact on LR exist. The use of portal vein embolization of the hepatic lobe that hosts the tumor to induce compensatory hypertrophy in the non-affected liver before major resection is controversial. Uncontrolled tumor progression because of the proliferation of malignant cells stimulated by this method and the risk of variceal bleeding resulting from acute portal hypertension are some of the concerns.
PVE blocks portal flow to the side of the liver ipsilateral to the lesion to be resected (Figure 1A and B) and causes an increase in size of the future liver remnant (FLR) (Figure 2A and B). The increased size is due to both clonal expansion and cellular hypertrophy[32]. The assumption that increase in liver volume correlates with increased function post-PVE has been demonstrated in studies showing increase in asialoglycoprotein receptor binding sites in the FLR before resection[33–36]. Percutaneous transhepatic PVE can be performed by either of two standard approaches: the transhepatic ipsilateral[37] and transhepatic contralateral[38].
Figure 1.
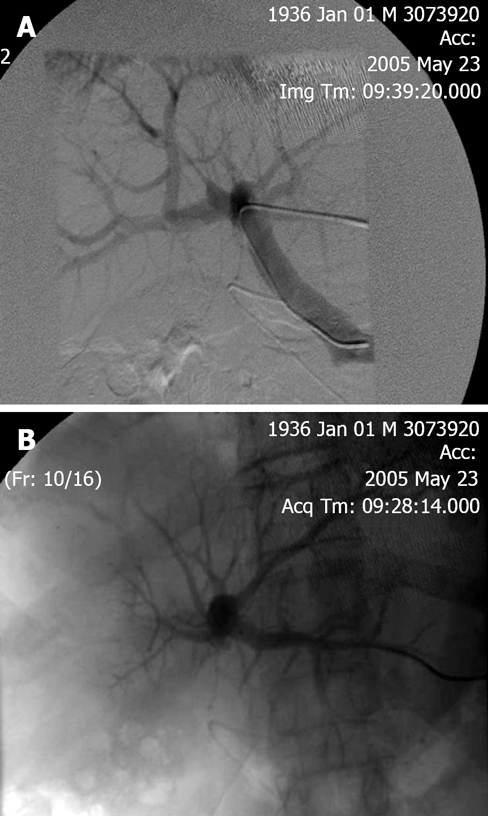
A: Before portal vein embolization (PVE); B: After portal vein embolization (PVE).
Figure 2.
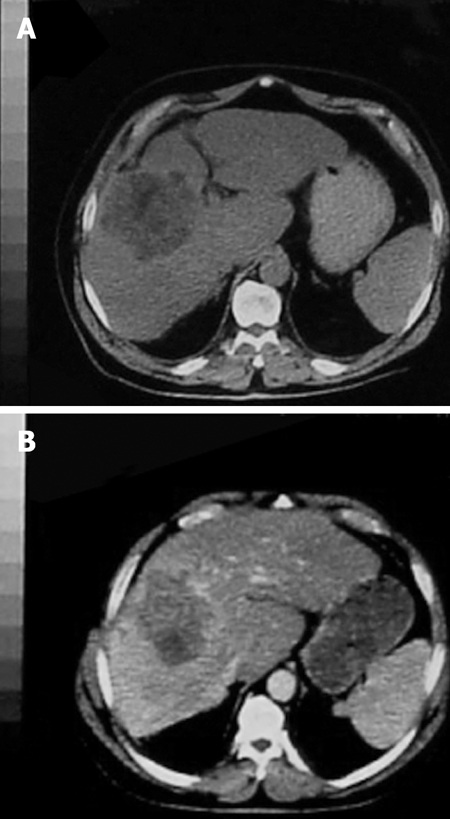
CT indicated, A: Before PVE; B: Six weeks after PVE on the same patient. Hypertrophy of the left hepatic lobe is obvious.
Many commercially available embolic agents have been used for preoperative PVE without significant differences in degrees or rates of hypertrophy of the nonembolized segments[39]. The ideal agent is well tolerated by the patient and causes permanent embolization of the portal vein and its branches with minimal risk of recanalization[40]. In addition, it should be widely available and easily administered without causing inflammatory reaction and be associated with a low risk of post-embolization syndrome and hepatic necrosis. Although each of the embolic agents has advantages and disadvantages, no single agent has been proven to be consistently superior[41,42].
All patients have a volumetric assessment of their liver volumes both before PVE and before surgery using CT imaging. Post-embolization CT is essential for assessment of liver volume change and planning of LR[39]. The average interval for the CT volumetric study from portal vein embolization to surgery is 4 to 6 wk. CT is used to make direct measurements of total liver volume, volume to be resected, and volume of the future liver remnant. The future liver remnant volume is considered to be the volume of the liver segments expected to remain after hepatectomy. More precisely Computed Tomography scans of the liver are necessary. Serial transverse scans at 1-cm intervals from the dome of the liver to the most inferior part of the organ must be obtained, with enhancement by intravenous bolus injection of contrast and with the patient suspending respiration in expiration. Each slice of the liver is traced with a cursor, and computer calculates the corresponding area. The middle hepatic vein and gallbladder are used as landmarks to define the borders between the right and left livers. Segment IV volume is measured using the middle hepatic vein and the umbilical portion of the left portal vein as landmarks. The total volumes measured (whole liver volume, tumor volume, and remnant liver volume) are calculated by multiplying the area of each part by the interval thickness and by adding all the interval volumes of each part. The estimated rate of remnant functional liver parenchyma (ERRFLP) is calculated by the formula: FLR volume = (remnant liver volume × 100)/(total liver volume - tumor volume)[43,44].
The increase in FLR volume after portal vein embolization can be calculated with the following for-mula: (volume of the FLR before surgery - volume of the FLR before PVE) × 100/(volume of the FLR before PVE). The increase in the %FLR after portal vein embolization can also be calculated from: (%FLR after PVE - %FLR before surgery). The total liver volume can be estimated on the basis of the body surface area using the formula; total liver volume = 706.2 × body surface area + 2.4. The calculated range of percentage increase of future liver remnant volume following portal vein embolization is 8% to 27% in different studies including our experience.
Preoperative imaging: Selection of candidates with HCC for LR requires adequate preoperative staging[1,2]. Sensitivity and diagnostic value of preoperative imaging (CT, MR) approximates 80% but is limited for satellite nodules or less than 1 cm lesions. MR angiography although is more sensitive for identification of 1-2 cm lesions has limitations for subcentimeter tumors. Intraoperative ultrasound is more accurate for very early less than 1 cm HCC. Positron emission tomography (PET) is also useful in the identification of extra-hepatic disease that considerably influence clinical decision-making. If there is any suspicion of lymph node metastasis or tumor dissemination, diagnostic laparoscopy with intraoperative ultra-sonography is useful to avoid an unnecessary laparotomy. There is widespread acceptance that liver biopsy is not necessary as a routine for lesions more than 2 cm with imaging compatible for HCC in a patient with history of underlying cirrhosis or high AFP levels.
PROGNOSTIC FACTORS
The most significant predictive factors for early recurrence are the size and number of tumors, the pre-sence of satellite nodules, the histologic grade, the severity of cirrhosis, and the serum AFP level[45–49]. Tumor size and the number of nodules are important factors that predict vascular invasion. However, tumor size is not an absolute contraindication for LR[50]. Long-term survival varies from 66% in cases of less than 5 cm lesions to 37% in the group of large (> 5 cm) HCC[46–50]. Vascular invasion is an independent factor affect pro-gnosis and is strongly associated with both size and histological grade. More precisely lesions less than 2 cm in diameter have a 20% rate of micro-vascular invasion although the rate increases progressively for lesions more than 5 cm in diameter (60%-90%). Even though vascular invasion is not easily identified preoperatively by imaging techniques, tumor size and grading are surrogate markers. According to the results of a study reported recently, a tumor size larger than 5 cm was an indicator of high histological grade in more than 40% of patients with HCC. Needle core biopsy (NCB) is notorious, unreliable to confirm preoperatively tumor grading due to heterogenous nature of HCC as reported by Pawlik and associates[51].
The number of lesions is strongly related with incidence of recurrence[49]. Long-term survival is 57% for solitary lesions but only 26% for multifocal HCC. Other factors affect prognosis includes AFP levels, age and concomitant diseases[46,49]. In addition, genetic signature of the tumor is attributed to both disease free and overall survival[46,49,50]. The role of hepatic resection for bilobar HCCs is controversial. Bilobar HCCs may represent advanced disease with intrahepatic metastasis or may represent multifocal HCCs derived from multicentric hepatocarcinogenesis. Major hepatectomy in one lobe combined with wedge resection for a smaller lesion in the other lobe is possible in some cases. Alternatively, hepatic resection in one lobe can be combined with local ablation of a smaller lesion in the other lobe using ethanol injection or newer ablative modalities such as radiofrequency ablation. In a recent study by Poon and associates, they demonstrated that hepatic resection for patients with bilobar HCCs resulted in a better survival outcome than non-resectional therapies[52]. Hence, in accordance with others we recommend that hepatic resection should be considered in selected patients with bilobar HCCs, especially those with a small solitary lesion in the contra lateral lobe that are amenable to wedge resection or local ablative therapy.
Although hepatic resection with removal of tumor thrombus in the inferior vena cava or main portal vein has been advocated by some authors[53], most liver surgeons consider the presence of tumor thrombus in the inferior vena cava or main portal vein a contraindication for hepatic resection because the prognosis is usually poor even with such an aggressive approach. However, hepatic resection for patients with tumor invasion of the hepatic veins or major intrahepatic branches of the portal vein is justified because favourable survival results may be expected compared with non-surgical treatment[54,55].
A major drawback of LR in the setting of HCC is the high recurrence rate (70%) due to intrahepatic dissemination or de novo appearance of new lesions. Molecular techniques differentiate widespread liver disease (60%-70%) from de novo HCC development (30%-40%). Intrahepatic metastases occurred early (less than 2 years) after LR and related to primary tumor biologic aggressiveness (low grade, vascular invasion, satellite nodules). De novo HCC occurrence is related mostly to the underlying liver disease and appears later.
TREATMENT STRATEGY
Although surgery remains the gold standard for HCC in patients with or without cirrhosis, most individuals are ineligible for surgical intervention. In fact, only 20% to 30% of cases are amenable to resection using the previous mentioned selection criteria. Despite the difficulty of exposing patients to the risks and consequences of transplantation-associated immune suppression, liver transplantation is the ultimate treatment option in patients with HCC who fulfil Milan selection criteria. Transplantation restores liver function and decrease tumor recurrence due to removal of the oncogenic potential dysplastic lesions. However when compared with LR the results of liver transplantation in patients with HCC and without cirrhosis are less favorable. Shortage of available donors is an additional limitation associated with a high dropout rate especially in large tumors that do not fulfil Milan criteria. Recently, several groups compared resection and transplantation demonstrated similar survival rates when the different tumor invasiveness was taken into account[47]. The inclusion of patients with more advanced cancer in the waiting list for transplantation result in a higher dropout rate that leads in turn to poor survival rates in an intent-to-treat analysis. In such cases, LR if feasible is the only curative option. The relative benefits of transplantation and resection are, therefore, likely to depend upon local organ allocation policy and waiting times. Another approach to reduce both waiting-list dropout rate and the demand for donor organs is primary resection followed by salvage transplantation in case of recurrence. Two studies have compared this strategy of primary transplantation with conflicting conclusions. Opposite results applying salvage transplantation have been published by two groups reporting peri-operative mortality ranging from 5% to 30%. The BCLC group proposed a policy of listing patients for liver transplant without evident HCC based on pathological risk of recurrence after resection (vascular invasion or satellites). However, in current clinical practice the applicability of this policy is low, about 10% of cases especially if there is underlying hepatitis C.
The recent development of laparoscopic LR has added new possibilities for the limited removal of peripheral lesions[56]. The avoidance of long sub-costal incisions seems to be associated with reduced morbidity and earlier recovery. Re-operations are easy after laparoscopic resection, which allows it to be used as a neoadjuvant treatment in those awaiting liver transplantation without compromising subsequent surgery. An additional advantage of resecting small HCCs is that it allows histopathological study of the whole tumour and identifies patients at high risk of intra-hepatic recurrence (those with poorly differentiated tumor, microvascular invasion, and satellite nodules). This raises the concept of pre-emptive transplantation when these criteria apply to the resected specimen.
CONCLUSION
In conclusion, the selection of the treatment options in patients with HCC must be based on the patient’s condition, the number and size of the hepatic tumors, the functional reserve capacity and the available resources. LR is strongly recommended in non-cirrhotic and selected cirrhotic individuals. Appropriate selection of candidates achieved by accurate estimation of hepatic liver reserve is of paramount importance. Technical advances in imaging and surgery facilitates resection and improves outcome.
Peer reviewer: Frank A Anania, Professor, Emory University School of Medicine, Division of Division Digestive Diseases, 615 Michael Street, Room 255 Whitehead Biomedical Research Building, Atlanta, GA 30322, United States
S- Editor Li DL L- Editor Alpini GD E- Editor Yin DH
References
- 1.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 3.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 4.Delis SG, Bakoyiannis A, Tassopoulos N, Athanasiou K, Madariaga J, Dervenis C. Radiofrequency-assisted liver resection. Surg Oncol. 2008;17:81–86. doi: 10.1016/j.suronc.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Delis SG, Madariaga J, Bakoyiannis A, Dervenis Ch. Current role of bloodless liver resection. World J Gastroenterol. 2007;13:826–829. doi: 10.3748/wjg.v13.i6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 7.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 8.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;94:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–1007. doi: 10.1245/s10434-007-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roayaie S, Llovet JM. Liver transplantation for hepatocellular carcinoma: is expansion of criteria justified? Clin Liver Dis. 2005;9:315–328. doi: 10.1016/j.cld.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 14.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701-703. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 16.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43. doi: 10.1097/01.sla.0000231758.07868.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. doi: 10.1016/s0002-9610(99)80227-x. [DOI] [PubMed] [Google Scholar]
- 19.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10:S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 20.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 21.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215; discussion 1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for End-Stage Liver Disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg. 2005;242:244–251. doi: 10.1097/01.sla.0000171327.29262.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140:650–654; discussion 655. doi: 10.1001/archsurg.140.7.650. [DOI] [PubMed] [Google Scholar]
- 24.Delis S, Biliatis I, Athanassiou K. Model for end-stage liver disease. MELD score as a prognostic factor for postoperative morbidity and mortality in cirrhotic patients undergoing hepatectomy for HCC. Gut. 2006;56:A145. doi: 10.1111/j.1477-2574.2009.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooby DA, Jarnagin WR. Surgical management of hepatic malignancy. Cancer Invest. 2004;22:283–303. doi: 10.1081/cnv-120030217. [DOI] [PubMed] [Google Scholar]
- 26.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799; discussion 799-800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha CH, Ruo L, Fong Y, Jarnagin WR, Shia J, Blumgart LH, DeMatteo RP. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315–321; discussion 321-323. doi: 10.1097/01.sla.0000086548.84705.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, Fong Y, D'Angelica MI, Blumgart LH, DeMatteo RP. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- 29.Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung D, Jarnagin W, Fong Y, Blumgart LH. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. doi: 10.1016/s1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 30.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 31.Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 33.Kubo S, Shiomi S, Tanaka H, Shuto T, Takemura S, Mikami S, Uenishi T, Nishino Y, Hirohashi K, Kawamura E, et al. Evaluation of the effect of portal vein embolization on liver function by (99m)tc-galactosyl human serum albumin scintigraphy. J Surg Res. 2002;107:113–118. doi: 10.1006/jsre.2002.6503. [DOI] [PubMed] [Google Scholar]
- 34.Kudo M, Todo A, Ikekubo K, Yamamoto K, Vera DR, Stadalnik RC. Quantitative assessment of hepatocellular function through in vivo radioreceptor imaging with technetium 99m galactosyl human serum albumin. Hepatology. 1993;17:814–819. [PubMed] [Google Scholar]
- 35.Vera DR, Topcu SJ, Stadalnik RC. In vitro quantification of asialoglycoprotein receptor density from human hepatic microsamples. Methods Enzymol. 1994;247:394–402. doi: 10.1016/s0076-6879(94)47030-8. [DOI] [PubMed] [Google Scholar]
- 36.Yumoto Y, Umeda M, Ohshima K, Ogawa H, Kurokawa T, Kajitani M, Yumoto E, Hanafusa T, Tsuboi H, Higashi T. Estimation of remnant liver function before hepatectomy by means of technetium-99m-diethylenetriamine-pentaacetic acid galactosyl human albumin. Cancer Chemother Pharmacol. 1994;33 Suppl:S1–S6. doi: 10.1007/BF00686659. [DOI] [PubMed] [Google Scholar]
- 37.Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, Wallace MJ, Morello FA Jr, Ahrar K, Murthy R, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–808. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 39.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680; discussion 680-681. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 40.de Baere T, Roche A, Vavasseur D, Therasse E, Indushekar S, Elias D, Bognel C. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73–77. doi: 10.1148/radiology.188.1.8511321. [DOI] [PubMed] [Google Scholar]
- 41.de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–1391. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 42.Imamura H, Shimada R, Kubota M, Matsuyama Y, Nakayama A, Miyagawa S, Makuuchi M, Kawasaki S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29:1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 43.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 44.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252–259. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Ramacciato G, Mercantini P, Nigri GR, Ravaioli M, Cautero N, Di Benedetto F, Masetti M, Grazi GL, Ziparo V, Ercolani G, et al. Univariate and multivariate analysis of prognostic factors in the surgical treatment of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2006;53:898–903. [PubMed] [Google Scholar]
- 50.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 51.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245:435–442. doi: 10.1097/01.sla.0000250420.73854.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu CL, Fan ST, Lo CM, Ng IO, Poon RT, Wong J. Hepatic resection for bilobar hepatocellular carcinoma: is it justified? Arch Surg. 2003;138:100–104. [PubMed] [Google Scholar]
- 53.Konishi M, Ryu M, Kinoshita T, Inoue K. Surgical treatment of hepatocellular carcinoma with direct removal of the tumor thrombus in the main portal vein. Hepatogastroenterology. 2001;48:1421–1424. [PubMed] [Google Scholar]
- 54.Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379–384. doi: 10.1097/00000658-200103000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poon RT, Fan ST, Ng IO, Wong J. Prognosis after hepatic resection for stage IVA hepatocellular carcinoma: a need for reclassification. Ann Surg. 2003;237:376–383. doi: 10.1097/01.SLA.0000055224.68432.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]


