Abstract
AIM: To investigate the phenotype of inflammatory bowel disease associated with primary sclerosing cholangitis (PSC-IBD).
METHODS: Data from 75 PSC-IBD patients evaluated in our tertiary center between 1963 and 2006 were collected and compared to 150 IBD patients without PSC, matched for sex, birth date, IBD diagnosis date and initial disease location regarding ileal, different colonic segments, and rectum, respectively.
RESULTS: While PSC-IBD patients received more 5-aminosalicylates (8.7 years/patient vs 2.9 years/patient, P < 0.001), they required less immuno-suppressors (24% vs 46% at 10 years; P < 0.001) and less intestinal resection (10% vs 44% at 10 years, P < 0.001). The 25-year cumulative rate of colectomy was 25.1% in PSC-IBD and 37.3% in controls (P = 0.004). The 25-year cumulative rate of colorectal cancer was 23.4% in PSC-IBD vs 0% in controls (P = 0.002). PSC was the only independent risk factor for the development of colorectal cancer (OR = 10.8; 95% CI, 3.7-31.3). Overall survival rate without liver transplantation was reduced in PSC-IBD patients (67% vs 91% in controls at 25 years, P = 0.001).
CONCLUSION: This study confirms that patients with PSC-IBD have a particular disease phenotype independent of the initial disease location. Although their disease is less active and they use more 5-aminosalicylates, they present a higher risk of colorectal cancer.
Keywords: Primary sclerosing cholangitis, Inflammatory bowel disease, Colorectal cancer, Ulcerative colitis, Crohn’s disease
INTRODUCTION
The association of primary sclerosing cholangitis (PSC) with ulcerative colitis (UC) was first described by Smith and Loe in 1965[1]. The association with Crohn’s disease (CD) was suggested by Atkinson and Caroll in 1964[2], but was found less common than with UC. PSC may appear many years after proctocolectomy for colitis, and onset of IBD can be seen many years after liver transplantation for complicated PSC[3–7]. There is no evident correlation between the severity of UC and that of the associated PSC[4,5].
Backwash ileitis, rectal sparing and low disease activity seem to characterize IBD when associated to PSC[8,9]. These characteristics may be partly linked as a proximal colonic disease and is usually less symptomatic than a distal one. Another crucial point regarding colitis is the increased risk of colorectal carcinoma. The two major risk factors for this complication are long duration of disease and the extent of colitis. Recently, family history of sporadic colorectal cancer, active inflammation within the colonic mucosa and presence of PSC have been shown to increase the risk of colorectal cancer or dysplasia[10–14]. In a meta-analysis involving 116 studies[15], the cumulative probability of cancer in UC patients regardless of disease extent or PSC association was 18% at 30 years. In a study from Broomé et al[16], the cumulative risk of colorectal neoplasia (cancer or dysplasia) after 25 years of disease duration was 50% in the PSC-IBD group and 10% in the group of patients with UC without PSC. Nevertheless, conflicting results have been published[17–20], but these studies had different sample size, end points and comparison groups. Moreover, patients with PSC may be misdiagnosed for IBD due to usual quiescent colitis in this sub-group of patients.
The patients with PSC associated UC are more likely to have extensive disease or pancolitis than those without PSC[8]. Moreover, the rectum seems to be less involved by inflammatory lesions[8]. Thus, comparing the colorectal cancer risk of PSC-UC patients with that of all coming UC patients without PSC may be debatable. Moreover, CD can also be associated with PSC, and there is no reason to restrain this comparison exclusively to UC patients. Besides the disease duration, the extent of disease is the most important matching criteria for comparing the colorectal cancer risk of PSC-IBD patients to IBD patients without PSC.
The aim of this study was to describe the clinical setting and outcome, in particular the risk of neoplasia, of a cohort of PSC associated IBD patients and to compare them to a group of IBD patients without PSC matched for disease location and extent at diagnosis. The patients were not matched for the type of IBD (i.e. CD or UC) because we considered that PSC-IBD patients represent a third IBD phenotype which have to be regarded without presumption[8].
MATERIAL AND METHODS
Patients
Patients in this study were obtained from the MICISTA Registry, a tertiary clinical database of all IBD patients evaluated by the same staff of physicians at Rothschild Hospital (1974-2002) and then at Saint-Antoine Hospital (since 2003 to present time). The registry was built during the year 1994, with data before 1994 collected retrospectively. Data were collected prospectively in patients entering the database after 1994. There were 51 patients with additional diagnosis of PSC in the MICISTA registry (n = 51 out of 5274, 0.97%). In addition, PSC-IBD patients followed in the Hepatology Department of the Saint-Antoine Hospital in the same period were also included (n = 24). In all patients, diagnosis of PSC was defined by the following criteria: persistently elevated serum gamma glutamyl transferase (GGT) or alkaline phosphatase levels for at least 3 mo, characteristic radiographic appearance of sclerosing cholangitis and/or histologic features consistent with PSC on liver biopsy and absence of conditions associated with secondary sclerosing cholangitis[21]. The diagnosis of CD, UC, indeterminate colitis (IC) or unclassified IBD (IBDu) were based on Lennard-Jones criteria and Montreal classification[22,23].
Matching
Controls were chosen randomly within the MICISTA registry to match to the identified cases (2 controls for one PSC case). In order to focus the analysis on colorectal cancer risk, we chose the following matching criteria: gender, birth year (± 2.5 years), IBD diagnosis calendar year (± 2.5 years) and initial disease location. Digestive tract was divided into the following seven segments: upper gastrointestinal tract, jejunum, ileum, caecum and ascending colon, transverse and descending colon, sigmoid colon, and rectum. Controls were matched to case considering each gastrointestinal tract segment.
Data collection
Data concerning demographic information, IBD clinical settings (duration, initial and cumulative extent, year-by-year disease activity (assessed prospectively over one year according to a score taking into account flares: score 0 to 3, hospitalisations: score 4, excision surgery: score 5) from 1995 to 2005, extra intestinal lesions, medical and surgical treatment), morphological examination results (endoscopy, radiology), histopathology findings (digestive and liver biopsies) and outcome information (including colorectal neoplasia, colectomy, PSC complications and death) were abstracted from medical files. Colorectal neoplasia included flat low or high grade dysplasia and adenocarcinoma. Low and high grade dysplasia occurring in non-flat lesion was not taken into consideration.
Statistical analysis
Comparisons between the two groups were made using the Student’s t test or Mann Whitney test when appropriate. A P value under 0.05 was considered significant. The cumulative risks for colorectal neoplasia, CRC, treatment by immunosuppressors, ileal or colonic surgery and colectomy were calculated using Kaplan-Meier survival curves. The calculation of the cumulative risk for colorectal neoplasia and CRC were done after censoring observations at the time of proctocolectomy or death or at the end of follow up. Risk factors for colorectal neoplasia and CRC were established on the entire population of the study (composed by the two groups). Seventeen variables were tested by univariate analysis using the log rank test. Those variables with P values below 0.20 were further tested in a logistic multivariate regression model using a backward stepwise procedure (Cox model). In addition, two therapeutic variables assessing a prolonged treatment with aminosalicylates and immunosuppressors (treatment of more than two years) was entered in the model. The final step retained independent factors with P values below 0.05 in a 2-tailed test. Results are given as odds ratios (OR) ± 95% confidence intervals (CI).
RESULTS
Characteristics of the cases and controls
Seventy five IBD patients with concomitant PSC were identified and were matched to 150 IBD patients without PSC. Demographic and clinical characteristics of the two groups are described in Table 1. The mean follow up was longer in the PSC-IBD group. Rectal sparing and ileal involvement were observed in 20% and 19% of PSC-IBD patients (cumulative topography). Systemic manifestations were equally reported in the two groups. The final IBD diagnosis differs between the two groups. Final diagnosis of CD was made more often in the control group than in the PSC-IBD group (P = 0.05). Although UC was finally diagnosed in the same proportion in the two groups, the proportion of IBD unclassified was significantly higher in the PSC-IBD group. PSC-IBD patients were significantly less likely to be current smokers (whether before or after IBD diagnosis) than controls without PSC.
Table 1.
Demographical and clinical characteristics of the two groups
| PSC-IBD (n = 75) | IBD without PSC (n = 150) | P | ||||
| Male (%) | 53.3 | 53.3 | 1 | |||
| Age at IBD diagnosis (yr) (mean + SD) | 27.8 (14) | 28.5 (14) | 0.75 | |||
| Age at PSC diagnosis (yr) (mean + SD) | 35.8 (15.6) | NA | ||||
| IBD Follow up (mo) (mean + SD) | 172 (133) | 132 (123) | 0.025 | |||
| Initial/Cumulative IBD location (%) | ||||||
| Pancolitis | 41 (55.4) | 49 (66.2) | 74 (49.3) | 91 (60.7) | 0.39 | 0.65 |
| Rectal sparing | 18 (24.3) | 15 (20.3) | 30 (20) | 20 (13.3) | 0.46 | 0.18 |
| Sigmoid colon involvement | 64 (86.5) | 68 (91.9) | 132 (88.6) | 138 (92.6) | 0.65 | 0.85 |
| Left colon involvement | 60 (81.1) | 68 (91.9) | 113 (75.8) | 130 (87.2) | 0.38 | 0.3 |
| Right colon involvement | 55 (74.3) | 61 (82.4) | 93 (62.4) | 109 (72.7) | 0.076 | 0.13 |
| Ileum involvement | 9 (12) | 14 (18.7) | 19 (12.7) | 36 (24) | 0.88 | 0.36 |
| Jejunum involvement | 0 | 1 (1.3) | 0 | 3 (2) | 1 | 0.59 |
| Upper gastrointestinal tract involvement | 1 (1.3) | 1 (1.3) | 4 (2.7) | 8 (5.3) | 0.46 | 0.14 |
| Extra-intestinal manifestations (%) | ||||||
| Articular | 16 (21.3) | 34 (22.7) | 0.82 | |||
| Oral aphtae | 3 (4) | 4 (2.7) | 0.58 | |||
| Skin | 8 (10.7) | 7 (4.7) | 0.09 | |||
| Muscular | 0 | 1 (0.7) | 0.67 | |||
| Ophthalmological | 0 | 4 (2.7) | 0.19 | |||
| At least one extra intestinal lesion | 22 (29.3) | 39 (26) | 0.59 | |||
| Initial/Final diagnosis (%) | ||||||
| CD | 19 (25.3) | 21 (28) | 43 (28.7) | 62 (41.3) | 0.6 | 0.051 |
| UC | 40 (53.3) | 42 (56) | 94 (62.7) | 80 (53.3) | 0.18 | 0.7 |
| IC | 1 (1.3) | 1 (1.3) | 1 (0.7) | 3 (2) | 0.61 | 0.72 |
| IBDu | 15 (20) | 11 (14.7) | 12 (8) | 5 (3.3) | 0.009 | 0.002 |
| Smoking At IBD diagnosis/After IBD diagnosis | 4 (5.3) | 6 (8) | 45 (30) | 49 (32.7) | < 0.001 | < 0.001 |
| Colorectal neoplasia (%) | ||||||
| Dysplasia | 2 (2.7) | 1 (0.7) | 0.2 | |||
| Carcinoma | 8 (10.7) | 1 (0.7) | < 0.001 | |||
| Appendectomy (%) | 9 (12.7) | 30 (20.3) | 0.25 | |||
PSC-IBD: Inflammatory bowel disease associated with primary sclerosing cholangitis; CD: Crohn’s disease; UC: Ulcerative colitis; IC: Indeterminate colitis; IBDu: Unclassified IBD; NA: Not applicable.
Characteristics of patients with PSC and IBD are presented in Table 2. The mean time from onset of IBD to discovery of associated PSC was 8 ± 15.6 years. 94.7% of PSC-IBD patients received ursodeoxycholic acid (UDCA), and the mean delay between PSC diagnosis and UDCA treatment was 13.5 mo. Thirteen patients underwent orthotopic liver transplantation (17.3%).
Table 2.
Clinical features and outcomes of PSC-IBD patients
| Clinical features | n = 75 | |
| Age at PSC diagnosis (yr) (SD) | 35.8 (15.6) | |
| Age at IBD diagnosis (yr) (SD) | 27.8 (14) | |
| Disease topography | Intrahepatic | 42% |
| Intra- and extrahepatic | 55.10% | |
| Extrahepatic | 2.90% | |
| Morphologic examinations | ERCP | 50.70% |
| MRCP | 77.30% | |
| Endoscopic ultrasound | 17.30% | |
| Liver biopsy | 81.30% | |
| Delay between diagnosis and UDCA treatment (mo) (SD) | 13.5 (38.8) | |
| Complications | Acute cholangitis | 15.10% |
| Cholangiocarcinoma | 2.80% | |
| Cirrhosis | 20.80% | |
| Ascites | 9.70% | |
| Gastrointestinal bleeding | 5.70% | |
| Non UDCA treatments | Corticosteroid | 12.70% |
| immunosuppressors | 7% | |
| Liver transplantation | 17.30% | |
| Death | 8.50% | |
PSC-IBD: Inflammatory bowel disease associated with primary sclerosing cholangitis; UDCA: Ursodeoxycholic acid; ERCP: Endoscopic retrograde cholangiopancreatography; MRCP: Magnetic resonance cholangiopancreatography.
IBD severity
IBD year-by-year activity: In controls, IBD was active during 351 out of 739 patient-years (47.5%) and hospitalization was required in 90 patient-years (12.2%), whereas PSC-IBD patients exhibited active IBD in 143 out of 529 patient-years (27%; P < 0.001) and hospitalization in 30 patient-years (5.7%; P < 0.001).
Medical treatment: PSC-IBD patients received significantly more 5-ASA (8.5 years/patient vs 3.0 years/patient in controls, P < 0.0001). In the PSC-IBD group, 48.6% of the patients required systemic steroids or immunosuppressors, as compared to 85.3% in the control group (P < 0.001; OR = 0.15; 95%CI, 0.07-0.31). In fact, in the PSC-IBD group and in the control group, 21.6% and 38% of patients, respectively, had at least once enteral nutrition or systemic steroid therapy (P = 0.014; OR = 0.5; 95% CI, 0.2-0.9). Moreover, 27% of PSC-IBD patients vs 47.3% of controls received immunosuppressors (P = 0.004; OR = 0.4; 95%CI, 0.2-0.8). Finally, cumulative incidence of immunosuppressors use at 10 years after the diagnosis of IBD was 24% in the PSC-IBD group and 46% in the control group (P < 0.001, Figure 1A).
Figure 1.

Cumulative probability of colorectal cancer or dysplasia (A), cumulative incidence of immunosuppressors use (B), cumulative incidence of colonic or ileal surgical resection (C), from the IBD diagnosis date in cases (those with inflammatory bowel disease -associated with primary sclerosing cholangitis) vs controls (those with IBD without PSC).
Surgery requirement: A colonic or a small bowel surgical resection was performed more frequently in the control group than in the PSC-IBD group (36.7% vs 17.3%; P = 0.003). The cumulative incidence was also lower. At 10 years, 10% of patients from the PSC-IBD group and 44% from the control group underwent surgical resection (P < 0.001, Figure 1B). This difference between the two groups was essentially due to colonic resection difference as cumulative incidence of small bowel resection was not different between the two groups (log rank test: P = 0.14), whereas cumulative incidence of colonic resection showed major differences (log rank test: P = 0.0004). Cumulative incidence of colectomy was significantly lower in the PSC-IBD group than in the control group (25.1% vs 37.3% at 25 years; P = 0.004). The most common indication of colectomy was failure of medical treatment in both groups (55.9% in the control group and 62.5% in the PSC-IBD group; P = 0.7). In the PSC-IBD group, the second indication was colorectal neoplasia (37.5%). In the control group the second and third indications were fulminant colitis (38.2%) and colorectal neoplasia (5.9%), respectively.
Overall survival: Six deaths were observed in the PSC-IBD group and five in the control group. Global actuarial survival rate at 25 years was similar in the two groups: 91.2% in the control group and 88.4% in the PSC-IBD group (P = 0.4). Nevertheless, when censoring observation at liver transplantation, the survival was shorter in PSC-IBD group with a 25-year survival rate of 91.2% in control group and 67.6% in PSC-IBD group (P < 0.001, Figure 2). In the PSC-IBD group, causes of death were most of the time (5/6) related to chronic liver disease complications including cholangiocarcinoma (2 cases), hepatocellular carcinoma (1 case), decom-pensated cirrhosis (1 case), and immediate post transplantation complication in one case. Death was of cardiac origin in the last case. In the control group, causes of death were never related to IBD and were due to tuberculosis, lung cancer, ischemic cardiac events and septic shock.
Figure 2.
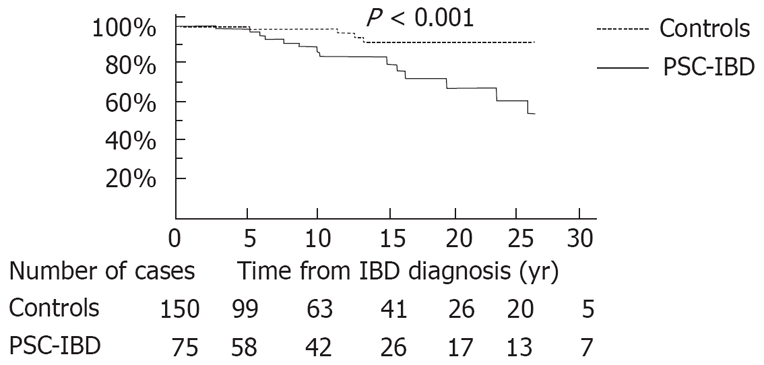
Overall survival from IBD diagnosis date in cases (those with inflammatory bowel disease associated with primary sclerosing cholangitis) vs controls (those with chronic ulcerative colitis). Follow up was censored for those who underwent orthotopic liver transplantation.
Colorectal neoplasia risk
Colorectal dysplasia or cancer risk: Crude incidence of colorectal neoplasia (dysplasia and cancer) was higher in the PSC-IBD group (n = 10, 13.3%) than in controls (n = 2, 1.3%). The cumulative rate of colorectal neoplasia was also higher in PSC-IBD group (25.6% vs 1.5% in control group at 25 years; P = 0.004; Figure 2). When restricted to cancer only, results were similar with colorectal cancer observed in 10.7% of patients in the PSC-IBD group versus 0.7% in the control group, and cumulative rates of colorectal cancer of 23.4 and 0% at 25 years in cases and controls, respectively (P = 0.002). The mean IBD duration at cancer diagnosis was 209 mo ± 93 mo in PSC-IBD group, whereas the only case of colorectal cancer in control group was diagnosed 534 mo after the IBD onset. Nevertheless, duration of the colitis in PSC-IBD patients may be underestimated due to the mild course of the disease.
UDCA exposition were 78.3 ± 23.7 mo and 71.2 ± 8.2 mo in PSC-IBD patients with and without neoplasia, respectively (P = 0.75). When taking into account only carcinoma, UDCA exposition were 74.6 ± 25.9 mo and 71.8 ± 8.1 mo in PSC-IBD patients with and without colorectal cancer, respectively (P = 0.91).
None of the patients with colorectal cancer had a familial history of colorectal cancer. Among the 8 colorectal cancers diagnosed in PSC-IBD group (6 PSC-UC and 2 PSC-CD patients), 7 had neither nodal involvement nor metastasis and were treated by proctocolectomy. The remaining patient had a nodal invasion and was treated with adjuvant chemotherapy (5FU and folinic acid). For one patient who underwent a proctocolectomy for uncontrolled active UC, colon cancer was unexpectedly diagnosed on histological examination of the colon. The only case of cancer diagnosed in the control group was localized (T4N0M0) and required colectomy with no adjuvant treatment. The 8 colorectal cancers in PSC-IBD group were located in the right colon, whereas the only case of colorectal cancer in the control group was located in the left colon. Among the 8 cases of colorectal cancer in the PSC-IBD group, 3 patients underwent orthotopic liver transplantation (2 liver transplantations after colorectal cancer diagnosis). Until the end of follow up, no death related to colon cancer was observed.
Risk factor of neoplasia and colorectal cancer: The univariate analyses for the risk of colorectal neoplasia and colorectal cancer were established on the entire population of the study and the variables tested are described in Table 3. Five variables were further tested in the multivariate regression model: initial sigmoid colon involvement, extraintestinal manifestations, association with PSC, treatment with 5-ASA for more than two years, and treatment with immunosuppressors for more than two years. After analysis, the only independent variable associated with colorectal neoplasia was association with PSC (OR = 6.9; 95% CI, 3.2-14.9). Similarly, PSC was the only independent variable associated with colorectal cancer (OR = 10.8; 95% CI, 3.7-31.3).
Table 3.
Univariate analysis for the risk of colorectal neoplasia n (%)
| Neoplasia | No neoplasia | Log rank | |
| (n = 12) | (n = 213) | P | |
| Male gender | 8 (66.7) | 112 (52.6) | > 0.20 |
| Age at IBD diagnosis below 16 yr | 3 (25) | 52 (24.4) | > 0.20 |
| Age at IBD diagnosis below 40 yr | 9 (75) | 165 (77.5) | > 0.20 |
| Initial diagnosis of CD | 2 (16.7) | 60 (28.2) | > 0.20 |
| IBD familial history | 1 (8.3) | 27 (12.7) | > 0.20 |
| Initial IBD location | |||
| Ileum involvement | 1 (8.3) | 27 (12.7) | > 0.20 |
| Right colon involvement | 9 (75) | 139 (65.3) | > 0.20 |
| Left colon involvement | 9 (75) | 164 (77) | > 0.20 |
| Sigmoid colon involvement | 12 (100) | 184 (86.4) | 0.12 |
| Rectum involvement | 10 (83.3) | 166 (77.9) | > 0.20 |
| Anoperineal involvement | 0 | 21 (9.9) | > 0.20 |
| 5 ASA during more than 2 yr | 6 (54.6) | 79 (42.5) | > 0.20 |
| Immunosuppressant during more than 2 yr | 3 (25) | 69 (32.4) | > 0.20 |
| Appendectomy before IBD onset | 2 (16.7) | 37 (17.4) | > 0.20 |
| Current smoking | 2 (16.7) | 53 (24.9) | > 0.20 |
| Extra-intestinal manifestations | 2 (16.7) | 61 (28.6) | 0.09 |
| PSC | 10 (83.3) | 65 (30.5) | 0.004 |
IBD: Inflammatory bowel disease associated; PSC: Primary sclerosing cholangitis; CD: Crohn’s disease.
DISCUSSION
In the present study, PSC-IBD patients had a less active IBD than patients without PSC, and PSC was the only independent risk factor for CRC (Odds Ratio 10.8). Our study was performed in a large cohort and displays a methodological design to address the role of PSC as a risk factor of colorectal cancer in IBD. This case-control study involves 75 PSC-IBD patients. Each case was matched to two IBD controls without PSC. Initial disease location accounts for a matching criterion. This matching procedure was chosen to limit confounding factors which could bias colorectal neoplasia risk evaluation. It is, therefore, noticeable that the cumulative disease location was not statistically different between the two groups. In a study from the Mayo Clinic published in 2005[8], a trend to an increase of colorectal neoplasia was observed, but the two groups compared had different rates of pancolitis : 87% in the PSC-IBD group vs 54% in the control group (P < 0.001). Thus, in this study, the trend observed could be due to the difference in colitis extent. In our study, this bias has been avoided by the matching procedure. Moreover, in the study of Loftus et al[8], matching criteria included the year of first visit at the Mayo clinic ± 10 years and the IBD duration before the first visit at the Mayo clinic ± 5 years, meaning that two matched patients could have a difference of 15 years regarding the IBD diagnosis date. In our study, this difference was at maximum of 5 years. Finally, our study is the only one to use the birth date as a matching criterion.
The increased risk of colorectal cancer in PSC-IBD patients has been discussed in the literature. Although some studies did not retrieve an excessive risk[17–20], many recent studies are in agreement with our results[14–16]. The fact that 33% of patients in the study by definition had PSC could hold back others factors. Nevertheless, our multivariate analysis showed that PSC remained an independent factor. In our study, cumulative incidences of colorectal neoplasia and cancer in the control group were low compared to recent data of the literature. In the study of Rutter et al focusing on ulcerative colitis[24], the cumulative incidences of colorectal neoplasia and cancer were higher than in our control group (7.7% and 2.5% vs 1.5% and 0% at 20 years). Furthermore, in our IBD group without PSC, CD was finally diagnosed in 40% of the cases and cumulative incidence of colectomy was higher than in the study of Rutter et al[24] (22.2% vs 9.5% at 20 years). High colectomy rates could play a protective role regarding colonic neoplasia. Indeed, in a middle 1980’s study on UC[25], with high colectomy rate (31% at 18 years), the cumulative incidence of colorectal cancer was closer to our results (1.4% at 18 years). More recently, in a population based cohort study from Copenhagen county[26], the overall cumulative probability for development of CRC in UC patients was 1.1% at 20 years with an overall cumulative colectomy probability after 20 years of 27.9%.
To assess IBD activity in the two groups, we took into account the number of active patient-years and the requirement of medical or surgical treatment. Our results pointed out a less active disease in PSC-IBD patients. Ten years after IBD diagnosis, 46% of the patients from the control group had required immunosuppressors (versus only 24% in the PSC-IBD group). The lesser severity of PSC associated IBD was also reflected by a lower surgery rate. Moreover, in the control group colectomy was indicated in 94.1% for uncontrolled active disease (with more than one third of severe acute colitis), whereas in PSC-IBD group, colectomy was performed for this indication in only 62.5% of cases (with no case of severe acute colitis). Five-aminosalicylates might prevent colorectal cancer in IBD[27] and difference between the two study groups regarding the 5-ASA consumption could have skew our conclusions on colorectal neoplasia and cancer risk. In fact, PSC-IBD patients received more 5-ASA than controls and despite larger use of 5-ASA, this assumed protective factor did not decrease the risk of colorectal neoplasia in this group. The larger use of 5-ASA in PSC-IBD patients can be explained by their weak IBD activity. While patients with PSC-IBD have often a quiescent IBD course and carry on their 5-ASA treatment lengthily, IBD patients without PSC can have a more active disease requiring immunosuppressors. Until recently, 5-ASA was not known to have a preventive effect on colorectal cancer risk in IBD[27], and patients in this situation stopped their 5-ASA treatment.
The reasons of the increased risk of colorectal neoplasia in PSC-IBD patients are still not well understood. The role of endogenous bile acids has been hypothesized, but remains speculative. The high frequency of colorectal cancer and of several other cancers in PSC patients suggest that other factors are involved in the carcinogenesis[28,29]. Moreover, two recent studies have suggested that UDCA treatment decrease the colorectal neoplasia risk in PSC-IBD patients[30,31]. As a consequence, it may be hypothesized that our PSC-IBD patients could have presented a higher rate of colorectal neoplasia in the absence of UDCA treatment.
In our patients with PSC, as in other series[8], the most frequent presentation was pancolitis. On the contrary, rectal sparing and backwash ileitis were retrieved in only 20% and 19%, respectively, whereas it was seen more often in several studies (52% and 51%, respectively, in the recent study by Loftus et al[8]). In our study, current smoker status was less frequent in PSC-IBD group than in control group. This has already been described and is probably emphasized by a strong association between PSC and UC compared to CD[32,33], and by the protective effect of smoking regarding the onset of UC and PSC[19,34–36].
In our study, most of the deaths in PSC-IBD patients were related to complications of a chronic liver disease. In both groups, death was directly linked neither to an IBD complication nor to a colorectal neoplasia or cancer.
In conclusion, this study confirms that PSC-IBD patients have a higher risk of colorectal neoplasia and cancer than IBD patients without PSC, despite a lower disease activity and a larger use of 5-ASA. It demonstrates that this higher risk is unrelated to disease location or extent. It suggests that a factor directly linked to PSC may increase the risk of neoplasia. Our finding strongly support that these patients require a close colorectal neoplasia tracking by endoscopy, and may be by other methods.
COMMENTS
Background
Inflammatory bowel disease associated with primary sclerosing cholangitis (PSC-IBD) may have a distinct IBD phenotype, with a low activity and a high risk of colorectal cancer. However, lower activity may be due to more proximal disease location and rectal sparing, and the high risk of colorectal cancer to the high frequency of pancolitis.
Research frontiers
IBD patients with colonic involvement have an increase risk of colorectal neoplasia. Some studies suggest that PSC-IBD patients might have an increased risk of colorectal neoplasia compared to patients with IBD alone. However, no previous studies comparing colorectal cancer risk between PSC-IBD and IBD patients and using rigorous matching criteria are available.
Innovations and breakthroughs
In this study, we confirmed that patients with PSC-IBD have a particular disease phenotype, with a high rate of pancolitis and a lower disease activity compared to IBD matched controls without PSC. Despite a weaker disease activity and a higher use of 5-aminosalicylates, we showed that PSC-IBD patients have a higher risk of colorectal cancer. Contrariwise to previous studies, we used a matching procedure taking into account the disease extent and the diagnosis date. Thus we avoided these major biases.
Applications
This study confirms that PSC-IBD patients have a higher risk of colorectal neoplasia and cancer than IBD patients without PSC, despite a lower disease activity and a larger use of 5-aminosalicylates. It demonstrates that this higher risk is unrelated to disease location or extent. It suggests that a factor directly linked to PSC may increase the risk of neoplasia. These results strongly support that PSC-IBD patients require a close colorectal neoplasia tracking by endoscopy, and may be by other methods.
Peer review
The paper by Sokol and co-workers investigated disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. This paper is interesting and it has clearly stated aims, the sample size and the overall designs of the study are fair, the results adequate to provide experimental evidence and to support valid conclusions. The authors conclusions support previous literature about increased risk of colon cancer in PSC-IBD patients, compared to patients with PSC alone.
Peer reviewer: Andrew Ukleja, MD, Assistant Professor, Clinical Assistant Professor of Medicine, Director of Nutrition Support Team, Director of Esophageal Motility Laboratory, Cleveland Clinic Florida, Department of Gastroenterology, 2950 Cleveland Clinic Blvd., Weston, FL 33331, United States
S- Editor Zhong XY L- Editor Rippe RA E- Editor Lin YP
References
- 1.Smith MP, Loe RH. Sclerosing Cholangitis; Review Of Recent Case Reports And Associated Diseases And Four New Cases. Am J Surg. 1965;110:239–246. doi: 10.1016/0002-9610(65)90018-8. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson AJ, Carroll WW. Sclerosing Cholangitis. Association With Regional Enteritis. Jama. 1964;188:183–184. [PubMed] [Google Scholar]
- 3.Aadland E, Schrumpf E, Fausa O, Elgjo K, Heilo A, Aakhus T, Gjone E. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol. 1987;22:655–664. doi: 10.3109/00365528709011139. [DOI] [PubMed] [Google Scholar]
- 4.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31–39. doi: 10.1055/s-2008-1040420. [DOI] [PubMed] [Google Scholar]
- 6.Riley TR, Schoen RE, Lee RG, Rakela J. A case series of transplant recipients who despite immunosuppression developed inflammatory bowel disease. Am J Gastroenterol. 1997;92:279–282. [PubMed] [Google Scholar]
- 7.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 8.Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moayyeri A, Daryani NE, Bahrami H, Haghpanah B, Nayyer-Habibi A, Sadatsafavi M. Clinical course of ulcerative colitis in patients with and without primary sclerosing cholangitis. J Gastroenterol Hepatol. 2005;20:366–370. doi: 10.1111/j.1440-1746.2005.03727.x. [DOI] [PubMed] [Google Scholar]
- 10.Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, Kowdley KV, Stevens AC, Crispin DA, Emond M, et al. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331–338. doi: 10.1053/gast.1996.v110.pm8566577. [DOI] [PubMed] [Google Scholar]
- 11.Broome U, Lindberg G, Lofberg R. Primary sclerosing cholangitis in ulcerative colitis--a risk factor for the development of dysplasia and DNA aneuploidy? Gastroenterology. 1992;102:1877–1880. doi: 10.1016/0016-5085(92)90308-l. [DOI] [PubMed] [Google Scholar]
- 12.D'Haens GR, Lashner BA, Hanauer SB. Pericholangitis and sclerosing cholangitis are risk factors for dysplasia and cancer in ulcerative colitis. Am J Gastroenterol. 1993;88:1174–1178. [PubMed] [Google Scholar]
- 13.Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522–525. doi: 10.1136/gut.41.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leidenius MH, Farkkila MA, Karkkainen P, Taskinen EI, Kellokumpu IH, Hockerstedt KA. Colorectal dysplasia and carcinoma in patients with ulcerative colitis and primary sclerosing cholangitis. Scand J Gastroenterol. 1997;32:706–711. doi: 10.3109/00365529708996522. [DOI] [PubMed] [Google Scholar]
- 15.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–1408. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 17.Choi PM, Nugent FW, Rossi RL. Relationship between colorectal neoplasia and primary sclerosing cholangitis in ulcerative colitis. Gastroenterology. 1992;103:1707–1709. doi: 10.1016/0016-5085(92)91211-l. [DOI] [PubMed] [Google Scholar]
- 18.Gurbuz AK, Giardiello FM, Bayless TM. Colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Dis Colon Rectum. 1995;38:37–41. doi: 10.1007/BF02053855. [DOI] [PubMed] [Google Scholar]
- 19.Loftus EV Jr, Sandborn WJ, Tremaine WJ, Mahoney DW, Zinsmeister AR, Offord KP, Melton LJ 3rd. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis. Gastroenterology. 1996;110:432–440. doi: 10.1053/gast.1996.v110.pm8566590. [DOI] [PubMed] [Google Scholar]
- 20.Nuako KW, Ahlquist DA, Sandborn WJ, Mahoney DW, Siems DM, Zinsmeister AR. Primary sclerosing cholangitis and colorectal carcinoma in patients with chronic ulcerative colitis: a case-control study. Cancer. 1998;82:822–826. doi: 10.1002/(sici)1097-0142(19980301)82:5<822::aid-cncr4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16-19. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 24.Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Hendriksen C, Kreiner S, Binder V. Long term prognosis in ulcerative colitis--based on results from a regional patient group from the county of Copenhagen. Gut. 1985;26:158–163. doi: 10.1136/gut.26.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 27.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 28.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzen H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 29.Stiehl A. Primary sclerosing cholangitis: neoplastic potential in bile ducts, colon and the pancreas? J Hepatol. 2002;36:433–434. doi: 10.1016/s0168-8278(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 30.Pardi DS, Loftus EV Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 31.Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, Brentnall TA. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 32.Olsson R, Danielsson A, Jarnerot G, Lindstrom E, Loof L, Rolny P, Ryden BO, Tysk C, Wallerstedt S. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319–1323. [PubMed] [Google Scholar]
- 33.Rasmussen HH, Fallingborg JF, Mortensen PB, Vyberg M, Tage-Jensen U, Rasmussen SN. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn's disease. Scand J Gastroenterol. 1997;32:604–610. doi: 10.3109/00365529709025107. [DOI] [PubMed] [Google Scholar]
- 34.Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96:2113–2116. doi: 10.1111/j.1572-0241.2001.03944.x. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell SA, Thyssen M, Orchard TR, Jewell DP, Fleming KA, Chapman RW. Cigarette smoking, appendectomy, and tonsillectomy as risk factors for the development of primary sclerosing cholangitis: a case control study. Gut. 2002;51:567–573. doi: 10.1136/gut.51.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Erpecum KJ, Smits SJ, van de Meeberg PC, Linn FH, Wolfhagen FH, vanBerge-Henegouwen GP, Algra A. Risk of primary sclerosing cholangitis is associated with nonsmoking behavior. Gastroenterology. 1996;110:1503–1506. doi: 10.1053/gast.1996.v110.pm8613056. [DOI] [PubMed] [Google Scholar]


