Abstract
Alamethicin is a 20-residue, hydrophobic, helical peptide, which forms voltage-sensitive ion channels in lipid membranes. The helicogenic, nitroxyl amino acid TOAC was substituted isosterically for Aib at residue positions 1, 8, or 16 in a F50/5 alamethicin analog to enable EPR studies. Electron spin-echo envelope modulation (ESEEM) spectroscopy was used to investigate the water exposure of TOAC-alamethicin introduced into membranes of saturated or unsaturated diacyl phosphatidylcholines that were dispersed in D2O. Echo-detected EPR spectra were used to assess the degree of assembly of the peptide in the membrane, via the instantaneous diffusion from intermolecular spin-spin interactions. The profile of residue exposure to water differs between membranes of saturated and unsaturated lipids. In monounsaturated dioleoyl phosphatidylcholine, D2O-ESEEM intensities decrease from TOAC1 to TOAC8 and TOAC16 but not uniformly. This is consistent with a transmembrane orientation for the protoassembled state, in which TOAC16 is located in the bilayer leaflet opposite to that of TOAC1 and TOAC8. Relative to the monomer in fluid bilayers, assembled alamethicin is disposed asymmetrically about the bilayer midplane. In saturated dimyristoyl phosphatidylcholine, the D2O-ESEEM intensity is greatest for TOAC8, indicating a more superficial location for alamethicin, which correlates with the difference in orientation between gel- and fluid-phase membranes found by conventional EPR of TOAC-alamethicin in aligned phosphatidylcholine bilayers. Increasing alamethicin/lipid ratio in saturated phosphatidylcholine shifts the profile of water exposure toward that with unsaturated lipid, consistent with proposals of a critical concentration for switching between the two different membrane-associated states.
Abbreviations used: Aib, α-aminoisobutyric acid; CW, continuous wave; DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOXYL, 4,4-dimethyl-oxazolidinyl-N-oxyl; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; ED, echo-detected; EPR, electron paramagnetic resonance; ESEEM, electron spin echo envelope modulation; Fol, l-phenylalaninol; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; TOACm-Alm, Glu(OMe)7,18,19 analogue of alamethicin F50/5 with TOAC substituted at residue position m; n-PCSL, 1-acyl-2-[n-(4,4-dimethyl-oxazolidinyl-N-oxyl)]stearoyl-sn-glycero-3-phosphocholine; TOAC, 2,2,6,6-tetramethylpiperidino-1-oxyl-4-amino-4-carboxylic acid
Introduction
Alamethicin is a hydrophobic α-helical peptaibol that induces voltage-dependent ion channels in lipid membranes (1,2). There are two variants that have either a glutamic acid or a glutamine residue at position 18. The latter variant, alamethicin F50/5, bears no electrical charge and is used here as the [Glu(OMe)7,18,19] analog. Substitution of an Aib residue at position 1, 8, or 16 by the equally helicogenic nitroxyl amino acid TOAC (3–5) produces a spin-labeled analog that can be used to investigate the membrane environment of alamethicin F50/5 by means of EPR spectroscopy. Crystal structure determination by x-ray diffraction shows that TOAC-substituted [Glu(OMe)7,18,19] alamethicin F50/5 has a kinked helical configuration that is very similar to that of native alamethicin, and functional studies show ion-channel activity of this alamethicin analog in membranes (6).
Previous studies have shown that alamethicin is predominantly monomeric in fluid lipid membranes, in the absence of a membrane potential (7), and this has been found to be the case also for the TOAC-labeled analogs (8,9). In the latter case, the isotropic hyperfine couplings, measured by means of CW-EPR, were used to establish the transmembrane orientation of TOAC-labeled alamethicin in phosphatidylcholine bilayers. This is done by using calibrations with spin-labeled phospholipids in fluid membranes (10,11). In the low-temperature gel phase, the peptide tends to aggregate, even in the absence of a membrane potential (8), which correlates with the large increase in ion channel formation in the gel phase (12). Recently, librational motions of the peptide backbone on a timescale that is kinetically competent to participate in ion conductance have been shown by pulsed EPR (13).
In this study, we use electron spin-echo spectroscopy to study the association of water with TOAC-labeled [Glu(OMe)7,18,19] alamethicin F50/5 in the low-temperature state of phospholipid membranes with either fully saturated (myristoyl) or monounsaturated (oleoyl) chains. (Note that DMPC and DOPC have similar bilayer thicknesses at the same temperature in the fluid phase (14,15)). Echo-detected spectra are used to assess the degree of aggregation/assembly of the peptide, from the instantaneous diffusion induced by intermolecular spin-spin interactions. Electron spin-echo envelope modulation (ESEEM) by D2O is used to study the localization and interaction of water relative to the peptide (16). Water penetration into phospholipid membranes has been characterized previously by this technique (17,18), and also the accessibility of water to spin-labeled lipids in the binding sites of serum albumin has been investigated (19). The 2H-ESEEM spectra of D2O are characterized by a broad component that corresponds to water molecules that are directly hydrogen-bonded to the spin-label nitroxide group, and a sharp component that corresponds to water molecules that are in the vicinity but located less closely to the nitroxide (18). For peptides that are not aggregated, these latter measurements may be used as a calibration to determine the position of the spin-labeled peptide in lipid membranes (20). Comparison with measurements in the fluid phase then establishes the effects of peptide assembly on the water accessibility. Because it has been found previously that alamethicin can adopt two different membrane-associated configurations, which depend not only on peptide/lipid ratio but also on the particular lipid and the temperature (21–23), we also examine membranes composed of saturated lipids with differing contents of alamethicin.
Materials and Methods
Materials
Phospholipids, DMPC, DPPC, DOPC, and POPC, were from Avanti Polar Lipids (Alabaster, AL). Spin-labeled derivatives of alamethicin F50/5, [TOACm, Glu(OMe)7,18,19] with m = 1, 8, and 16, which were synthesized according to Peggion et al. (24,25), were kindly supplied by Prof. C. Toniolo and M. de Zotti of the University of Padua. The complete amino-acid sequences of the three analogs are:
Ac-TOAC-Pro-Aib-Ala-Aib-Ala-Glu(OMe)-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu(OMe)-Glu(OMe)-Fol [TOAC1, Glu(OMe)7,18,19]
Ac-Aib-Pro-Aib-Ala-Aib-Ala-Glu(OMe)-TOAC-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu(OMe)-Glu(OMe)-Fol [TOAC8, Glu(OMe)7,18,19]
Ac-Aib-Pro-Aib-Ala-Aib-Ala-Glu(OMe)-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-TOAC-Aib-Glu(OMe)-Glu(OMe)-Fol [TOAC16, Glu(OMe)7,18,19],
where Aib is α-aminoisobutyric acid and Fol is l-phenylalaninol. These TOAC-labeled alamethicin analogs are abbreviated here as TOACm-Alm. The crystal structure of TOAC16-Alm shows a conformation very similar to that of native alamethicin, and electrophysiological experiments show functional ion channel activity for this TOAC-alamethicin analog (6).
Sample preparation
Phosphatidylcholine and the required mole ratio of TOAC spin-labeled alamethicin (in MeOH) were codissolved in CHCl3, and the solution was then evaporated with dry nitrogen. (Note that the oligomeric state of alamethicin depends on solvent polarity (26).) After keeping under vacuum overnight, the dry mixture was dispersed at a concentration of ∼100 mg/ml in D2O by vortex mixing while heating to above the chain-melting transition of the phosphatidylcholine. The hydrated lipid bilayers were transferred to a standard 4 mm-diameter, quartz EPR tube, concentrated by pelleting in a bench-top centrifuge, and the excess water was removed. The samples were then incubated overnight at 4°C. Immediately before pulse-EPR measurements, the samples were frozen rapidly in liquid nitrogen and then transferred to the spectrometer that was pre-equilibrated at 77 K. This protocol should result in trapping of the DMPC samples in the gel phase, and of the DOPC samples in a (metastable) disordered state.
Aligned planar phospholipid membranes were formed by evaporating a CH2Cl2 solution of phospholipid plus 1 mol% of TOAC8-Alm onto the internal surfaces of a quartz flat cell (60 × 10 mm, with 0.3 mm spacing) by using a stream of dry nitrogen. Residual solvent was removed under vacuum overnight. The oriented lipid film was hydrated with excess buffer containing 150 mM NaCl. The cells were drained and sealed immediately before measurement, retaining sufficient buffer to ensure complete hydration throughout the measurement.
EPR spectroscopy
Pulsed EPR data were collected at 77 K on an ELEXSYS E580 9-GHz Fourier Transform FT-EPR spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with an MD5 dielectric resonator and a CF 935P cryostat (Oxford Instruments, UK). To obtain ESEEM spectra, three-pulse, stimulated echo (π/2-τ-π/2-T-π/2-τ-echo) decays were collected by using microwave pulse widths of 12 ns, with the microwave power adjusted to give π/2-pulses. The time delay T between the second and the third pulses was incremented from To = 20 ns by N = 700 steps of ΔT = 12 ns, while maintaining the separation τ between the first and the second pulses constant at 168 ns. A four-step phase-cycling program, +(x,x,x), −(x,−x,x), −(−x,x,x), +(−x,−x,x), where the initial sign indicates the phase of the detection (±y), was used to eliminate unwanted echoes.
The time-dependent echo amplitudes, V(τ,T), were processed to yield standardized ESEEM intensities, according to the protocol developed previously (18). The average experimental echo decay, <V(τ,T)>, was obtained by fitting V(τ,T) versus T with a bi-exponential function. The normalized ESEEM signal was then obtained as:
| (1) |
A complex Fourier transform of this normalized time-domain signal can be evaluated numerically:
| (2) |
where ωk = 2πk/(NΔT) with k = −N/2 to +N/2. The real and imaginary Fourier coefficients are then used to obtain the absolute-value ESEEM spectrum. Note that the discrete Fourier-transform software supplied with the ELEXSYS FT-EPR spectrometer does not take account of different dwell times, ΔT, in determining amplitudes of the ESEEM spectrum. Definition according to Eq. 2 provides machine-independent spectral densities, with the dimensions of time, which can be used for comparing different D2O-containing systems.
Calibration of the local water concentration at different depths in the membrane is taken from D2O-ESEEM measurements with phospholipids spin-labeled at different C-atom positions in the acyl chain, which are reported in Erilov et al. (18). A multiplicative factor of ×12 ns is required to convert the intensities reported in the latter reference to absolute values consistent with Eq. 2. Otherwise, ESEEM intensities should be obtained from three-pulse echo decays recorded with increments in the time delay ΔT = 12 ns (together with τ = 168 ns, To = 20 ns), which were used in Erilov et al. (18) for recording the D2O-ESEEM calibration spectra, or be scaled to this value of ΔT according to Eq. 2 from the dwell time actually used. For instance, the ESEEM amplitudes in Salnikov et al. (20) should be multiplied by a factor of 16/12 = 1.333× to be compatible with the calibration data of Erilov et al. (18) from spin-labeled lipids.
Conventional CW-EPR spectra were recorded either on an ESP-300 spectrometer (Bruker) or on a Varian Century-Line spectrometer (Varian, Palo Alto, CA), both operating at 9 GHz with 100-kHz field modulation. CW-spectra from aligned bilayers were obtained with quartz flat cells in a TE102 rectangular microwave cavity mounted with the H1-field horizontal. Either spectra were recorded at room temperature (20°C), or the entire cavity system was thermostated with nitrogen gas-flow.
Results
Aligned membranes
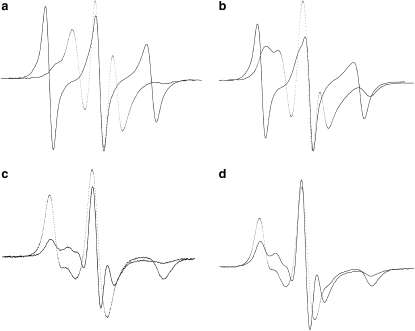
Fig. 1 shows the conventional CW-EPR spectra of the alamethicin analog that contains TOAC in the middle of the peptide chain (TOAC8-Alm), in aligned multibilayers of fully hydrated phosphatidylcholines in the gel and fluid phases (lower and upper rows, respectively). Spectra are shown for the static magnetic field parallel (solid lines) and perpendicular (dashed lines) to the normal to the orienting quartz substrate on which the multibilayers are deposited. Although the alignment of the sample is probably not completely homogeneous (as evidenced by apparent broadening of the central line for the parallel orientation and outer shoulders in the perpendicular orientation), there is clear anisotropy between spectra recorded with the parallel and perpendicular orientations of the magnetic field.
Figure 1.
Conventional CW-EPR spectra of the [TOAC8, Glu(OMe)7,18,19] alamethicin analog in aligned phosphatidylcholine bilayers in the fluid (upper row) and gel (lower row) phases. (Solid lines) magnetic field parallel to the membrane normal; (dashed lines) magnetic field perpendicular to the membrane normal. (a) in DOPC at 20°C; (b) in POPC at 20°C; (c) in DMPC at 12°C; and (d) in DPPC at 20°C. Peptide/lipid = 1:100 mol/mol. Total scan width = 100 Gauss.
For DOPC and POPC bilayers in the fluid phase at 20°C (Fig. 1, a and b), the largest hyperfine splitting of the first derivative-like single absorption lines is obtained in the parallel orientation with the magnetic field lying along the substrate normal (27). This shows that the director, N, for the uniaxial rotational diffusion in the fluid phase lies along the membrane normal. Because the principal z axis corresponding to the largest hyperfine splitting of TOAC lies close to the axis of an α-helix (28), the spectra from aligned samples are consistent with a transmembrane orientation of the alamethicin molecule in fluid-phase DOPC and POPC. This conclusion is also borne out by the spectra of the TOAC1-Alm and TOAC16-Alm analogs in aligned membranes of DOPC and POPC (data not shown).
For aligned DMPC in the gel phase at 12°C (Fig. 1 c), the largest hyperfine splitting is found with the magnetic field directed perpendicular to the bilayer normal, and the lineshape for this orientation approximates that of a two-dimensional powder pattern (29). This implies that the helical axis of alamethicin lies preferentially parallel to the plane of the membrane, when associated with gel-phase DMPC. The situation is quite unlike the transmembrane orientation found with fluid-phase phosphatidylcholines (Fig. 1, a and b, (8)). Correspondingly, the smallest hyperfine splitting is found with the magnetic field directed along the bilayer normal in Fig. 1 c, although the overall lineshape for this orientation evidences a considerable spread of disorder. For aligned DPPC in the gel phase at 20°C (Fig. 1 d), the spectra of TOAC8-Alm are similar to those for gel-phase DMPC (Fig. 1 c), indicating that alamethicin has the same membrane orientation in the gel phases of the two saturated lipids.
Note that the transmembrane orientation of alamethicin in DOPC and POPC is a general feature of fluid-phase phosphatidylcholines and not a specific feature of 9,10 cis-unsaturation, because a similar orientation is found when DMPC is in the fluid phase (8).
ESEEM spectra
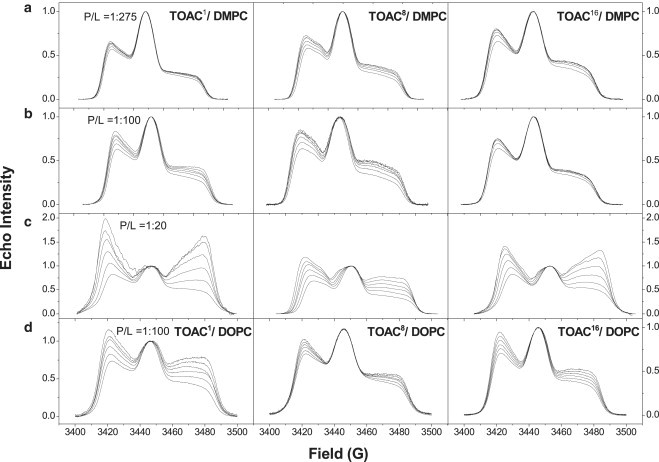
2H-ESEEM spectroscopy was used to investigate the degree of accessibility to water of the TOAC residues, for alamethicin in phospholipid bilayer membranes that are dispersed in D2O (16). Fig. 2 shows absolute-value ESEEM spectra of TOAC1-Alm, TOAC8-Alm, and TOAC16-Alm. The spin-labeled alamethicin is incorporated either in DMPC bilayer membranes, which have saturated chains, or in DOPC bilayer membranes, which have unsaturated chains. The interpulse separation, τ, between the first and second pulses in the stimulated echo sequence was set equal to 168 ns to maximize the deuterium and proton modulations simultaneously.
Figure 2.
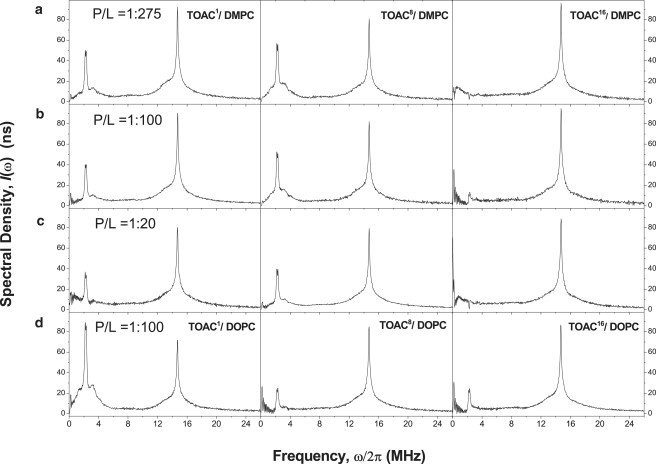
Absolute-value Fourier-transform ESEEM spectra of [Glu(OMe)7,18,19] alamethicin F50/5 analogs with TOAC substituted for: (left column) residue 1 (TOAC1-Alm), (center column) residue 8 (TOAC8-Alm), (right column) residue 16 (TOAC16-Alm), in: (a) DMPC bilayers at mole ratio 1:275; (b) DMPC bilayers at 1:100 mol/mol; (c) DMPC bilayers at 1:20 mol/mol; and (d) DOPC bilayers at 1:100 mol/mol.
Peaks are seen in the ESEEM spectra of TOAC1-Alm and TOAC8-Alm (Fig. 2, left and center columns, respectively) that are centered around the deuterium Larmor frequency at ∼2.6 MHz and around that of protons at 14.6 MHz. The latter originates primarily from matrix protons. The ESEEM spectra of TOAC16-Alm in DMPC (Fig. 2, right column) are essentially devoid of intensity in the region of the 2H-Larmor frequency, but have a spectral component at the proton frequency that is similar in shape and amplitude to that of TOAC1-Alm or TOAC8-Alm in DMPC. Water (i.e., D2O) therefore is apparently absent at the location of the 16-residue position of alamethicin with DMPC bilayers, even at a peptide/lipid ratio of 1:20 mol/mol.
The 2H-ESEEM spectra of TOAC1-Alm and TOAC8-Alm (Fig. 2, left and center columns) consist of a sharp doublet superimposed on a much broader component. The sharp doublet arises from free intramembranous D2O, and the broad component arises from D2O that is hydrogen bonded to the spin-label nitroxide group (18). Increasing the concentration of the TOAC-labeled alamethicin from a peptide/DMPC ratio of 1:275 mol/mol to 1:100 mol/mol decreases the normalized 2H-ESEEM intensity only slightly (compare Fig. 2, a and b). Increasing the concentration further to a peptide/DMPC ratio of 1:20 mol/mol (Fig. 2 c) produces a marked decrease in the normalized 2H-ESEEM intensity. Under these conditions, the proton ESEEM intensity hardly changes, therefore suggesting that the increased peptide concentration in saturated-chain DMPC is accompanied by a decreased accessibility of alamethicin to water.
The 2H-ESEEM spectra of TOAC1-Alm in DMPC (Fig. 2, left column) differ quantitatively from those of TOAC8-Alm (Fig. 2, center column). The overall intensity is lower and the ratio of the broad to narrow 2H-ESEEM components is also lower. These differences follow the trend seen also with increasing concentration of the TOAC1 peptide. Evidently, in saturated-chain DMPC, TOAC at residue position 1 has a lower probability of hydrogen bonding to D2O than does TOAC at residue position 8, although the local concentrations of free D2O (narrow component) are more similar.
The 2H-ESEEM spectra of TOACm-Alm in unsaturated-chain DOPC (Fig. 2 d) differ qualitatively from those in saturated-chain DMPC at the same peptide/lipid ratio (Fig. 2 b). The intensity decreases from TOAC1-Alm to TOAC8-Alm and TOAC16-Alm in DOPC, whereas it is largest for TOAC8-Alm in DMPC. To a certain extent, this mirrors the change on increasing the peptide/lipid ratio from 1:100 to 1:20 mol/mol in DMPC, in that the 2H-ESEEM intensity of TOAC1-Alm then becomes more comparable to that of TOAC8-Alm. In general, the difference in exposure to D2O between saturated and unsaturated phosphatidylcholines that is found by ESEEM correlates with the difference in peptide orientation that is found between the phosphatidylcholine gel and fluid phases, respectively, in aligned samples (Fig. 1).
Table 1 summarizes the normalized intensities of the total and broad 2H-ESEEM components determined for the different TOACm-Alm analogs in the two phosphatidylcholine lipid hosts. At low peptide concentration, the difference between the hydration profiles of alamethicin in saturated and unsaturated lipids is clearly evident. For DMPC, values are given for samples with different peptide/lipid ratios. These latter reflect the preferential decrease in 2H-ESEEM intensities with increasing content of spin-labeled alamethicin and the redistribution of relative intensities at high concentrations in the saturated lipid.
Table 1.
Normalized intensities of the total (Itot) and the broad (Ibroad) components in the 2H-ESEEM spectra from TOACm-Alm in D2O-lipid dispersions
| Lipid/TOACm | Peptide/lipid (mol/mol) | Itot (ns) | Ibroad (ns) | Ibroad/Io∗ |
|---|---|---|---|---|
| DMPC/TOAC1 | 1:275 | 46 | 8 | 0.08 |
| 1:100 | 35 | 5 | 0.04 | |
| 1:20 | 31 | 4 | 0.03 | |
| DMPC/TOAC8 | 1:275 | 54 | 14 | 0.13 |
| 1:100 | 49 | 12 | 0.11 | |
| 1:20 | 36 | 6 | 0.05 | |
| DMPC/TOAC16 | 1:275,100,20 | 0 | 0 | — |
| DOPC/TOAC1 | 1:100 | 83 | 23 | 0.20 |
| DOPC/TOAC8 | 1:100 | 20 | 2 | 0.02 |
| DOPC/TOAC16 | 1:100 | 19 | 1 | 0.01 |
Io is the theoretical intensity for one D2O hydrogen bonded to the nitroxide (18).
CW-EPR powder spectra
The outer hyperfine splittings, 2Azz, in rigid-limit nitroxide spectra depend on the local polarity (30). Fig. 3 shows the conventional CW-EPR spectra of the three TOACm-Alm spin-labeled peptides in DMPC or DOPC bilayer membranes at 77 K. The peptide/lipid ratio is 1:100 mol/mol. Some inhomogeneous and/or spin-spin broadening is evident in these spectra. (Note that the broadening may not be due entirely to spin-spin interactions, because environmental microheterogeneity and conformational substates that are frozen-in can also contribute substantially to Gaussian line widths in powder spectra.) The rigid-limit values of the Azz-hyperfine coupling deduced from these spectra are given in Table 2. To correct for intrinsic differences between the residue positions, values of Azz are referred to those of the same TOACm-Alm in methanol. (Note that polarity-dependent contributions to hyperfine couplings are additive (32,33).) The corrected values (ΔAzz) differ for the three TOAC positions, indicating differences in polarity of the local spin-label environment. However, the relative polarities in the saturated DMPC and DPPC lipids are not in the same order as those reflected by the isotropic hyperfine couplings Δao measured in the fluid phase at high temperature ((9), Fig. 4). The highest polarity is found for TOAC8-Alm in DMPC. For the unsaturated DOPC lipid, on the other hand, the values of ΔAzz are closer to the order found for Δao in fluid bilayers, which is TOAC1 > TOAC16 > TOAC8 for fluid-phase phosphatidylcholines with chain lengths from C12 to C18 (9). In particular, TOAC8-Alm does not correspond to a higher polarity than TOAC1-Alm. These sequences for ΔAzz also correspond largely to the order of D2O-ESEEM intensities in DOPC and DMPC (Table 1).
Figure 3.
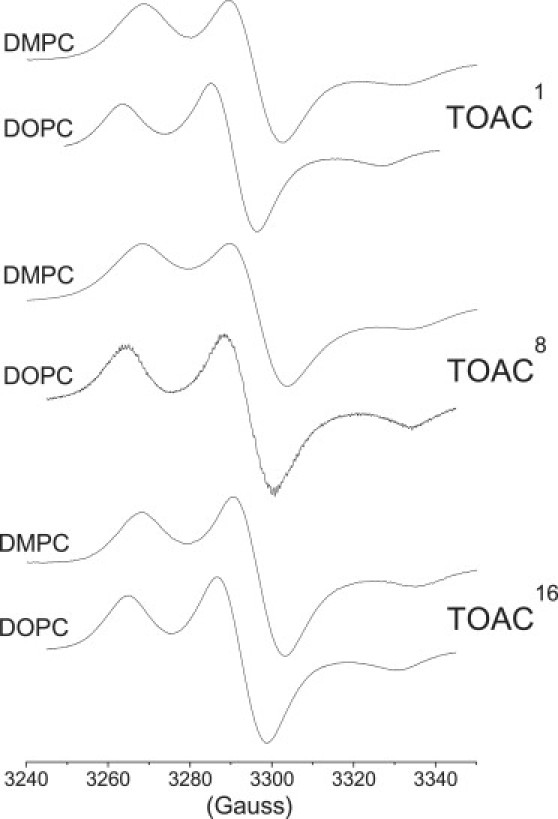
Conventional CW-EPR spectra of [Glu(OMe)7,18,19] alamethicin F50/5 analogs with TOAC substituted for: residue 1, TOAC1-Alm; residue 8, TOAC8-Alm; and residue 16, TOAC16-Alm; in bilayers of DMPC (upper of each pair) or DOPC (lower of each pair) at 77 K. Peptide/lipid = 1:100 mol/mol.
Table 2.
Rigid-limit hyperfine couplings, Azz, of spin-labeled alamethicin in different PC bilayers, with TOAC at residue position, m (TOACm-Alm)
| TOAC1 |
TOAC8 |
TOAC16 |
||||
|---|---|---|---|---|---|---|
| Azz (Gauss) | ΔAzz(Gauss) | Azz (Gauss) | ΔAzz(Gauss) | Azz (Gauss) | ΔAzz(Gauss) | |
| DMPC | 32.6 | −3.0 | 33.4 | −2.6 | 31.4 | −3.7 |
| DPPC | 33.0 | −2.6 | 34.1 | −1.9 | 32.8 | −2.3 |
| DOPC | 34.5 | −1.1 | 34.8 | −1.2 | 33.0 | −2.1 |
ΔAzz is referred to Azz in methanol, i.e.: ΔAzz = Azz(PC) − Azz(MeOH).
Figure 4.
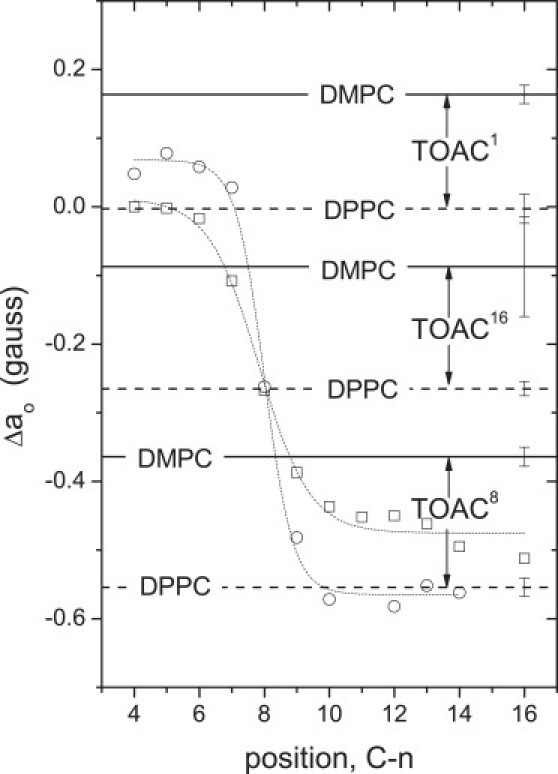
Effective isotropic hyperfine couplings, ao, for [Glu(OMe)7,18,19] alamethicin TOAC1, TOAC8, and TOAC16 analogs, as indicated, in fluid-phase DMPC (solid horizontal lines) and fluid-phase DPPC (dashed horizontal lines) bilayers (8,9). To establish the transmembrane location of the TOAC labels, the dependence of ao on nitroxide position, n, in the sn-2 chain of spin-labeled phosphatidylcholine, n-PCSL, in DMPC (circles) and DPPC (squares) fluid-phase bilayers is given by the dotted lines (10). All values of ao are given relative to those in methanol, i.e.: Δao = ao(PC) − ao(MeOH), and are constant over a wide range of temperature in the respective fluid phases. The relative order for Δao of TOACm-Alm in fluid-phase phosphatidylcholines is: TOAC1 > TOAC16 > TOAC8, for chain lengths C12–C18 (9).
Fluid-phase bilayers
The isotropic hyperfine couplings, ao, of the TOACm-Alm analogs have been determined at high temperatures in the fluid phase of DMPC and DPPC bilayers, for which the CW-spectra are in the fast motional regime (8,9). These values should depend only on the polarity of the environment in which the spin label is situated (11,32). Fig. 4 gives the isotropic couplings, Δao, relative to those measured in methanol, to correct for intrinsic differences between the three TOAC positions. For the purposes of calibration, the dependence of ao on position in the sn-2 chain of spin-labeled phosphatidylcholine, n-PCSL, is also included in Fig. 4. These values are likewise given relative to those in methanol, i.e., Δao = ao(PC) − ao(MeOH), to correct for the intrinsic difference in isotropic couplings of DOXYL nitroxides (in n-PCSL) and TOAC nitroxides (34). Note that the transbilayer polarity profile is not disturbed by the presence of a membrane-spanning α-helical peptide, WALP, which is both hydrophobic and uncharged, as are the alamethicin analogs used here (35). Therefore, the transmembrane profiles of the peptide-free bilayer are appropriate references to determine the location of the TOACm-Alm analogs.
For all three TOAC positions of alamethicin in fluid membranes, the effective isotropic hyperfine splitting constants correspond to a lower polarity in DPPC bilayers than in the thinner DMPC bilayers. In DMPC, the TOAC1 residue is situated in a more polar location than that of the 4 C-atom of the phosphatidylcholine sn-2 chain, but in the region of the 4–6 C-atom in DPPC. The TOAC8 residue in DPPC, is situated toward the center of the bilayer, in the region of the 10–14 C-atom of the lipid chains. In DMPC, TOAC8 is located at a similar depth as the 8–9 C-atom of the chain. The TOAC16 residue is located close to the 8 C-atom of the lipid chains in DPPC, and between the 7 and 8 C-atoms in DMPC. Clearly, TOAC16 resides in the opposite bilayer leaflet from that of TOAC8 and TOAC1, because the various crystal structures (6,36) and solution NMR structure (37,38) show that alamethicin adopts a predominantly extended helical conformation.
ED-EPR spectra
Echo-detected (ED)-EPR spectra are sensitive to spin-spin interactions that cause so-called instantaneous (spin) diffusion, even in the absence of molecular motion (39). This is therefore a sensitive means to investigate aggregation or cluster formation of spin-labeled peptides.
Fig. 5 shows the ED-EPR spectra of the TOACm-Alm analogs in DMPC bilayers at different peptide/lipid ratios and in DOPC bilayers at 1:100 mol/mol. The dependence of the ED-lineshapes on echo delay-time at 77 K is characteristic of instantaneous diffusion arising from spin-spin interactions. For this process, the central region of the spectrum decays faster with increasing τ than do the outer wings (40). (Note that the ED-spectra in Fig. 5 are normalized to the central line height.) The ED-spectra of TOACm-Alm in DMPC are rather similar at the two lower peptide/lipid ratios of 1:275 and 1:100 mol/mol, and show only limited instantaneous diffusion. At the higher alamethicin/DMPC mole ratio of 1:20, instantaneous diffusion is much more rapid corresponding to a greater degree of spin-spin interaction between TOAC-labeled alamethicins.
Figure 5.
Echo-detected EPR spectra of: (left column) residue 1 (TOAC1-Alm), (center column) TOAC8-Alm, and (right column) TOAC16-Alm, in: (a) DMPC bilayers at 1:275 mol/mol; (b) DMPC bilayers at 1:100 mol/mol; (c) DMPC bilayers at 1:20 mol/mol; and (d) DOPC bilayers at 1:100 mol/mol. ED-spectra are recorded for increasing interpulse spacings of (bottom to top) τ = 168, 296, 424, 552, 680, and 804 ns, and are normalized to the maximum central line height. T = 77 K.
Of decisive significance is the much higher instantaneous diffusion at the TOAC1 position than at the TOAC8 and TOAC16 positions (Fig. 5 c). In principle, the weaker spin-spin interactions for the latter two positions could arise from longer-range dipolar interactions between aggregates, which should be the same at all TOAC spin-label positions. However, the much stronger spin-spin interactions at the TOAC1 position must be attributed to specific aggregate formation at an alamethicin/DMPC ratio of 1:20 mol/mol, in which the TOAC1 labels are located closer to each other than are the TOAC8 or TOAC16 labels. Evidently, TOACm-Alm is predominantly monomeric at peptide/lipid ratios of 1:275 and 1:100 mol/mol in the DMPC preparations, but is appreciably aggregated at the higher concentration of 1:20 mol/mol. A possible reason for the preferential interaction at the residue 1 position in DMPC might be a tendency to head-to-head association of the partially inserted peptide.
In DOPC at a peptide/lipid ratio of 1:100 mol/mol, all three TOACm-Alm peptides show stronger spin-spin interactions than in DMPC at 1:100 mol/mol, with the greatest extent for TOAC1-Alm. Again, the discrimination between different TOAC label positions is diagnostic for specific aggregation. From this it can be concluded that TOACm-Alm has a greater tendency to self-assemble in DOPC than in DMPC. In DOPC, TOAC1 might be oriented preferentially toward the center of a transmembrane helix bundle and hence experience greater spin-spin interactions. Possibly, this may also apply in DMPC at high peptide concentrations.
Table 3 lists values of C/L, the line height of the central peak in the ED-spectra, relative to that at low-field, for TOACm-Alm at different peptide/lipid ratios in the saturated and unsaturated phosphatidylcholine lipid hosts. The C/L line height ratio (41) is a sensitive monitor of instantaneous diffusion. It decreases progressively with increasing echo delay time τ in an approximately exponential fashion (41), and the rate of decay is a measure for the strength of spin-spin interactions (cf. above). Thus, C/L decreases with increasing peptide/lipid ratio, at all values of τ. Furthermore, it is greater for TOACm-Alm in DOPC than in DMPC at the same peptide/lipid ratio (and same value of τ).
Table 3.
Central line height (C), normalized to that at low field (L), in the echo-detected EPR spectra from TOACm-Alm in phosphatidylcholine membranes, for different delay times τ
| Lipid/TOACm | Peptide/lipid (mol/mol) |
C/L |
|
|---|---|---|---|
| τ = 168 ns | τ = 804 ns | ||
| DMPC/TOAC1 | 1:275 | 1.76 | 1.52 |
| 1:100 | 1.58 | 1.20 | |
| 1:20 | 1.21 | 0.58 | |
| DMPC/TOAC8 | 1:275 | 1.72 | 1.38 |
| 1:100 | 1.57 | 1.17 | |
| 1:20 | 1.45 | 0.85 | |
| DMPC/TOAC16 | 1:275 | 1.52 | 1.24 |
| 1:100 | 1.56 | 1.34 | |
| 1:20 | 1.21 | 0.71 | |
| DOPC/TOAC1 | 1:100 | 1.36 | 0.87 |
| DOPC/TOAC8 | 1:100 | 1.31 | 1.11 |
| DOPC/TOAC16 | 1:100 | 1.42 | 1.05 |
Discussion
Membrane topography of alamethicin
At low peptide contents, the D2O-ESEEM spectra show that the pattern of alamethicin exposure to water differs qualitatively between membranes composed of saturated and unsaturated lipids (Table 1). In the unsaturated lipid (DOPC), maximum water exposure is found for TOAC1-Alm at the N-terminus, whereas in the saturated lipid (DMPC), maximum water exposure is found for TOAC8-Alm toward the middle of the peptide. A similar distinction is found in the environmental polarities registered by the rigid-limit hyperfine couplings (ΔAzz), which are largest for TOAC1-Alm in DOPC, and for TOAC8-Alm in DMPC (Table 2). This distinction between unsaturated and saturated lipid membranes in the frozen state parallels the difference in membrane orientation of alamethicin that is found between DOPC and POPC in the fluid phase, on the one hand, and DMPC and DPPC in the gel phase, on the other hand, at near-ambient temperatures (Fig. 1). In the fluid phase of the unsaturated lipids, alamethicin adopts a transmembrane orientation, parallel to the membrane normal, as found previously for the fluid phase of DMPC (8). In the gel phase of the saturated lipids, however, alamethicin is oriented more closely parallel to the membrane surface.
Huang and co-workers (42) have shown previously that alamethicin can adopt two different states when bound to lipid bilayer membranes: one in which the helix axis is oriented parallel to the membrane surface, and the other in which the helix orientation is transmembrane. The preferred orientational state is determined by a critical peptide/lipid ratio that depends both on the particular lipid, and on the temperature and degree of hydration (21–23). The EPR results presented here with TOAC-alamethicin in aligned membranes (Fig. 1) are in agreement with these conclusions. It is therefore reasonable to suggest that the different hydration profiles found here may also reflect different orientations of alamethicin: parallel to the membrane surface in DMPC, and transmembrane in DOPC, at the low temperatures that are required for the ESEEM experiments. Further support for this proposal comes from the relative D2O-ESEEM intensities at higher peptide/lipid ratio (1:20 mol/mol) in the saturated DMPC lipid (Table 1). These exhibit a positional profile that is much closer to that obtained with the unsaturated DOPC lipid than is found at lower peptide/lipid ratios, suggesting that the critical concentration for transmembrane insertion in saturated lipids has been achieved at 1:20 mol/mol.
Hydration profiles
The echo-detected EPR spectra at 77 K indicate an increasing degree of spin-spin interaction with increasing concentration of TOAC-labeled alamethicin (Fig. 5). This suggests that oligomerization of alamethicin partially takes place at low temperature in the frozen state. It is therefore of direct interest to compare the water accessibility in this proto-assembled state with that in the fluid membrane state, where it is known both from conventional CW-EPR and from saturation transfer EPR that alamethicin is in a predominantly monomeric state (7–9). This comparison is made here below. Distinction must be made between ESEEM results from unsaturated and saturated lipids because, as seen already, the alamethicin configuration differs between these two classes of host membrane.
Measurements of isotropic hyperfine couplings in fluid membranes (Fig. 4) indicate clearly that TOAC-labeled alamethicin adopts a transmembrane orientation in DMPC and DPPC bilayers when they are above their respective chain-melting transitions. This is also true of alamethicin in fluid-phase phosphatidylcholine bilayers of other acyl chain lengths (9). In fluid-phase DMPC, the N-terminal TOAC1 residue resides in the lipid polar headgroup region of the membrane, and the C-terminal phenylalaninol has a similar location but at the opposite surface of the bilayer (9). In fluid-phase DPPC, the N-terminal TOAC1 residue is situated close to the polar-apolar interface of the membrane, and the C-terminal phenylalaninol again has a similar location at the opposite side of the membrane. Thus, in fluid membranes of different thicknesses, alamethicin occupies approximately the same position relative to the bilayer mid-plane (9).
Fig. 6 gives the D2O-ESEEM amplitudes for the TOACm-Alm analogs in bilayers of the unsaturated lipid DOPC (solid lines) and of the saturated lipid DMPC (dashed lines). Calibration ESEEM data from spin-labeled lipid chains, n-PCSL, in DPPC bilayers are included for comparison. The latter have been converted from the values reported originally (18), according to the standardized procedure defined by Eqs. 1 and 2. The D2O-ESEEM amplitude from the N-terminal TOAC1 residue in DOPC correlates with the lipid polar headgroup region of the membrane. The D2O-ESEEM amplitudes for the TOAC8 and TOAC16 analogs in DOPC are similar, indicating that these residues are situated at similar locations on opposite sides of the bilayer midplane. The amplitudes correlate approximately with the C12 atoms of the lipid sn-2 chain, which are expected to have a separation of 1.29 nm between the two bilayer leaflets in the gel phase of DPPC, allowing for the terminal methyl groups and sn-1/sn-2 chain mismatch (43) and a 35° chain tilt (44). This separation is therefore sufficient to accommodate the distance between TOAC8 and TOAC16 in a fully extended helix.
Figure 6.
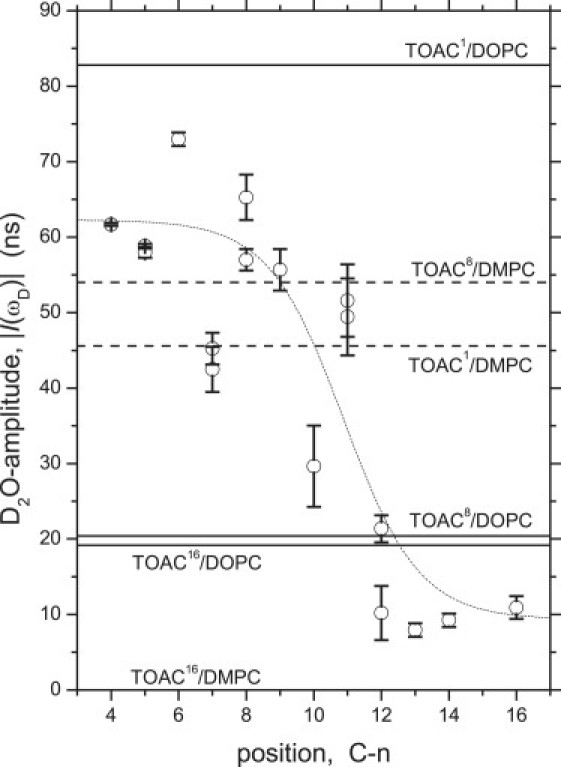
Dependence of D2O-ESEEM amplitudes on position, n, in the sn-2 chain for spin-labeled phosphatidylcholine (n-PCSL) in DPPC bilayers. Data (open circles) from Erilov et al. (18) are standardized according to Eqs. 1 and 2, and are fitted with a trough-like Boltzmann sigmoid (dotted line) according to Marsh (10). Horizontal lines are the corresponding D2O-amplitudes for 1:100 mol/mol TOACm-Alm analogs in DOPC (solid lines) and DMPC (dashed lines) bilayers, as indicated. The value for TOAC16-Alm in DMPC lies along the baseline.
Comparing Figs. 4 and 6, it is thus seen that, for TOACm-Alm analogs in frozen DOPC membranes, the TOAC1 and TOAC8 residues correlate with positions similar, relative to the lipid chains, to those found in fluid bilayers. The TOAC16 residue, on the other hand, although situated in the apposing bilayer leaflet, correlates with a position closer to the bilayer midplane in the thicker frozen membranes of DOPC than in fluid bilayers. This indicates a more asymmetric location of alamethicin with respect to the membrane midplane in frozen DOPC membranes than in fluid bilayers. The results on spin diffusion (Fig. 5 and Table 3) indicate preferential self-assembly of TOACm-Alm in DOPC, relative to DMPC. Therefore, the asymmetric offset within the membrane may be a distinguishing feature of the protoassembled channel complex, as compared with the peptide monomer in fluid bilayers.
The alamethicin hydration profile in the saturated lipid DMPC (Fig. 6, dashed lines) correlates neither with that in the frozen unsaturated lipid DOPC nor with the polarity sensed at the different TOAC positions in fluid lipid membranes (Fig. 4). The highest water accessibility at the TOAC8 position is consistent with a surface or interfacial location, as suggested from the orientation results with aligned membranes of saturated lipids in the gel phase (Fig. 1). However, the D2O-ESEEM amplitude of TOAC8-Alm in DMPC, although greater than that at the TOAC8 position in DOPC, is less than that of TOAC1-Alm in the unsaturated DOPC lipid. The reason for this, in addition to the different orientation relative to the membrane normal, is probably a combination of partial embedding in the membrane and limited aggregation of the peptide.
The D2O-ESEEM amplitude at the TOAC1 position in DMPC, although smaller than that at the TOAC8 position, is of a similar magnitude. On the other hand, the amplitude at the TOAC16 position is much smaller, practically zero. This difference can be understood from the relative orientations of the residue positions about the long axis of the alamethicin molecule. Fig. 7 shows one of the molecules in the crystal structure of native alamethicin (36) to which the predominant crystal conformer of the TOAC residue has been grafted at position 1, 8, or 16 (8). The view is down the axis of the longer (N-terminal) helix. It is seen that TOAC1 and TOAC8 are situated on the same face of the molecule in the N-terminal helix, whereas TOAC16, which resides in the shorter C-terminal helix after Pro14, faces in a different direction. Thus, TOAC1 and TOAC8 are likely to have more similar locations in surface aggregates than does TOAC16, which might penetrate rather deeper into the membrane and be involved in peptide-peptide contacts that more efficiently exclude water.
Figure 7.
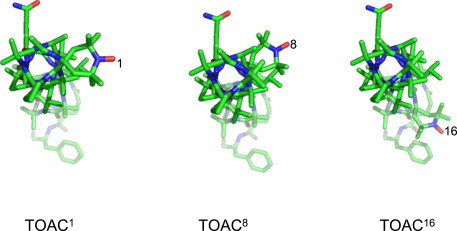
Crystal structure for molecule A of native alamethicin ((36); PDB: 1amt) with the TOAC structure from molecule B of Z-TOAC-(L-Ala)2-NHtBu (45) substituted for Aib at residue position 1, 8, or 16 (8). The alamethicin molecule is oriented with the axis of the longer helix perpendicular to the plane of the figure and the N-terminus faces upward from the plane of the figure.
Hydrogen-bonded water
It is possible to use previous calibrations of D2O-ESEEM intensities to give further information on the association of water with the TOAC-alamethicins that is rather more quantitative than the comparisons made above. Density functional (DFT) calculations predict that the normalized 2H-ESEEM intensity for one D2O molecule hydrogen bonded to the nitroxide is Io ≈ 115 ns (18). Thus, from the intensities of the broad D2O-ESEEM components given in Table 1, it can be estimated, for example, that the fraction of TOAC1-Alm nitroxides that are hydrogen bonded to water is ∼0.20 in DOPC and 0.04 in DMPC, at a peptide/lipid ratio of 1:100 mol/mol. Further, the product of the equilibrium constant, K, for hydrogen bonding and the effective concentration of free water, [W], is given by (18):
| (3) |
where Ibroad is the normalized intensity of the broad D2O-ESEEM component. This yields values of K[W] = 0.11 and 0.02 for TOAC1-Alm in DOPC and DMPC, respectively. Correspondingly, the fraction of TOAC that is hydrogen bonded by a single water molecule is given by (18):
| (4) |
that yields f1W ≈ 0.18 and 0.04 for TOAC1-Alm in DOPC and DMPC, respectively. The fraction of TOAC that is hydrogen bonded by two water molecules is then given by:
| (5) |
that yields f2W ≈ 0.01 and 0.0005 for TOAC1-Alm in DOPC and DMPC, respectively.
Table 4 summarizes the values of K[W], f1W, and f2W that are obtained for all three TOAC positions in the two lipid hosts. For comparison, the values that are obtained from ESEEM with spin-labeled lipids in DPPC bilayers are: K[W] = 0.12 and 0.02, with fractions of nitroxides hydrogen bonding to one water of f1W = 0.20 and 0.04, and fractions hydrogen bonding to two waters of f2W = 0.01 and 0.00, at chain positions C4 and C14, respectively, where the transition between these two regions takes place at chain positions C10 to C12 (18). As for overall hydration, there is a clear distinction in the positional profiles of water hydrogen-bonded to the TOAC residues between the unsaturated and saturated lipid hosts. In unsaturated DOPC, there is appreciable hydrogen bonding to TOAC1 with levels comparable to those at the top of the lipid chains in peptide-free bilayers. There is little water hydrogen bonded to TOAC8 and TOAC16, however, in the case of DOPC. In the saturated DMPC lipid, water is hydrogen bonded to both TOAC8 and TOAC1 and at levels for the former that approach those for TOAC1 in the unsaturated DOPC lipid. Neither configuration approaches levels that might be expected for a large water-filled channel (for which the total fractional population of spin label H-bonded by water is f1W + 2f2W ∼ 0.8 (19)), possibly in part because of orientational restrictions on optimum hydrogen bonding and also none of the TOAC side chains may be directed optimally into the channel lumen (cf. Fig. 7).
Table 4.
Fractions of TOACm-Alm hydrogen bonded by one (f1W) and two (f2W) water molecules, and product of the equilibrium constant for H-bonding, K, with the effective free water concentration, [W], from 2H-ESEEM spectra of D2O-lipid dispersions
| Lipid/TOACm | Peptide/lipid (mol/mol) | K[W] | f1W | f2W |
|---|---|---|---|---|
| DMPC/TOAC1 | 1:275 | 0.04 | 0.08 | 0.002 |
| 1:100 | 0.02 | 0.04 | 0.0005 | |
| DMPC/TOAC8 | 1:275 | 0.07 | 0.12 | 0.004 |
| 1:100 | 0.06 | 0.10 | 0.003 | |
| DMPC/TOAC16 | 1:275,100 | — | 0 | — |
| DOPC/TOAC1 | 1:100 | 0.11 | 0.18 | 0.01 |
| DOPC/TOAC8 | 1:100 | 0.01 | 0.02 | 0.0 |
| DOPC/TOAC16 | 1:100 | 0.005 | 0.01 | 0.0 |
Values deduced from Eqs. 3–5.
Conclusions
In conclusion, alamethicin associates differently with gel- and fluid-phase lipids at near-ambient temperatures, and with saturated and unsaturated lipids at low (cryogenic) temperatures. Aligned membranes at ambient temperatures indicate a transmembrane orientation of alamethicin in fluid phase lipids, but an orientation more parallel to the membrane surface in the gel phase. Water contact monitored by D2O-ESEEM at low temperature indicates a transmembrane location for alamethicin in unsaturated lipids (DOPC), but a more superficial location in saturated lipids (DMPC). Spin-spin interaction, monitored from ED-spectra, indicates a greater tendency to form specific oligomers for the transmembrane orientation of alamethicin in DOPC than for the more superficial location in DMPC. None of the TOAC1, TOAC8 and TOAC16 residue positions experience the maximum degree of water contact that can be expected at the center of a large water-filled channel. However, orientational restrictions make such a high degree of exposure unlikely.
Acknowledgments
We are extremely grateful to Professor Claudio Toniolo and Marta de Zotti (University of Padua) for providing the TOAC alamethicin analogues that were used in this study. We also wish to thank Professor Sergei Dzuba (Russian Academy of Science, Novosibirsk) for helpful discussions during the course of this work. The authors are members of the COST P15 Action of the European Union.
References
- 1.Sansom M.S. The biophysics of peptide models of ion channels. Prog. Biophys. Mol. Biol. 1991;55:139–235. doi: 10.1016/0079-6107(91)90004-c. [DOI] [PubMed] [Google Scholar]
- 2.Marsh D. Peptide models for membrane channels. Biochem. J. 1996;315:345–361. doi: 10.1042/bj3150345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakaie C.R., Goissis G., Schreier S., Paiva A.C.M. pH-dependence of electron paramagnetic resonance spectra of nitroxides containing ionizable groups. Braz. J. Med. Biol. Res. 1981;14:173–180. [PubMed] [Google Scholar]
- 4.Nakaie C.R., Schreier S., Paiva A.C.M. Synthesis and properties of spin-labeled angiotensin derivatives. Biochim. Biophys. Acta. 1983;742:63–71. doi: 10.1016/0167-4838(83)90359-x. [DOI] [PubMed] [Google Scholar]
- 5.Marchetto R., Schreier S., Nakaie C.R. A novel spin-labeled amino acid derivative for use in peptide synthesis—(9-fluorenylmethyloxycarbonyl)-2,2,6,6-tetramethylpiperidine-N-oxyl-4-amino-4-carboxylic acid. J. Am. Chem. Soc. 1993;115:11042–11043. [Google Scholar]
- 6.Crisma M., Peggion C., Baldini C., MacLean E.J., Vedovato N. Crystal structure of a spin-labeled, channel-forming, alamethicin analogue. Angew. Chem. Int. Ed. 2007;46:2047–2050. doi: 10.1002/anie.200604417. [DOI] [PubMed] [Google Scholar]
- 7.Barranger-Mathys M., Cafiso D.S. Collisions between helical peptides in membranes monitored using electron paramagnetic resonance: evidence that alamethicin is monomeric in the absence of a membrane potential. Biophys. J. 1994;67:172–176. doi: 10.1016/S0006-3495(94)80466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh D., Jost M., Peggion C., Toniolo C. TOAC spin labels in the backbone of alamethicin: EPR studies in lipid membranes. Biophys. J. 2007;92:473–481. doi: 10.1529/biophysj.106.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh D., Jost M., Peggion C., Toniolo C. Lipid chainlength dependence for incorporation of alamethicin in membranes: EPR studies on TOAC-spin labeled analogues. Biophys. J. 2007;92:4002–4011. doi: 10.1529/biophysj.107.104026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh D. Polarity and permeation profiles in lipid membranes. Proc. Natl. Acad. Sci. USA. 2001;98:7777–7782. doi: 10.1073/pnas.131023798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh D. Membrane water-penetration profiles from spin labels. Eur. Biophys. J. 2002;31:559–562. doi: 10.1007/s00249-002-0245-z. [DOI] [PubMed] [Google Scholar]
- 12.Boheim G., Hanke W., Eibl H. Lipid phase transition in planar bilayer membrane and its effect on carrier- and pore-mediated ion transport. Proc. Natl. Acad. Sci. USA. 1980;77:3403–3407. doi: 10.1073/pnas.77.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartucci R., Guzzi R., De Zotti M., Toniolo C., Sportelli L. Backbone dynamics of alamethicin bound to lipid membranes: spin-echo EPR of TOAC spin labels. Biophys. J. 2008;94:2698–2705. doi: 10.1529/biophysj.107.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucerka N., Tristram-Nagle S., Nagle J.F. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. 2006;208:193–202. doi: 10.1007/s00232-005-7006-8. [DOI] [PubMed] [Google Scholar]
- 15.Marsh D. Energetics of hydrophobic matching in lipid-protein interactions. Biophys. J. 2008;94:3996–4013. doi: 10.1529/biophysj.107.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartucci R., Erilov D.A., Guzzi R., Sportelli L., Dzuba S.A. Time-resolved electron spin resonance studies of spin-labeled lipids in membranes. Chem. Phys. Lipids. 2006;141:142–157. doi: 10.1016/j.chemphyslip.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Bartucci R., Guzzi R., Marsh D., Sportelli L. Intramembrane polarity by electron spin echo spectroscopy of labeled lipids. Biophys. J. 2003;84:1025–1030. doi: 10.1016/S0006-3495(03)74918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erilov D.A., Bartucci R., Guzzi R., Shubin A.A., Maryasov A.G. Water concentration profiles in membranes measured by ESEEM of spin-labeled lipids. J. Phys. Chem. B. 2005;109:12003–12013. doi: 10.1021/jp050886z. [DOI] [PubMed] [Google Scholar]
- 19.De Simone F., Guzzi R., Sportelli L., Marsh D., Bartucci R. Electron spin-echo studies of spin-labeled lipid membranes and free fatty acids interacting with human serum albumin. Biochim. Biophys. Acta. 2007;1768:1541–1549. doi: 10.1016/j.bbamem.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Salnikov E.S., Erilov D.A., Milov A.D., Tsvetkov Yu D., Peggion C. Location and aggregation of the spin-labeled peptide trichogin GA IV in a phospholipid membrane as revealed by pulsed EPR. Biophys. J. 2006;91:1532–1540. doi: 10.1529/biophysj.105.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller W.T., He K., Ludtke S.J., Harroun T.A., Huang H.W. Effect of changing the size of lipid headgroup on peptide insertion into membranes. Biophys. J. 1997;73:239–244. doi: 10.1016/S0006-3495(97)78064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F.Y., Lee M.T., Huang H.W. Sigmoidal concentration dependence of antimicrobial peptide activities: a case study on alamethicin. Biophys. J. 2002;82:908–914. doi: 10.1016/S0006-3495(02)75452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M.T., Hung W.C., Chen F.Y., Huang H.W. Many-body effect of antimicrobial peptides: on the correlation between lipid's spontaneous curvature and pore formation. Biophys. J. 2005;89:4006–4016. doi: 10.1529/biophysj.105.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peggion C., Jost M., Baldini C., Formaggio F., Toniolo C. Total synthesis in solution of TOAC-labeled alamethicin F50/5 analogues. Chem. Biodivers. 2007;4:1183–1199. doi: 10.1002/cbdv.200790104. [DOI] [PubMed] [Google Scholar]
- 25.Peggion C., Coin I., Toniolo C. Total synthesis in solution of alamethicin F50/5 by an easily tunable segment condensation approach. Biopolymers. 2004;76:485–493. doi: 10.1002/bip.20161. [DOI] [PubMed] [Google Scholar]
- 26.Marsh D., Jost M., Peggion C., Toniolo C. Solvent dependence of the rotational diffusion of TOAC-spin-labeled alamethicin. Chem. Biodivers. 2007;4:1269–1274. doi: 10.1002/cbdv.200790109. [DOI] [PubMed] [Google Scholar]
- 27.Schreier-Muccillo S., Marsh D., Dugas H., Schneider H., Smith I.C.P. A spin probe study of the influence of cholesterol on motion and orientation of phospholipids in oriented multibilayers and vesicles. Chem. Phys. Lipids. 1973;10:11–27. doi: 10.1016/0009-3084(73)90037-6. [DOI] [PubMed] [Google Scholar]
- 28.Marsh D. Orientation of TOAC amino-acid spin labels in α-helices and β-strands. J. Magn. Reson. 2006;180:305–310. doi: 10.1016/j.jmr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Marsh D. Molecular motion in phospholipid bilayers in the gel phase: long axis rotation. Biochemistry. 1980;19:1632–1637. doi: 10.1021/bi00549a017. [DOI] [PubMed] [Google Scholar]
- 30.Kurad D., Jeschke G., Marsh D. Lipid membrane polarity profiles by high-field EPR. Biophys. J. 2003;85:1025–1033. doi: 10.1016/S0006-3495(03)74541-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reference deleted in proof.
- 32.Marsh D. Polarity contributions to hyperfine splittings of hydrogen-bonded nitroxides—the microenvironment of spin labels. J. Magn. Reson. 2002;157:114–118. doi: 10.1006/jmre.2002.2572. [DOI] [PubMed] [Google Scholar]
- 33.Marsh D. Reaction fields and solvent dependence of the EPR parameters of nitroxides: the microenvironment of spin labels. J. Magn. Reson. 2008;190:60–67. doi: 10.1016/j.jmr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Marsh D., Toniolo C. Polarity dependence of EPR parameters for TOAC and MTSSL spin labels: correlation with DOXYL spin labels for membrane studies. J. Magn. Reson. 2008;190:211–221. doi: 10.1016/j.jmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Marsh D., Dzikovski B.G., Livshits V.A. Oxygen profiles in membranes. Biophys. J. 2006;90:L49–L51. doi: 10.1529/biophysj.106.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox R.O., Jr., Richards F.M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5 Å resolution. Nature. 1982;300:325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- 37.Esposito G., Carver J.A., Boyd J., Campbell I.D. High-resolution 1H NMR study of the solution structure of alamethicin. Biochemistry. 1987;26:1043–1050. doi: 10.1021/bi00378a010. [DOI] [PubMed] [Google Scholar]
- 38.Bak M., Bywater R.P., Howy M., Thomsen J.K., Adelhorst K. Conformation of alamethicin in oriented phospholipid bilayers determined by 15N solid-state nuclear magnetic resonance. Biophys. J. 2001;81:1684–1698. doi: 10.1016/S0006-3495(01)75822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erilov D.A., Bartucci R., Guzzi R., Marsh D., Dzuba S.A. Echo-detected electron paramagnetic resonance spectra of spin-labeled lipids in membrane model systems. J. Phys. Chem. B. 2004;108:4501–4507. [Google Scholar]
- 40.Salikov K.M., Tsvetkov Y.D. Electron spin-echo studies of spin-spin interactions in solids. In: Kevan L., Schwartz R.N., editors. Time Domain Electron Spin Resonance. Wiley-InterScience; New York: 1979. pp. 231–278. [Google Scholar]
- 41.Bartucci R., Guzzi R., Marsh D., Sportelli L. Chain dynamics in the low-temperature phases of lipid membranes by electron spin-echo spectroscopy. J. Magn. Reson. 2003;162:371–379. doi: 10.1016/s1090-7807(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 42.Huang H.W. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim. Biophys. Acta. 2006;1758:1292–1302. doi: 10.1016/j.bbamem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Dzikovski B.G., Livshits V.A., Marsh D. Oxygen permeation profile in lipid membranes: non-linear spin-label EPR. Biophys. J. 2003;85:1005–1012. doi: 10.1016/S0006-3495(03)74539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsh D. CRC Press; Boca Raton, FL: 1990. Handbook of Lipid Bilayers. [Google Scholar]
- 45.Flippen-Anderson J.L., George C., Valle G., Valente E., Bianco A. Crystallographic characterization of geometry and conformation of TOAC, a nitroxide spin-labeled Cα,α-disubstituted glycine, in simple derivatives and model peptides. Int. J. Pept. Protein Res. 1996;47:231–239. doi: 10.1111/j.1399-3011.1996.tb01350.x. [DOI] [PubMed] [Google Scholar]