Abstract
Liver cell transplantation is an attractive technique to treat liver-based inborn errors of metabolism. The feasibility and efficacy of the procedure has been demonstrated, leading to medium term partial metabolic control of various diseases. Crigler-Najjar is the paradigm of such diseases in that the host liver is lacking one function with an otherwise normal parenchyma. The patient is at permanent risk for irreversible brain damage. The goal of liver cell transplantation is to reduce serum bilirubin levels within safe limits and to alleviate phototherapy requirements to improve quality of life. Preliminary data on Gunn rats, the rodent model of the disease, were encouraging and have led to successful clinical trials. Herein we report on two additional patients and describe the current limits of the technique in terms of durability of the response as compared to alternative therapeutic procedures. We discuss the future developments of the technique and new emerging perspectives.
Keywords: Hepatocyte transplantation, Cell therapy, Inborn error of metabolism, Crigler-Najjar, Liver regeneration, Animal models
INTRODUCTION
Crigler-Najjar (CN) syndrome is the paradigm of an inborn error of liver metabolism affecting the function of one enzyme, the 1A1 isoform of the bilirubin-uridine diphosphate glucuronosyltransferase (UGT1A1)[1]. The parenchyma and thousands of other metabolic functions are normal, but the patient is at risk for severe neurological complications. Quality of life is deeply impaired, requiring phototherapy up to 12 h daily with efficacy lessening with ageing (probably due to unfavorable body surface/weight ratio and to increased skin thickness and pigmentation). Orthotopic liver transplantation (OLT) is a curative for the disorder[2,3], but seems disproportionate to correct one single missing enzymatic function in an otherwise normal liver. Patients and physicians are often reluctant to undertake such an irreversible procedure and are seeking less invasive alternative options. Indeed, up to 15% of OLT patients require re-transplantation, and progressive fibrosis of the graft is a subject of concern at long term[4].
Auxiliary liver transplantation (ALT) is another curative approach that has the advantage of being reversible. However, ALT remains associated with major pitfalls. In addition to being an invasive surgical procedure, the technique is difficult mainly because of perilous anastomosis that can hamper the venous in- or outflow and can lead to graft atrophy/ischemia or vascular thrombosis. Another complication is the small-for-size liver syndrome, defined as liver impairment, following inadequate liver mass replacement[5]. The diagnosis of rejection is difficult because of minimal enzyme elevation.
Successful long-term results were recently obtained with gene therapy in Gunn rats[6,7]. This technique was described to depend on vector serotypes and allowed a reduction of serum bilirubin up to 64% after one year[8]. Globally, this technique is still facing with anti-UGT1A1 antibody production in the host organism, impeding the perpetuation of the metabolic effect[9]. Although encouraging, ex vivo gene transfer and cell injection is closely related to the quality of cell preparation[10,11] and has not been documented in CN patients.
Other experimental protocols have been described, such as tin-mesoporphyrin treatment, for which feasibility has been demonstrated in two 17 year old patients[12], or treatment with chimeric oligonucleotides that allowed a significant reduction of serum bilirubin in Gunn rats for up to 11 mo[13].
Since the princeps report by Fox et al[14], liver cell therapy (LCT) appeared as a new alternative treatment, which is intermediate between whole organ transplantation and gene therapy. Cells can be infused safely in the diseased liver, and are expected to bring sufficient enzyme activity to restore bilirubin metabolism, setting the patients within safer metabolic limits and improving quality of life. LCT has been shown to be able to restore metabolic function not only in CN patients[15], but also in disorders of ammonium metabolism[16,17], glucose metabolism[18], clotting factor deficiencies[19], and even complex enzyme systems such as Refsum disease[20].
However, the technique remains insufficient; metabolic control is partial and durability of the result is limited to less than one year in most cases. Our aim is to review the current knowledge on the role of LCT to treat CN patients, report two additional patients, and review animal experiments performed as preclinical studies.
LCT FOR CN DISEASE TYPE I
Lessons from the animal model
The Gunn rat model represents the rodent equivalent of CN disease and is characterized by a single mutation in the ugt1A1 gene. In this model, many experimental protocols using free or encapsulated liver cells have been designed with syngeneic/congeneic or allogeneic transplantation procedures[21–32]. Table 1 summarizes representative experiments. The best results were obtained when a hepatic injury was caused before LCT to create a niche and a regenerative stimulus for engrafting cells. The explanation for why the injury was beneficial is Gunn rats global liver function is normal, except for bilirubin conjugation, and the lack of host hepatocyte impairment fails to provide to donor cells a proliferative advantage. The repopulation rate necessary to observe a metabolic efficacy ranges from 5% to 10%[33]. Significant lowering of serum bilirubin could be observed up to 12 mo while using congenic procedures[27].
Table 1.
Representative liver cell transplantation experiments in the Gunn rat model
| Donor cells | Injection site | Hepatic injury | Outcome | Cell tracking | References |
| 50 × 106 free or encapsulated congeneic Hc | Peritoneum | None | 34.8% serum bilirubin reduction with encapsulated Hc vs 13.5% with free Hc at 1 mo | Light and electron microscopy | 22 |
| 10 × 106 syngeneic Hc | Liver | Hepatectomy | Significant reduction of serum bilirubin up to 4 wk | ND | 24 |
| Apparition of conjugates in bile | |||||
| 10 × 106 congeneic Hc | Spleen | None | Significant reduction of serum bilirubin up to 12 mo | ND | 27 |
| Apparition of bile conjugates at 4 mo | |||||
| 2-20 × 106 congeneic Hc | Portal vein | Right portal vein ligation | Significant reduction of serum bilirubin when injury with 2 × 106 Hc or with 20 × 106 without injury up to 30 d | UGT1A1 activity, WB, PCR for ugt1 gene | 28 |
| Conjugates in bile after 10 d | |||||
| 5 × 106 congeneic Hc | Spleen | Hepatic irradiation ± Hepatectomy | Normalization of serum bilirubin only with combined injury | UGT1A1 activity, WB, IHC | 29 |
| Conjugates in bile detected up to 5 mo | |||||
| 10 × 106 congeneic Hc | Spleen | Hepatic irradiation ± FasL-induced apoptosis | Normalization of serum bilirubin up to 160 d | UGT1A1 activity, WB, IHC | 31 |
| Conjugates in bile at 150 d | |||||
| Estimation of repopulation at 52 ± 15% when combined injury | |||||
| 40 × 106 fetal or adult syngeneic Hc | Spleen | Retrorsine + Triiodothyronine | Significant reduction of serum bilirubin (+ conjugates in bile) up to 90 d (no difference between fetal and adult cells) | PCNA | 32 |
Hc: Hepatocyte; IHC: Immunohistochemistry; PCNA: Proliferating cell nuclear antigen; WB: Western blot.
On the clinical side
Reports of human LCT for CN disease have shown encouraging results. The first demonstration of the efficacy of the technique was provided by Fox et al[14]. In this case, 7.5 × 109 viable liver cells were infused in a 10-year-old patient and the effect was a significant decrease of bilirubin levels for up to 11 mo (Table 2). UGT1A1 enzyme activity was detected in the host liver and glucuronoconjugates were found in bile confirming the integration of functional, healthy hepatocytes. Dhawan et al reported two additional patients ages 18 mo and 3 years, in which the reduction of serum bilirubin reached up to 50% and 30%, respectively over a follow-up period up of 7 mo (Table 2). Donor hepatocyte engraftment was illustrated by short tandem repeat analysis at 8 mo follow-up. Ambrosino et al also described a decrease of bilirubin levels up to 3 mo post-LCT, whereas they did not detect donor cells by using a short tandem repeat assay at 40 d follow-up[34].
Table 2.
Summary of clinical liver cell transplantation procedures for liver-based inborn errors of metabolism
| Indication | n | Patient age | Cell amount (% liver cell mass) | Follow-up | References |
| Familial hypercho-lesterolemia | 5 | 7-41 yr | Partial reduction of LDL (3/5 patients) | 69 | |
| Donor hepatocytes detected by ISH at 4 mo | |||||
| CN disease type I | 1 | 10 yr | 7.5 × 109 (5%) | Decrease of bilirubin levels up to 11 mo | 14 |
| Detection of UGT1A1 enzyme activity and of glucurono-conjugates in bile | |||||
| 1 | 9 yr | 7.5 × 109 (5%) | 50%-65% reduction of bilirubin up to 3 mo | 34 | |
| Donor hepatocytes not detected by short tandem repeat analysis at 40 d | |||||
| 2 | 18 mo/3 yr | ND | 50%/30% reduction of serum bilirubin over 7 mo/ND follow-up | 33 | |
| Donor hepatocytes detected in one case by short tandem repeat analysis at 8 mo | |||||
| Infantile refsum disease | 1 | 4 yr | 2 × 109 | Donor Y-chromosomes detected by PCR at 7 d | 20 |
| Inherited coagulation factor VII deficiency | 2 | 3 mo/2 yr | 1.1 × 109/2.2 × 109 (4%/3%) | Decrease in the factor VII requirements | 19 |
| PFIC 2 | 2 | ND | 0.3 × 109 | No improvement | 33 |
| Glycogen storage disease type Ia | 1 | 47 yr | 2 × 109(1%) | Fasting tolerance: up to 7 h | 18 |
| Increase of glycemia | |||||
| Improvement of diet | |||||
| G6Pase activity detected | |||||
| Urea cycle disease | 1 (OTC) | 5 yr | 1 × 109 | Improvement of ammonia levels | 70 |
| Detection of enzyme activity | |||||
| 1 (OTC) | 0 d | 10.5 × 109 | Transient metabolic improvement between 20 and 31 d of life | 71 | |
| 1 (OTC) | 1 d | 1.9 × 109 | Improvement of ammonia levels | 72 | |
| Increased urea synthesis | |||||
| 1 (OTC) | 14 mo | 2.4 × 109 (6%) | Improvement of psychomotor development and of ammonia levels | 16 | |
| Urea neo-synthesis | |||||
| 1 (ASL) | 3.5 yr | 4.7 × 109 (9%) | Improvement of psychomotor development and of ammonia levels | 17 | |
| Donor hepatocytes detected by FISH at 12 mo and by enzyme activity at 8 mo |
ASL: Arginino-succinate lyase; (F)ISH: (Fluorescent) in situ hybridization; LDL: Low density lipoproteins; ND: Not documented; OTC: Ornithine transcarbamylase; PCR: Polymerase chain reaction; PFIC: Progressive familial intrahepatic cholestasis.
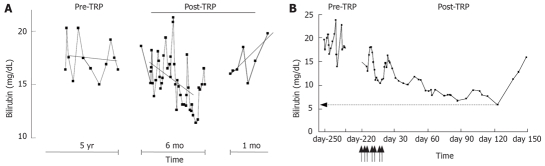
We performed LCT in two CN pediatric cases (Table 3, Figure 1). The first patient was a 9 year old girl in whom a port-a-cath was placed in the jejunal vein. She received 18 cell infusions from three different donors over a period of 5 mo for a total of 4% of her estimated liver cell mass. Mean cell viability was high (80%) and no adverse events were noticed during the procedure. Pre-transplant serum bilirubin values attained 17.5 ± 0.49 mg/dL (mean ± SD) and dropped after LCT to the lowest value of 11.4 mg/dL (mean ± SD: 13.6 ± 0.42 mg/dL, P < 0.001). After a period of 6 mo, bilirubin values increased suddenly without a concomitant event and the patient was scheduled for OLT. For the second patient, the protocol was revised in order to provide a higher amount of cells within a shorter infusion period. She was 1 year old at the time of the procedure and received 14 infusions from one single donor over 15 d to reach a total of 8.6% of her estimated liver cell mass. Cells were infused via a broviac catheter surgically inserted via a colonic vein to the spleno-mesaraïc confluent. Cell viability (mean 83%) and clinical tolerance were optimal. With pre-LCT levels of 17.6 ± 3.5 mg/dL (mean ± SD), the serum bilirubin dramatically decreased to values of 13.3 ± 2.4 mg/dL (mean ± SD) with the lowest value at 6 mg/dL. Skin jaundice reduced rapidly and the daily phototherapy schedule was alleviated from 10 to 8 h without any influence on the bilirubin levels. After 4 mo of progressive decrease of serum bilirubin, the values increased suddenly following an intercurrent Epstein-Barr virus (EBV) infection. The child underwent OLT without complications related to the previous LCT. Both patients received a methylprednisolone bolus and tacrolimus the day before and for 12 d after LCT. Subsequently they were given tacrolimus as long-term monotherapy.
Table 3.
Presentation of LCT procedures in two Crigler-Najjar disease type I patients
| Patient 1 | Patient 2 | |
| Age/Gender | 9 yr/Female | 1 yr/Female |
| Infusion procedure | Porth-a-cath in jejunal vein | Broviac in portal vein |
| Timing of infusions | 18 infusions/5 mo | 14 infusions/15 d |
| Donor cells | Fresh and cryopreserved from 3 donors | Fresh and cryopreserved from 1 donor |
| Cell amount | 6.1 billion | 2.6 billion |
| 0.16 billion/kg | 0.35 billion/kg | |
| % Liver cell mass | 4% | 8% |
| Mean viability | 80% | 83% |
Figure 1.
Evolution of serum bilirubin before and after LCT in two CN patients performed in our center. A: After fluctuating over a period of 5 yr, serum bilirubin of patient 1 decreased significantly to the lesser value of 11.4 mg/dL in 6 mo. Subsequently, increasing values were observed and the patient was listed for OLT. B: For patient 2, after cell infusions, the serum bilirubin dramatically decreased to the value of 6 mg/dL in 4 mo. At this time, concomitantly to an EBV infection, higher values were observed and the patient underwent OLT. Arrows indicate the timing of cell infusions. TRP: Transplantation.
PERSPECTIVES
At present, LCT remains limited by incomplete and time-limited metabolic control, mainly due to unfavorable immunological cell interactions, impaired donor cell quality and poor repopulation rates. Whereas the immunogenicity of liver cells is quite different compared to whole liver[35], the same immunosuppression protocols are applied for LCT and OLT. Additional fundamental in vivo studies are necessary for the development of the optimal immunosuppression protocol. In that way, Wu et al recently compared the effects of tacrolimus, rapamycin and mycophenolate mofetil on the engraftment and proliferation of engrafted liver cells in a allogeneic setting[36]. They observed a deleterious effect of rapamycin on the proliferation of the transplanted cells. Serrano et al reported the lack of toxicity of tacrolimus and methylprednisolone on human hepatocytes in vitro[37]. Other experimental protocols were designed to reduce the immunological pressure occurring in LCT procedures. For example, Mashalova et al obtained similar engraftment levels with syngeneic or allogeneic hepatocytes after their transduction with adenoviral early region 3 genes, suggesting a protective effect against rejection[38]. This was related to the down-expression of Fas receptor at the cell surface leading to inhibition of Fas-mediated apoptosis. Protocols combining LCT with bone marrow transplantation with[39] or without[40] elimination of natural killer cells are being investigated. Liver cell encapsulation aiming to protect cells from the immune system has demonstrated promising results in Gunn rats[41–43]. The technique is reversible and allows delivery of the cells to extrahepatic sites that are easy to access for sampling. However, major remaining hurdles are the creation of an adequate ‘intracapsular’ microenvironment allowing long-term cell functionality and the restriction of this technique to an enzyme-delivery role. Host immunity can be modulated by co-transplantation of immunomodulatory cells, as developed by Le Blanc et al, using mesenchymal stem cells to control graft versus host disease in the bone marrow transplant setting[44,45]. These cells and others, as non-parenchymal cells[46] or liver-derived mesenchymal lineages[47,48], could provide permissive factors or a microenvironment allowing more favorable immunological cell interactions, although this has not been tested so far in LCT protocols. Study of inner mechanisms of cell rejection may also lead to improved clinical efficiency of LCT. For example, it has been shown recently that human hepatocytes exert a procoagulant activity depending on tissue factor expression[49], as previously demonstrated with pancreatic islet cells[50,51]. In this work, Stéphenne et al demonstrated the improvement of the procoagulant activity by incubating the cells with N-acetylcysteine, making this drug valuable for additional in vivo studies.
Enhancement of liver cell engraftment capacity is another challenge. Engraftment depends on liver cell quality and host liver environment. While LCT is highly dependent on banking of cryopreserved cells, this procedure has been demonstrated to deteriorate cell quality. Indeed, although cryopreserved/thawed hepatocytes have been shown to possess in vivo clonal replicative potential identical to freshly isolated cells[52], their in vivo potential seems to be restricted in time[53–55] and their in vitro functionality remains lower than that of freshly isolated hepatocytes[56]. Furthermore, we recently demonstrated that, with the current protocols, cryopreservation/thawing of hepatocytes induces cell alteration and especially mitochondrial defects (complex 1 impairment)[57]. Intracellular ice formation remains the major factor affecting the quality of cells. Protection delivered by non-permeating cryoprotectants must be further analyzed in terms of cell death and mitochondrial functions. New perspectives, such as vitrification, to avoid the crystalline state, coupled or not with encapsulation, must be validated in the future while considering the problem of hepatocyte de-differentiation at long term that could occur in this type of configuration.
Actions on the liver microenvironment have been evaluated in a recent report using monocrotaline, which is an alkaloid showing toxicity against liver endothelial and Kupffer cells[58]. Authors reported an enhanced liver cell engraftment in a syngeneic background mainly related to endothelial cell damage. Comparable studies were performed on dipeptidyl peptidase IV-/-F344 rats using doxorubicin, irinotecan, or vincristine[59]. In this study, Kim et al showed improved cell engraftment after doxorubicin treatment attributed to endothelial cell disruption. While interesting, these approaches will not be applicable in a clinical setting. Physical alteration of the liver architecture was studied by Dagher et al on nonhuman primates using partial portal vein ligation or embolization in an autologous LCT procedure[60]. The authors reported hepatic regeneration rates up to 10% obtained at short term (15 d) after embolization of the portal vein. Others have successfully used chemicals as vascular endothelial growth factor delivered in situ[61] or by peripheral route[62] to promote cell engraftment.
As stem cells were recently described to have a hepatocyte differentiation potential[63,64], these are currently considered with growing interest for liver cell therapy. The most potent candidates are mesenchymal stem cells isolated from various tissues, with predilection for bone marrow[65] and umbilical cord[66]. Liver progenitor cells[67] or mesenchymal-like cells[47,48] also deserve detailed attention. However, stem cells only display partial hepatocyte-like functionality[64,68] and further advance is necessary to consider such cell types for therapy.
CONCLUSION
While LCT seems currently efficient and safe to improve the quality of life of CN diseased patients for a medium period of time, the technique still requires development to be considered for longer term or curative purposes. Advances must be focused on the quality of cell preparations together with the management of immunological barriers hampering reliable cell engraftment. Furthermore, other research areas, such as gene or stem cell therapy, are currently encountering exciting expansion, and combined therapeutic approaches would be justified in the near future.
Peer reviewers: Jian Wu, MD, PhD, Internal Medicine/Transplant Research Program, University of California, Davis Medical Center, Sacramento, CA 95817, United States; Dr. J Michael Millis, Department of Surgery, University of Chicago, Chicago 60637, United States; Roger Williams, Professor, The Institute of Hepatology, 69-75 Chenies Mews, London, WC1E 6HX, United Kingdom
S- Editor Liu JN L- Editor Lutze M E- Editor Yin DH
References
- 1.Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem. 1994;269:17960–17964. [PubMed] [Google Scholar]
- 2.Kaufman SS, Wood RP, Shaw BW Jr, Markin RS, Rosenthal P, Gridelli B, Vanderhoof JA. Orthotopic liver transplantation for type I Crigler-Najjar syndrome. Hepatology. 1986;6:1259–1262. doi: 10.1002/hep.1840060606. [DOI] [PubMed] [Google Scholar]
- 3.Sokal EM, Silva ES, Hermans D, Reding R, de Ville de Goyet J, Buts JP, Otte JB. Orthotopic liver transplantation for Crigler-Najjar type I disease in six children. Transplantation. 1995;60:1095–1098. doi: 10.1097/00007890-199511270-00006. [DOI] [PubMed] [Google Scholar]
- 4.Evans HM, Kelly DA, McKiernan PJ, Hubscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 5.Heaton N. Small-for-size liver syndrome after auxiliary and split liver transplantation: donor selection. Liver Transpl. 2003;9:S26–S28. doi: 10.1053/jlts.2003.50197. [DOI] [PubMed] [Google Scholar]
- 6.Bellodi-Privato M, Aubert D, Pichard V, Myara A, Trivin F, Ferry N. Successful gene therapy of the Gunn rat by in vivo neonatal hepatic gene transfer using murine oncoretroviral vectors. Hepatology. 2005;42:431–438. doi: 10.1002/hep.20794. [DOI] [PubMed] [Google Scholar]
- 7.van der Wegen P, Louwen R, Imam AM, Buijs-Offerman RM, Sinaasappel M, Grosveld F, Scholte BJ. Successful treatment of UGT1A1 deficiency in a rat model of Crigler-Najjar disease by intravenous administration of a liver-specific lentiviral vector. Mol Ther. 2006;13:374–381. doi: 10.1016/j.ymthe.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Seppen J, Bakker C, de Jong B, Kunne C, van den Oever K, Vandenberghe K, de Waart R, Twisk J, Bosma P. Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther. 2006;13:1085–1092. doi: 10.1016/j.ymthe.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Seppen J, van Til NP, van der Rijt R, Hiralall JK, Kunne C, Elferink RP. Immune response to lentiviral bilirubin UDP-glucuronosyltransferase gene transfer in fetal and neonatal rats. Gene Ther. 2006;13:672–677. doi: 10.1038/sj.gt.3302681. [DOI] [PubMed] [Google Scholar]
- 10.Seppen J, Tada K, Ottenhoff R, Sengupta K, Chowdhury NR, Chowdhury JR, Bosma PJ, Oude Elferink RP. Transplantation of Gunn rats with autologous fibroblasts expressing bilirubin UDP-glucuronosyltransferase: correction of genetic deficiency and tumor formation. Hum Gene Ther. 1997;8:27–36. doi: 10.1089/hum.1997.8.1-27. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TH, Birraux J, Wildhaber B, Myara A, Trivin F, Le Coultre C, Trono D, Chardot C. Ex vivo lentivirus transduction and immediate transplantation of uncultured hepatocytes for treating hyperbilirubinemic Gunn rat. Transplantation. 2006;82:794–803. doi: 10.1097/01.tp.0000234675.56598.35. [DOI] [PubMed] [Google Scholar]
- 12.Galbraith RA, Drummond GS, Kappas A. Suppression of bilirubin production in the Crigler-Najjar type I syndrome: studies with the heme oxygenase inhibitor tin-mesoporphyrin. Pediatrics. 1992;89:175–182. [PubMed] [Google Scholar]
- 13.Kren BT, Parashar B, Bandyopadhyay P, Chowdhury NR, Chowdhury JR, Steer CJ. Correction of the UDP-glucuronosyltransferase gene defect in the gunn rat model of crigler-najjar syndrome type I with a chimeric oligonucleotide. Proc Natl Acad Sci USA. 1999;96:10349–10354. doi: 10.1073/pnas.96.18.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 15.Najimi M, Sokal E. Liver cell transplantation. Minerva Pediatr. 2005;57:243–257. [PubMed] [Google Scholar]
- 16.Stephenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058–2061. doi: 10.1111/j.1600-6143.2005.00935.x. [DOI] [PubMed] [Google Scholar]
- 17.Stephenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 19.Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812–1814. doi: 10.1097/01.tp.0000146386.77076.47. [DOI] [PubMed] [Google Scholar]
- 20.Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- 21.Cobourn CS, Makowka L, Falk JA, Falk RE. Allogeneic intrasplenic hepatocyte transplantation in the Gunn rat using cyclosporine A immunosuppression. Transplant Proc. 1987;19:1002–1003. [PubMed] [Google Scholar]
- 22.Dixit V, Darvasi R, Arthur M, Brezina M, Lewin K, Gitnick G. Restoration of liver function in Gunn rats without immunosuppression using transplanted microencapsulated hepatocytes. Hepatology. 1990;12:1342–1349. doi: 10.1002/hep.1840120615. [DOI] [PubMed] [Google Scholar]
- 23.te Velde AA, Bosman DK, Oldenburg J, Sala M, Maas MA, Chamuleau RA. Three different hepatocyte transplantation techniques for enzyme deficiency disease and acute hepatic failure. Artif Organs. 1992;16:522–526. doi: 10.1111/j.1525-1594.1992.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Miescher-Clemens E, Drugas G, Lee SM, Colombani P. Intrahepatic hepatocyte transplantation following subtotal hepatectomy in the recipient: a possible model in the treatment of hepatic enzyme deficiency. J Pediatr Surg. 1992;27:312–315; discussion 315-316. doi: 10.1016/0022-3468(92)90853-y. [DOI] [PubMed] [Google Scholar]
- 25.Holzman MD, Rozga J, Neuzil DF, Griffin D, Moscioni AD, Demetriou AA. Selective intraportal hepatocyte transplantation in analbuminemic and Gunn rats. Transplantation. 1993;55:1213–1219. doi: 10.1097/00007890-199306000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Albani AP, Campanati L, Arosio E, Gatti S, Gridelli B, Orsenigo R, Grizzi F, Doglia M, Fassati LR, Galmarini D. Hepatocyte injection in Gunn rats' thymus and spleen. Transplant Proc. 1994;26:3443–3445. [PubMed] [Google Scholar]
- 27.Kokudo N, Otsu I, Okazaki T, Takahashi S, Sanjo K, Adachi Y, Makino S, Nozawa M. Long-term effects of intrasplenically transplanted adult hepatocytes and fetal liver in hyperbilirubinemic Gunn rats. Transpl Int. 1995;8:262–267. doi: 10.1007/BF00346878. [DOI] [PubMed] [Google Scholar]
- 28.Ilan Y, Roy-Chowdhury N, Prakash R, Jona V, Attavar P, Guha C, Tada K, Roy-Chowdhury J. Massive repopulation of rat liver by transplantation of hepatocytes into specific lobes of the liver and ligation of portal vein branches to other lobes. Transplantation. 1997;64:8–13. doi: 10.1097/00007890-199707150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, Roy-Chowdhury N, Vikram B, Roy-Chowdhury J. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354–362. doi: 10.1053/jhep.2002.34516. [DOI] [PubMed] [Google Scholar]
- 30.Kim BH, Han YS, Dong SH, Kim HJ, Chang YW, Lee JI, Chang R. Temporary amelioration of bilirubin conjugation defect in Gunn rats by transplanting conditionally immortalized hepatocytes. J Gastroenterol Hepatol. 2002;17:690–696. doi: 10.1046/j.1440-1746.2002.02744.x. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi M, Deb NJ, Kawashita Y, Lee SW, Furgueil J, Okuyama T, Roy-Chowdhury N, Vikram B, Roy-Chowdhury J, Guha C. A novel strategy for in vivo expansion of transplanted hepatocytes using preparative hepatic irradiation and FasL-induced hepatocellular apoptosis. Gene Ther. 2003;10:304–313. doi: 10.1038/sj.gt.3301909. [DOI] [PubMed] [Google Scholar]
- 32.Cubero FJ, Maganto P, Mula N, Ortiz A, Barrutia MG, Codesal FJ, Arahuetes RM. Hepatic proliferation in Gunn rats transplanted with hepatocytes: effect of retrorsine and tri-iodothyronine. Cell Prolif. 2005;38:137–146. doi: 10.1111/j.1365-2184.2005.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis. 2006;29:431–435. doi: 10.1007/s10545-006-0245-8. [DOI] [PubMed] [Google Scholar]
- 34.Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, Caenazzo L, Basso S, Carraro P, Valente ML, et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005;14:151–157. doi: 10.3727/000000005783983250. [DOI] [PubMed] [Google Scholar]
- 35.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu YM, Joseph B, Gupta S. Immunosuppression using the mTOR inhibition mechanism affects replacement of rat liver with transplanted cells. Hepatology. 2006;44:410–419. doi: 10.1002/hep.21277. [DOI] [PubMed] [Google Scholar]
- 37.Serrano T, Mitry RR, Terry C, Lehec SC, Dhawan A, Hughes RD. The effects of immunosuppressive agents on the function of human hepatocytes in vitro. Cell Transplant. 2006;15:777–783. doi: 10.3727/000000006783981530. [DOI] [PubMed] [Google Scholar]
- 38.Mashalova EV, Guha C, Roy-Chowdhury N, Liu L, Fox IJ, Roy-Chowdhury J, Horwitz MS. Prevention of hepatocyte allograft rejection in rats by transferring adenoviral early region 3 genes into donor cells. Hepatology. 2007;45:755–766. doi: 10.1002/hep.21525. [DOI] [PubMed] [Google Scholar]
- 39.Wesolowska A, Olszewski WL, Durlik M. Transplantation of hepatocytes: elimination of recipient natural killer cells with irradiation and bone marrow reconstitution prevent early graft dysfunction. Transplant Proc. 2003;35:2358–2360. doi: 10.1016/s0041-1345(03)00780-2. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida N, Kawahara T, Futagawa S. Induction of donor-specific tolerance to allogeneic hepatocytes by allogeneic bone marrow transplantation. Hepatol Res. 2003;26:148–153. doi: 10.1016/s1386-6346(02)00326-1. [DOI] [PubMed] [Google Scholar]
- 41.Dixit V, Darvasi R, Arthur M, Lewin K, Gitnick G. Cryopreserved microencapsulated hepatocytes--transplantation studies in Gunn rats. Transplantation. 1993;55:616–622. doi: 10.1097/00007890-199303000-00028. [DOI] [PubMed] [Google Scholar]
- 42.Gomez N, Balladur P, Calmus Y, Baudrimont M, Honiger J, Delelo R, Myara A, Crema E, Trivin F, Capeau J, et al. Evidence for survival and metabolic activity of encapsulated xenogeneic hepatocytes transplanted without immunosuppression in Gunn rats. Transplantation. 1997;63:1718–1723. doi: 10.1097/00007890-199706270-00003. [DOI] [PubMed] [Google Scholar]
- 43.Liu ZC, Chang TM. Coencapsulation of hepatocytes and bone marrow stem cells: in vitro conversion of ammonia and in vivo lowering of bilirubin in hyperbilirubemia Gunn rats. Int J Artif Organs. 2003;26:491–497. doi: 10.1177/039139880302600607. [DOI] [PubMed] [Google Scholar]
- 44.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 45.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 46.Ding XM, Xue WJ, Ji ZZ, Tian PX. Infusion of donor hepatic non-parenchymal cells prolongs survival of skin allografts in mice: role of microchimerism and IL-4. Hepatobiliary Pancreat Dis Int. 2007;6:34–37. [PubMed] [Google Scholar]
- 47.Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- 48.Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007;16:717–728. doi: 10.3727/000000007783465154. [DOI] [PubMed] [Google Scholar]
- 49.Stephenne X, Vosters O, Najimi M, Beuneu C, Dung KN, Wijns W, Goldman M, Sokal EM. Tissue factor-dependent procoagulant activity of isolated human hepatocytes: relevance to liver cell transplantation. Liver Transpl. 2007;13:599–606. doi: 10.1002/lt.21128. [DOI] [PubMed] [Google Scholar]
- 50.Beuneu C, Vosters O, Movahedi B, Remmelink M, Salmon I, Pipeleers D, Pradier O, Goldman M, Verhasselt V. Human pancreatic duct cells exert tissue factor-dependent procoagulant activity: relevance to islet transplantation. Diabetes. 2004;53:1407–1411. doi: 10.2337/diabetes.53.6.1407. [DOI] [PubMed] [Google Scholar]
- 51.Beuneu C, Vosters O, Ling Z, Pipeleers D, Pradier O, Goldman M, Verhasselt V. N-Acetylcysteine derivative inhibits procoagulant activity of human islet cells. Diabetologia. 2007;50:343–347. doi: 10.1007/s00125-006-0529-4. [DOI] [PubMed] [Google Scholar]
- 52.Jamal HZ, Weglarz TC, Sandgren EP. Cryopreserved mouse hepatocytes retain regenerative capacity in vivo. Gastroenterology. 2000;118:390–394. doi: 10.1016/s0016-5085(00)70221-6. [DOI] [PubMed] [Google Scholar]
- 53.David P, Alexandre E, Chenard-Neu MP, Audet M, Wolf P, Jaeck D, Azimzadeh A, Richert L. Engraftment and function of freshly isolated and cryopreserved Sprague Dawley rat hepatocytes after intrasplenic transplantation in analbuminemic rats. Transplant Proc. 2000;32:2796–2797. doi: 10.1016/s0041-1345(00)01890-x. [DOI] [PubMed] [Google Scholar]
- 54.David P, Alexandre E, Audet M, Chenard-Neu MP, Wolf P, Jaeck D, Azimzadeh A, Richert L. Engraftment and albumin production of intrasplenically transplanted rat hepatocytes (Sprague-Dawley), freshly isolated versus cryopreserved, into Nagase analbuminemic rats (NAR) Cell Transplant. 2001;10:67–80. [PubMed] [Google Scholar]
- 55.Fuller BJ, Lewin J, Sage L. Ultrastructural assessment of cryopreserved hepatocytes after prolonged ectopic transplantation. Transplantation. 1983;35:15–18. doi: 10.1097/00007890-198301000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Loven AD, Olsen AK, Friis C, Andersen B. Phase I and II metabolism and carbohydrate metabolism in cultured cryopreserved porcine hepatocytes. Chem Biol Interact. 2005;155:21–30. doi: 10.1016/j.cbi.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 57.Stephenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16:409–419. doi: 10.3727/000000007783464821. [DOI] [PubMed] [Google Scholar]
- 58.Joseph B, Kumaran V, Berishvili E, Bhargava KK, Palestro CJ, Gupta S. Monocrotaline promotes transplanted cell engraftment and advances liver repopulation in rats via liver conditioning. Hepatology. 2006;44:1411–1420. doi: 10.1002/hep.21416. [DOI] [PubMed] [Google Scholar]
- 59.Kim KS, Joseph B, Inada M, Gupta S. Regulation of hepatocyte engraftment and proliferation after cytotoxic drug-induced perturbation of the rat liver. Transplantation. 2005;80:653–659. doi: 10.1097/01.tp.0000173382.11916.bf. [DOI] [PubMed] [Google Scholar]
- 60.Dagher I, Boudechiche L, Branger J, Coulomb-Lhermine A, Parouchev A, Sentilhes L, Lin T, Groyer-Picard MT, Vons C, Hadchouel M, et al. Efficient hepatocyte engraftment in a nonhuman primate model after partial portal vein embolization. Transplantation. 2006;82:1067–1073. doi: 10.1097/01.tp.0000236103.99456.8f. [DOI] [PubMed] [Google Scholar]
- 61.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11:715–722. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 62.Shani-Peretz H, Tsiperson V, Shoshani G, Veitzman E, Neufeld G, Baruch Y. HVEGF165 increases survival of transplanted hepatocytes within portal radicles: suggested mechanism for early cell engraftment. Cell Transplant. 2005;14:49–57. doi: 10.3727/000000005783983331. [DOI] [PubMed] [Google Scholar]
- 63.Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M, et al. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006;45:144–159. doi: 10.1016/j.jhep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Lysy PA, Campard D, Smets F, Najimi M, Sokal EM. Stem cells for liver tissue repair: current knowledge and perspectives. World J Gastroenterol. 2008;14:864–875. doi: 10.3748/wjg.14.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lysy PA, Campard D, Smets F, Malaise J, Mourad M, Najimi M, Sokal EM. Persistence of a chimerical phenotype after hepatocyte differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2008;41:36–58. doi: 10.1111/j.1365-2184.2007.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Fiegel HC, Lange C, Kneser U, Lambrecht W, Zander AR, Rogiers X, Kluth D. Fetal and adult liver stem cells for liver regeneration and tissue engineering. J Cell Mol Med. 2006;10:577–587. doi: 10.1111/j.1582-4934.2006.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hengstler JG, Brulport M, Schormann W, Bauer A, Hermes M, Nussler AK, Fandrich F, Ruhnke M, Ungefroren H, Griffin L, et al. Generation of human hepatocytes by stem cell technology: definition of the hepatocyte. Expert Opin Drug Metab Toxicol. 2005;1:61–74. doi: 10.1517/17425255.1.1.61. [DOI] [PubMed] [Google Scholar]
- 69.Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ 3rd, Stein EA, Lupien PJ, Brewer HB Jr, Raper SE. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 70.Strom SC, Fisher RA, Rubinstein WS, Barranger JA, Towbin RB, Charron M, Mieles L, Pisarov LA, Dorko K, Thompson MT, et al. Transplantation of human hepatocytes. Transplant Proc. 1997;29:2103–2106. doi: 10.1016/s0041-1345(97)00252-2. [DOI] [PubMed] [Google Scholar]
- 71.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 72.Mitry RR, Dhawan A, Hughes RD, Bansal S, Lehec S, Terry C, Heaton ND-, Karani JB, Mieli-Vergani G, Rela M. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77:1614–1616. doi: 10.1097/01.tp.0000122224.98318.19. [DOI] [PubMed] [Google Scholar]