Abstract
AIM: To study the influence and mechanisms of dexamethasone on mesenteric lymph node of rats with severe acute pancreatitis (SAP).
METHODS: The SAP rats were assigned to model, treated or sham-operated groups. The mortality, pathological changes of mesenteric lymph nodes, expression levels of NF-κB, P-selectin, Bax, Bcl-2 and caspase-3 protein and changes in apoptotic indexes in lymph nodes were observed at 3, 6 and 12 h after operation. The blood levels of endotoxin, superoxide dismutase (SOD), malondialdehyde (MDA), and endothelin-1 (ET-1) in blood were determined.
RESULTS: SOD content, expression of Bax protein and apoptotic index were significantly higher in the treated group than in the model group at different time points (P < 0.05 or P < 0.01). Other blood-detecting indexes and histopathological scores of mesenteric lymph nodes were lower in the treated than in the model group (P < 0.05, P < 0.01 or P < 0.01). NF-κB protein expression was negative in all groups. Comparing P-selectin and caspase-3 expression levels among all three groups, there was no marked difference between the model and treated group.
CONCLUSION: Dexamethasone can protect mesenteric lymph nodes. The mechanism may be by reducing the content of inflammatory mediators in the blood and inducing lymphocyte apoptosis.
Keywords: Apoptosis, Dexamethasone, Lymph node, Rats, Severe acute pancreatitis, Tissue microarrays
INTRODUCTION
Severe acute pancreatitis (SAP) with a high incidence of complications and high mortality has been a difficult disease in medical research for many years. Recent studies have confirmed that the release of manifold inflammatory mediators is an important part of the inflammatory cascade reaction. Apart from anti-inflammatory and anti-allergic activity, dexamethasone can improve microcirculation and inhibit enzyme and inflammatory mediators. Dexamethasone has sound therapeutic effects on SAP[1]. The mesenteric lymph nodes are very important for the protection of abdominal organs from infection, and perform an important role in maintaining immune balance.
In this experiment, the influence of dexamethasone on changes in inflammatory mediator levels in the blood, pathological changes of mesenteric lymph nodes, and on changes in expression level of NF-κB, P-selectin, Bax, Bcl-2 and caspase-3 proteins, as well as on apoptotic index, has been observed. This is to explore the protective mechanism of dexamethasone on SAP complicated with mesenteric lymph node injury. This study is believed to be the first to apply tissue microarrays for histopathological determination of mesenteric lymph node disease severity. With advantages such as time and energy saving and high efficiency, tissue microarrays can significantly improve pathological study efficiency.
MATERIALS AND METHODS
Materials
Clean grade, healthy male Sprague-Dawley (SD) rats weighing 250-300 g were purchased from the Experimental Animal Center of the Medical School, Zhejiang University. Sodium taurocholate and sodium pentobarbital were purchased from Sigma (USA). Dexamethasone (injection) was purchased from Zhejiang Xinchang Pharmaceutical Company (China). Malondialdehyde (MDA), superoxide dismutase (SOD) assay kits were purchased from Nanjing Jiancheng Bioengineering Research Institute (China), with calculation units of nmol/mL and U/mL, respectively. The serum Endothelin-1 ELA kit (ET-1) was purchased from Cayman Chemical Company (Catalog Number: 583151, USA) and the calculation unit for content was ng/L (or pg/mL). The NF-κB, Bax, Bcl-2 and P-selectin antibody were purchased from Santa Cruz (Santa Cruz, CA). Caspase-3 antibodies was purchased from NeoMarkers. The DNA in situ nick end-labeling (TUNEL) kit was purchased from Takara (Japan).
Animal grouping and rat SAP model preparation
Ninety clean grade, healthy male SD rats were prepared into the SAP models and randomly divided into the model and treated groups (45 rats each). Another 45 were selected to be the sham-operated group. Next, the above groups were randomly divided into 3, 6 and 12-h groups, with 15 rats in each group. The treated group was injected with dexamethasone via the tail vein: 0.5 mg/100 g body weight, single dose, 15 min after successful preparation of the SAP model. The sham operation consisted of abdominal opening, pancreas and duodenum turning over, and finally abdominal closure. The sham-operated and model groups were injected with saline via the tail vein 15 min after the operation. SAP preparation was as follows. The rats were anesthetized by intraperitoneal injection of 2% sodium pentobarbital (0.25 mL/100 g). In the model group, we identified the duodenal papilla inside the duodenum duct wall, and then used a No. 5 needle to drill a hole in the mesenterium avascular area. After inserting a segmental epidural catheter into the duodenum cavity via the hole, it was inserted into the biliary-pancreatic duct, in the direction of the papilla, in a retrograde position. This was followed by retrograde transfusion of 3.5% sodium taurocholate 0.1 mL/100 g by microinjection pump at the speed of 0.2 mL/min, and then the hole in the duodenum lateral wall was sutured[2–6].
Survival rate and pathological changes
Rat mortality was measured at 3, 6 and 12 h after operation, and survival was calculated. We observed the pathological changes in the mesenteric lymph nodes. After euthanasia with sodium pentobarbital, we collected mesenteric lymph node samples from the rats. The lymph node pathological score was determined according to a set of standards developed by Zhang (Table 1).
Table 1.
Pathological score standard of lymph node
| Score | Observation indexes |
| 1 | Follicle Germinal center dilated, lymphatic sinus dilated, sinus cell hyperplasia or only lymphatic sinus dilated, sinus cell hyperplasia |
| 2 | Follicle Germinal center dilated, lymphatic sinus dilated, sinus cell hyperplasia, spotty necrosis in mantle zone and Germinal center or only lymphatic sinus dilated, and sinus cell hyperplasia, infiltration of neutrophil, eosinophile granulocyte and plasmocyte |
| 3 | Follicle Germinal center dilated, lymphatic sinus dilated, sinus cell hyperplasia, spotty necrosis in mantle zone and Germinal center, infiltration of neutrophil, eosinophile granulocyte and plasmocyte |
Observation index
The levels of plasma endotoxin, and serum SOD, MDA and endothelin-1 (ET-1) were determined from blood taken from the heart. The above indexes were all detected according to the manufacturers’ instructions.
Tissue microarrays and staining
Prepared tissue microarrays of mesenteric lymph node and adopted SP (streptavidin-perosidase) method for immunohistochemical staining, observed the NF-κB, Bax and Bcl-2 protein expression respectively. Tunel Staining was carried out as follows[7]. NF-κB, P-selectin, Bax, Bcl-2 and Caspase-3 protein expression: We applied tissue microarrays to prepare microarray sections of mesenteric lymph node; Adopted SP (streptavidin peroxidase) method for immunohistochemical = staining. We observed protei expression in mesenteric lymph nodes under light microscope, as follows: (-) < 10% positive cells; (+) 10%-20% positive cells; (++) 20%-50% positive cells; and (+++) > 50% positive cells. We applied the tissue microarrays to prepare the microarray sections of mesenteric lymph node. TUNEL staining was used to visualize the number of apoptotic cells in the lymph node tissue microarrays, and the apoptotic index (%) was calculated.
Statistical analysis
Statistical analysis was conducted by using SPSS 11.5 software (SPSS, USA). The Kruskal-Wallis test or analysis of variance was applied for comparison of the three groups. The Bonfferoni test was also applied. P ≤ 0.05 was considered statistically significant.
RESULTS
Survival
In the model group, the mortality was 0, 0 and 13.33% (2/15) at 3, 6 and 12 h, respectively. The sham-operated and treated groups showed 100% survival, while there was no significant difference between the model and treated groups.
Comparison of serum MDA, SOD and ET-1 levels
Serum MDA was significantly higher in the model and treated groups than in the sham-operated group at all time points (P < 0.01). Serum MDA in the treated group was significantly lower than in the model group at 3 and 6 h (P < 0.01) (Table 2).
Table 2.
Comparison of different indexes level in blood (M (QR))
| Index |
Sham-operated group (h) |
Model group (h) |
Treated group (h) |
||||||
| 3 | 6 | 12 | 3 | 6 | 12 | 3 | 6 | 12 | |
| Endotoxin (EU/mL) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.04 (0.02) | 0.06 (0.03) | 0.06 (0.02) | 0.03 (0.01) | 0.04 (0.01) | 0.04 (0.02) |
| MDA (nmol/mL) | 9.9 (9.9) | 13.2 (6.6) | 13.2 (9.9) | 29.7 (6.6) | 33.0 (9.9) | 29.7 (14.9) | 19.8 (9.9) | 26.4 (13.2) | 26.4 (13.2) |
| SOD (U/mL) | 105.6 (8.3) | 103.6 (6.2) | 99.2 (16.2) | 76.6 (13.0) | 73.0 (24.5) | 77.6 (12.6) | 90.8 (13.4) | 91.9 (11.6) | 88.7 (13.7) |
| ET-1 (ng/L) | 14.05 (1.78) | 14.53 (2.082) | 14.78 (2.28) | 17.97 (5.57) | 19.21 (7.02) | 18.31 (5.06) | 13.64 (1.54) | 13.86 (2.64) | 13.66 (2.47) |
Serum SOD in the model and treated groups was significantly lower than in the sham-operated group at different time points (P < 0.01). Serum SOD in the treated group was significantly higher than in the model group at different time points (P < 0.01) (Table 2).
Serum ET-1 differed significantly between the treated and sham-operated groups at various time points (P < 0.05), and the level in the model group significantly higher than that in the sham-operated group at various time points (P < 0.01). Serum ET-1 in the treated group was significantly lower than that in the model group at 3 and 12 h (P < 0.01), and the level in the treated group was significantly lower than that in the model group at 6 h (P < 0.01) (Table 2).
Pathological changes in lymph nodes, seen by light microscopy
The mesenteric lymph nodes in the sham-operated group had normal morphology and structure. In the model and treated groups, swollen lymph nodes, dilated follicular germinal centers and expanded lymphatic sinuses were seen in most cases. Sinus cell hyperplasia and spotty necrosis in the mantle zone and germinal center of the lymph nodes were clearly visible, and neutrophil and plasmocyte infiltration was seen in a few cases. There were no marked differences in the pathological changes between the model and treated groups, but spotty necrosis in the mantle zone and germinal centers, as well as inflammatory cell infiltration only occurred in a few rats in the treated group.
Comparison of lymph node pathological scores
The pathological score in the model group was significantly higher than in the sham-operated group at different time points (6 h, P < 0.05, 3 and 12 h, P < 0.01). The score in the treated group was significantly higher than in the sham-operated group at 12 h (P < 0.01). The pathological score was significantly lower in the treated group than in the model group at 6 h (P < 0.05) (Table 3).
Table 3.
Comparison of different pathological indexes in the lymph node (M (QR) )
| Indexes |
Sham-operated group (h) |
Model group (h) |
Treated group (h) |
||||||
| 3 | 6 | 12 | 3 | 6 | 12 | 3 | 6 | 12 | |
| Pathological score | 2.0 (1.0) | 2.0 (1.0) | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (0.0) | 2.0 (0.0) | 2.0 (0.0) |
| P-selection | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Bax protein | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (1.0) | 0.0 (1.0) |
| Bcl-2 protein | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Caspase-3 protein | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (0.0) | 0.0 (1.0) | 0.0 (1.0) |
| Apoptotic index | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.03) | 0.0 (0.0) | 0.0 (0.3) | 0.0 (0.4) |
Changes in lymph node expression of NF-κB, P-selectin, Bax, Bcl-2 and caspase-3
Expression of NF-κB was negative in all groups at all times (Figure 1A). Lymphocytes were stained positively for P-selectin. There was no significant difference in staining in any of the groups. Lymphocytes were stained positively for Bax. Expression of Bax was significantly higher in the treated group than in the model group at 12 h (P < 0.01). There was no marked difference in the other groups (Tables 3 and 4, Figure 1B and C). ymphocytes were stained positively for Bcl-2. There was no significant difference among any of the groups at different time points (Tables 3 and 4, Figure 1D).
Figure 1.
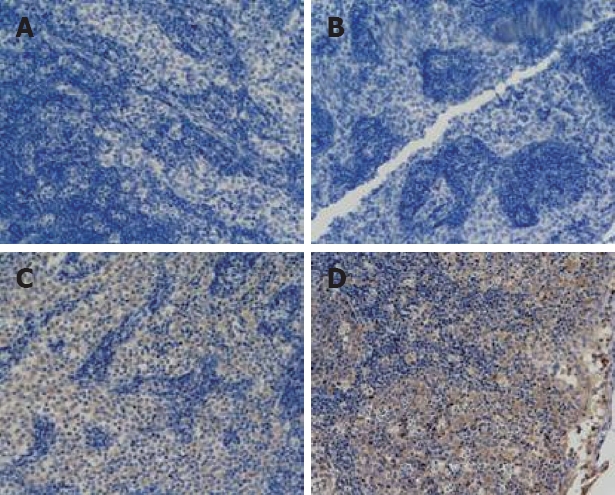
Tissue microarrays of mesenteric lymph nodes were prepared and stained for mmunohistochemistry (× 200). (A) NF-κB expression in the treated group (6 h); (B) Bax protein expression in the sham-operated group (3 h); (C) Bax protein expression in the treated group (12 h); (D) Bcl-2 protein expression in the treated group (3 h).
Table 4.
Expression of Bax, Bcl-2 and P-selectin caspase-3 pathologic grade
| Groups | Cases |
Bax |
Bcl-2 |
P-selectin |
Caspase-3 |
|||||||
| - | + | ++ | +++ | - | + | ++ | - | + | - | + | ||
| Sham-operated group-3 h | 15 | 12 | 3 | 15 | 15 | 12 | 3 | |||||
| Sham-operated group-6 h | 15 | 12 | 0 | 2 | 15 | 15 | 11 | 4 | ||||
| Sham-operated group-12 h | 15 | 13 | 1 | 1 | 15 | 15 | 12 | 3 | ||||
| Model group-3 h | 15 | 13 | 2 | 15 | 14 | 1 | 11 | 4 | ||||
| Model group-6 h | 15 | 13 | 1 | 1 | 15 | 12 | 3 | 11 | 4 | |||
| Model group-12 h | 13 | 13 | 13 | 12 | 1 | 9 | 4 | |||||
| Treated group-3 h | 15 | 12 | 3 | 12 | 1 | 2 | 15 | 12 | 3 | |||
| Treated group-6 h | 15 | 9 | 4 | 2 | 15 | 14 | 1 | 9 | 6 | |||
| Treated group-12 h | 15 | 8 | 5 | 1 | 1 | 15 | 14 | 1 | 5 | 10 | ||
Comparison of caspase-3 expression level of lymph node
Lymphocytes were stained positive for caspase-3. There was no significant difference between the sham-operated and model groups at all time points. Caspase-3 expression in the sham-operated group was significantly lower than in the treated group at 12 h (P < 0.05). There was no significant difference between the model and treated groups at all time-points (Tables 3 and 4).
Comparison of apoptotic index in lymph nodes
The apoptotic cells in lymph nodes were the lymphocytes. Apoptotic cells appeared in three rats in the sham-operated group at 6 h, with an apoptotic index between 10 and 20 per 10 000 cells. Apoptotic cells appeared in four rats in the model group at 12 h, with an apoptotic index between 6 and 50 per 10 000 cells. Apoptotic cells appeared in three, eight and nine rats at 3, 6 and 12 h, respectively, with an apoptotic index between 2 and 160 per 10 000 cells in treated group. There was no significant difference among any of the groups at 3 h. The apoptotic index was significantly higher in the treated group than in the model group at 6 h (P < 0.05). The apoptotic index was significantly higher in the treated group than in the sham-operated group at 12 h (P < 0.01) (Table 4 and Figure 2).
Figure 2.
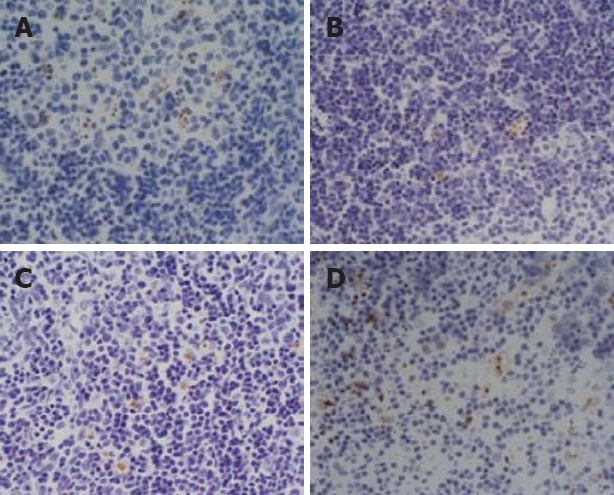
TUNEL staining, showing the pathological changes in the mesenteric lymph nodes (× 400). (A) In the sham-operated group (6 h), there were no apoptotic cells; (B) In the model group (12 h), several apoptotic cells appeared; (C) treated group (6 h); (D) apoptotic cells were increased in the treated group (12 h).
DISCUSSION
At present, the pathogenesis of SAP has not been fully elucidated[7,8]. We are attracted to the barrier function of mesenteric lymph nodes during the onset and progression of SAP[9]. The normal mesenteric lymph node barrier can effectively prevent intestinal bacteria and endotoxin entering the body and maintain homeostasis[10–12], while the function of the lymphocytes in the mesenteric lymph nodes reflects the function of the whole intestinal immune barrier[13]. If the function of the immune barrier is injured, the mesenteric lymph nodes are damaged and permeability of mucous membrane of small intestine is increased[14], which reduces their capacity to phagocytose ectopic bacteria and endotoxin. The excessive release of inflammatory mediators during SAP is the main cause of mesenteric lymph node injury. According to the results of this experiment, after inducing SAP, the level of inflammatory mediators such as ET-1 and MDA in the blood was significantly higher in the treated group than in the sham-operated group, and the level of inflammatory mediators was positively correlated with the severity of mesenteric lymph node injury.
Endotoxin can invade the body and cause gut-origin endotoxemia, increase permeability of mesenteric lymph nodes, and promote invasion of intestinal bacteria and endotoxin. As one of the important common mediators in inflammatory mediator cascade reaction of inflammatory reaction[15–17], Nitric oxide (NO) can be regarded as an index of SAP. However, some researchers[18] believe that a small amount of endogenous NO can protect the body from ischemia-reperfusion injury, and prevent any increase in intestinal vasopermeability induced by endotoxemia and bacteria translocation.
NF-κB is a transcription factor that participates in the regulation of expression of inflammatory molecules. Its activation is a key initial step in the inflammatory reaction[19–21]. However, NF-κB was found to be negative in this experiment, which indicates that there is no direct relation between inflammatory reactions in the mesenteric lymph nodes and NF-κB. This study also showed that dexamethasone decreased the level of inflammatory mediators. It was demonstrated that dexamethasone exerted its anti-inflammatory effect by lowering the level of several inflammatory factors in the serum, inhibiting their production, and blocking the cascade reaction of the inflammatory mediators. P-Selectin is a member of the family of cell adhesion molecules. However, the content is very low and its expression is significantly increased with acute inflammation[22,23]. It is also an important indicator of inflammation[22,24]. In this study, there was no marked difference in P-selectin expression. Therefore, we suppose that P-selectin expression is not related to lymph node injury.
Apoptosis also plays an important role during the onset, progression and prognosis of AP. It has been found[25,26] that when necrosis and apoptosis coexist and necrosis prevails, inducing apoptosis results in body protection. Both necrosis and apoptosis are mechanisms of death for injured cells[27]. Unlike necrosis, apoptosis does not release harmful substances in lysosomes or cause intense inflammatory reactions[28]. The present study showed that expression of Bax protein was significantly higher in the treated group than in the model group at 12 h. The expression of Bcl-2 protein was reduced. Bcl-2 expression was positively correlated with apoptosis level of mesenteric lymph nodes, and negatively correlated with SAP severity. The expression of Bax and Bcl-2 proteins was not significant in the model group at any time, with less apoptosis, which indicates that dexamethasone can protect lymph nodes by increasing Bax protein, inhibiting Bcl-2 protein, and inducing apoptosis of mesenteric lymph nodes. The apoptotic index was significantly higher in the treated group than in the model group, while the pathological score was significantly lower in the treated group than in the model group, which indicates that dexamethasone can promote the apoptosis of mesenteric lymph nodes and protect the lymph nodes. The massive apoptosis of lymph node cells certainly inhibits excessive inflammation, and protects several organs from SAP-related lymph node injury. Caspase-3 is one of the important proteases that can induce apoptosis, and is also the final effector in the caspase cascade effect, which is involved in apoptosis. Moreover, it has a pivotal position in the process of stopping the protease cascade. Caspase-3 is a marker of apoptosis and it is also involved in the process. It can destroy a variety of protease complexes in cells with the digestive way, activate intranuclear nuclease to cause DNA schizolysis, form DNA fragments, undermine cell calcium pump function, and lead to intracellular calcium overload[29,30]. Inhibiting caspase-3 activity can reduce the occurrence of apoptosis[31]. In this study, there was no marked difference in caspase-3 expression level in the model and treated groups; therefore, we suppose that caspase-3 expression is not related to severity of lymph node injury.
Nuñez et al[32] found that apoptosis occurs with high concentrations of NO. Increasing NO in serum can alleviate the inflammatory reaction of AP. Apoptosis during SAP is the result of complex action of various factors whose relationships form a network structure. Since 1952, when Stephensen et al[33] first reported the therapeutic effect of glucocor-ticoids on AP, many empirical studies have confirmed that glucocorticoids can improve survival of animals with pancreatitis[33–35], although the precise mechanism is unclear. As a long-acting glucocorticoid, dexamethasone can be applied extensively in a clinical setting. It can regulate inflammatory mediators[36,37], improve microcirculation[38], eliminate OFR (oxygen free radical)[39], and inhibit NF-κB[40,41]. There have now been several studies on the mechanism of dexamethasone in SAP, but there has still been no study on the effect of dexamethasone on mesenteric lymph nodes. We are of the opinion that a few apoptotic lymphocytes in lymph nodes mean that the function of lymphocytes has been slightly injured. However, the significance of lymphocyte apoptosis is that its immunological function is severely inhibited, which can have a protective effect.
Since it was first reported by Kononen et al[42] in 1998, tissue microarray has been extensively used[43–45], but there have been no reports on its application in the study of pancreatitis. In this experiment, the diameter of microarray tissue was only 2 mm. Super sensitive SP immunohistochemistry and TUNEL technique were used. The observation indexes of this experiment are satisfactory. This study demonstrates that tissue arrays of 2.0 mm diameter have advantages such as reliability, savings in time, energy and reagents, and convenient control[46,47]. This study provides a new theoretical basis for the application of tissue microarray in the pathological examination of AP.
COMMENTS
Background
The normal mesenteric lymph node barrier can effectively prevent intestinal bacteria and endotoxin entering the human body and maintain homeostasis. The function of lymphocytes in the mesenteric lymph nodes reflects the function of the whole intestinal immune barrier. If the function of the immune barrier is injured, the mesenteric lymph nodes will be damaged, which reduces the capacity for phagocytosis of ectopic bacteria and endotoxin. The excessive release of inflammatory mediators during SAP is the main cause of mesenteric lymph node injury.
Research frontiers
SAP has a high incidence of complications and high mortality, and has been a difficult disease in medical research for many years. Recent studies have confirmed that the release of many inflammatory mediators is an important reason for the inflammatory cascade reaction. Apart from anti-inflammatory and anti-allergic activity, dexamethasone can improve microcirculation and inhibit enzymes and inflammatory mediators. Dexamethasone has sound therapeutic effects on SAP. In this study, the influence of dexamethasone on changes in content of inflammatory mediators in blood, pathological changes of mesenteric lymph nodes, and changes of expression of NF-κB, P-selectin, Bax, Bcl-2 and caspase-3 proteins, as well as apoptotic index, was investigated, to explore the protective mechanism of dexamethasone on SAP complicated with injury of mesenteric lymph nodes.
Innovations and breakthroughs
This study applied tissue microarrays for histopathological examination of mesenteric lymph nodes, which is believed to be the first report of the pathological category for severity of lymph nodes around the world.
Applications
With advantages such as time and energy savings and high efficiency, tissue microarrays can significantly improve study efficiency. We applied tissue microarrays to pathological detection and obtained good results.
Terminology
NF-κB is a transcription factor that participates in regulating expression of inflammatory molecules. Its activation is a key initial step in the inflammatory reaction. NF-κB can regulate the expression of inflammatory mediators.
Peer review
This is a very interesting study. The authors studied the influences and mechanisms of dexamethasone on mesenteric lymph nodes in rats with SAP. It is believed to be the first report of the pathological classification of severity of lymph node injury around the world.
Supported by Technological Foundation Project of Traditional Chinese Medicine Science of Zhejiang Province, No. 2003C130 and No. 2004C142; Foundation Project for Medical Science and Technology of Zhejiang Province, No. 2003B134; Grave Foundation Project for Technological and Development of Hangzhou, No. 2003123B19; Intensive Foundation Project for Technology of Hangzhou; No. 2004Z006; Foundation Project for Medical Science and Technology of Hangzhou; No. 2003A004; and Foundation Project for Technology of Hangzhou, No. 2005224
Peer reviewers: James H Grendell, Professor of Medicine, Chief, Division of Gastroenterology, Hepatology & Nutrition, Winthrop University Hospital, 222 Station Plaza N. #429, Mineola, New York 11501, United States; Yoshiharu Motoo, MD, PhD, FACP, FACG, Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Li DL L- Editor Kerr C E- Editor Lin YP
References
- 1.Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang JM, Cai W, Shen HP, Cai J. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:548–556. doi: 10.3748/wjg.v13.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XP, Chen L, Hu QF, Tian H, Xu RJ, Wang ZW, Wang KY, Cheng QH, Yan W, Li Y, et al. Effects of large dose of dexamethasone on inflammatory mediators and pancreatic cell apoptosis of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:5506–5511. doi: 10.3748/wjg.v13.i41.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang XP, Zhang L, Xu HM, Xu YP, Cheng QH, Wang JM, Shen HP. Application of tissue microarrays to study the influence of dexamethasone on NF-kappaB expression of pancreas in rat with severe acute pancreatitis. Dig Dis Sci. 2008;53:571–580. doi: 10.1007/s10620-007-9867-4. [DOI] [PubMed] [Google Scholar]
- 4.Xiping Z, Li C, Miao L, Hua T. Protecting effects of dexamethasone on thymus of rats with severe acute pancreatitis. Mediators Inflamm. 2007;2007:72361. doi: 10.1155/2007/72361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang XP, Zhang L, Wang Y, Cheng QH, Wang JM, Cai W, Shen HP, Cai J. Study of the protective effects of dexamethasone on multiple organ injury in rats with severe acute pancreatitis. JOP. 2007;8:400–412. [PubMed] [Google Scholar]
- 6.Zhang XP, Tian H, Lu B, Chen L, Xu RJ, Wang KY, Wang ZW, Cheng QH, Shen HP. Tissue microarrays in pathological examination of apoptotic acinar cells induced by dexamethasone in the pancreas of rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:527–536. [PubMed] [Google Scholar]
- 7.Lankisch PG, Weber-Dany B, Doobe C, Finger T, Maisonneuve P, Lowenfels AB, Keim V. Pankrin: a new parameter for the diagnosis of acute pancreatitis in cases of late clinical presentation. Pancreas. 2006;32:330–331. doi: 10.1097/01.mpa.0000218317.89706.6e. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto M, Takada T, Yasuda H. A new experimental pancreatitis by incomplete closed duodenal loop: the influence of pancreatic microcirculation on the development and progression of induced severe pancreatitis in rats. Pancreas. 2004;28:e112–e119. doi: 10.1097/00006676-200405000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Gianotti L, Braga M, Alexander JW. [The intestine: a central organ in the pathogenesis of septic complications in acute pancreatitis] Chir Ital. 1995;47:14–24. [PubMed] [Google Scholar]
- 10.Garside P, Millington O, Smith KM. The anatomy of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:9–15. doi: 10.1196/annals.1309.002. [DOI] [PubMed] [Google Scholar]
- 11.Harari Y, Weisbrodt NW, Moody FG. Ileal mucosal response to bacterial toxin challenge. J Trauma. 2000;49:306–313. doi: 10.1097/00005373-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Kiyono H, Kweon MN, Hiroi T, Takahashi I. The mucosal immune system: from specialized immune defense to inflammation and allergy. Acta Odontol Scand. 2001;59:145–153. doi: 10.1080/000163501750266738. [DOI] [PubMed] [Google Scholar]
- 13.Kiyono H, Kweon MN, Hiroi T, Takahashi I. The mucosal immune system: from specialized immune defense to inflammation and allergy. Acta Odontol Scand. 2001;59:145–153. doi: 10.1080/000163501750266738. [DOI] [PubMed] [Google Scholar]
- 14.Lou TJ, Li N, Gao JZ. Intestinal barrier function of rats injured by glucocorticoid. Zhonghua Qigian Yizhi Zazhi. 2005;26:610–611. [Google Scholar]
- 15.Uehara S, Gothoh K, Handa H, Tomita H, Tomita Y. Immune function in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:363–370. doi: 10.1046/j.1440-1746.2003.02979.x. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Cambronero L, Camps B, de La Asuncion JG, Cerda M, Pellin A, Pallardo FV, Calvete J, Sweiry JH, Mann GE, Vina J, et al. Pentoxifylline ameliorates cerulein-induced pancreatitis in rats: role of glutathione and nitric oxide. J Pharmacol Exp Ther. 2000;293:670–676. [PubMed] [Google Scholar]
- 17.Um SH, Kwon YD, Kim CD, Lee HS, Jeen YT, Chun HJ, Lee SW, Choi JH, Ryu HS, Hyun JH. The role of nitric oxide in experimental cerulein induced pancreatitis. J Korean Med Sci. 2003;18:520–526. doi: 10.3346/jkms.2003.18.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz HU, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736–2750. [PubMed] [Google Scholar]
- 19.Liu X, Nakano I, Yamaguchi H, Ito T, Goto M, Koyanagi S, Kinjoh M, Nawata H. Protective effect of nitric oxide on development of acute pancreatitis in rats. Dig Dis Sci. 1995;40:2162–2169. doi: 10.1007/BF02209000. [DOI] [PubMed] [Google Scholar]
- 20.Grisham MB. NF-kappaB activation in acute pancreatitis: protective, detrimental, or inconsequential? Gastroenterology. 1999;116:489–492. doi: 10.1016/s0016-5085(99)70148-4. [DOI] [PubMed] [Google Scholar]
- 21.Suk K, Yeou Kim S, Kim H. Regulation of IL-18 production by IFN gamma and PGE2 in mouse microglial cells: involvement of NF-kB pathway in the regulatory processes. Immunol Lett. 2001;77:79–85. doi: 10.1016/s0165-2478(01)00209-7. [DOI] [PubMed] [Google Scholar]
- 22.Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197–G1208. doi: 10.1152/ajpgi.2001.280.6.G1197. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg AH, Granger DN, Russell J, Sabek O, Henry J, Gaber L, Kotb M, Gaber AO. Quantitative measurement of P- and E-selectin adhesion molecules in acute pancreatitis: correlation with distant organ injury. Ann Surg. 2000;231:213–222. doi: 10.1097/00000658-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kameda H, Morita I, Handa M, Kaburaki J, Yoshida T, Mimori T, Murota S, Ikeda Y. Re-expression of functional P-selectin molecules on the endothelial cell surface by repeated stimulation with thrombin. Br J Haematol. 1997;97:348–355. doi: 10.1046/j.1365-2141.1997.522700.x. [DOI] [PubMed] [Google Scholar]
- 25.Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
- 26.Pei HH, Liu RL, Jiang CQ, Ma LL, Fang XY. Influence of emodin on pancreatitic acinar cell apoptosis in rats with acute pancreatitis. Bengbu Yixueyuan Xuebao. 2005;30:112–113. [Google Scholar]
- 27.Pei HH, Dai W, Zhou J. Effects of somatostatin on apoptosis of pancreatitic acinar cell apoptosis in acute necrotizing pancreatitis in rats. Guangdong Yixue Zazhi. 2004;25:138–140. [Google Scholar]
- 28.Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med. 2004;8:402–409. doi: 10.1111/j.1582-4934.2004.tb00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuilov VD, Oleskin AV, Lagunova EM. Programmed cell death. Biochemistry (Mosc) 2000;65:873–887. [PubMed] [Google Scholar]
- 30.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 31.Henaff M, Antoine S, Mercadier JJ, Coulombe A, Hatem SN. The voltage-independent B-type Ca2+ channel modulates apoptosis of cardiac myocytes. FASEB J. 2002;16:99–101. doi: 10.1096/fj.01-038fje. [DOI] [PubMed] [Google Scholar]
- 32.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 33.Stephenson HE Jr, Pfeffer RB, Saupol GM. Acute hemorrhagic pancreatitis; report of a case with cortisone treatment. AMA Arch Surg. 1952;65:307–308. [PubMed] [Google Scholar]
- 34.Kaneto H, Fujii J, Seo HG, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes. 1995;44:733–738. doi: 10.2337/diab.44.7.733. [DOI] [PubMed] [Google Scholar]
- 35.Schiller WR, Duprez A, Iams WB, Suwa M, Anderson MC. Experimental pancreatitis. Treatment by colloid replacement and adrenocorticosteroid therapy combined with thoracic duct drainage. Arch Surg. 1969;98:698–702. doi: 10.1001/archsurg.1969.01340120046004. [DOI] [PubMed] [Google Scholar]
- 36.Kimura T, Zuidema GD, Cameron JL. Steroid administration and acute pancreatitis: studies with an isolated, perfused canine pancreas. Surgery. 1979;85:520–524. [PubMed] [Google Scholar]
- 37.McKay CJ, Gallagher G, Brooks B, Imrie CW, Baxter JN. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg. 1996;83:919–923. doi: 10.1002/bjs.1800830712. [DOI] [PubMed] [Google Scholar]
- 38.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 39.Yue MX, Zhang GX, Li CL, Li XB, Zhang LC, Zhang SL, Xue L, Wang XM. The effect of anisodaminum and dexamethasone on microcirculation in rabbit with multiple organ dysfunction syndrome. Wei xuanhuan Zazhi. 1997;7:10–11. [Google Scholar]
- 40.Liu JS, Wei XG, Fu J, Liu Jin, Yuan YZ, Wu YL. Stady of the relationship among endothelin, nitric oxide, oxgen free radical and acute pancreatitis. Zhongguo Yishi Zazhi. 2003;5:28–29. [Google Scholar]
- 41.Meduri GU. New rationale for glucocorticoid treatment in septic shock. J Chemother. 1999;11:541–550. doi: 10.1179/joc.1999.11.6.541. [DOI] [PubMed] [Google Scholar]
- 42.Lanza L, Scudeletti M, Monaco E, Monetti M, Puppo F, Filaci G, Indiveri F. Possible differences in the mechanism(s) of action of different glucocorticoid hormone compounds. Ann N Y Acad Sci. 1999;876:193–197. doi: 10.1111/j.1749-6632.1999.tb07638.x. [DOI] [PubMed] [Google Scholar]
- 43.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Younes M, Frolov A, Wheeler TM, Scardino P, Ohori M, Ayala G. Expression of neutral amino acid transporter ASCT2 in human prostate. Anticancer Res. 2003;23:3413–3418. [PubMed] [Google Scholar]
- 45.Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC, Mihatsch MJ, Gelmann EP, Bubendorf L. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003;55:20–29. doi: 10.1002/pros.10209. [DOI] [PubMed] [Google Scholar]
- 46.Wulfing P, Diallo R, Muller C, Wulfing C, Poremba C, Heinecke A, Rody A, Greb RR, Bocker W, Kiesel L. Analysis of cyclooxygenase-2 expression in human breast cancer: high throughput tissue microarray analysis. J Cancer Res Clin Oncol. 2003;129:375–382. doi: 10.1007/s00432-003-0459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB. Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol. 2002;117:723–728. doi: 10.1309/PEF8-GL6F-YWMC-AG56. [DOI] [PubMed] [Google Scholar]


