Abstract
Integrins are transmembrane receptors for cell adhesion to the extracellular matrix. In cell migration, integrins are endocytosed from the plasma membrane or the cell surface, transported in vesicles and exocytosed actively at the cell front. In the current study, we examined the roles of VAMP3, a SNARE protein that mediates exocytosis, in cell migration and integrin trafficking. Small interfering RNA (siRNA)-induced silencing of VAMP3 inhibited chemotactic cell migration by more than 60% without affecting cell proliferation. VAMP3 silencing reduced the levels of β1 integrin at the cell surface but had no effect on total cellular β1 integrin, indicating that VAMP3 is required for trafficking of β1 integrin to the plasma membrane. Furthermore, VAMP3 silencing diminished cell adhesion to laminin but not to fibronectin or collagen. Taken together, these data suggest that VAMP3-dependent integrin trafficking is crucial in cell migration and cell adhesion to laminin.
Keywords: SNARE, VAMP3, cell migration, integrin, cell adhesion
Introduction
Cell migration is essential not only for embryonic development and maintenance of multicellular organisms, but also for various pathological conditions including inflammation, wound healing and cancer metastasis. Migration begins when a cell responds to an external signal by polarizing and extending a protrusion in the direction of movement [1]. The integrin receptors, which mediate cell adhesion to the extracellular matrix proteins including laminin, fibronectin and collagen [2], stabilize the protrusion and serve as traction points to move the cell body forward. Finally, release of adhesion and retraction at the cell rear completes the migration cycle.
Emerging evidence indicates that intracellular vesicle trafficking exerts temporal and spatial control over integrin functions [3; 4]. As transmembrane proteins, the α and β subunits of integrins are synthesized and paired in the endoplasmic reticulum [5; 6], transported in vesicles and delivered to the plasma membrane or the cell surface by exocytosis. In migrating cells, the net forward movement of the cell would lead to an accumulation of integrins towards the cell rear. To enhance adhesion at the cell front or the leading edge, integrins at the plasma membrane are endocytosed, transported forward by vesicles and exocytosed at the cell front [7; 8; 9]. Protein kinase Cα (PKCα) and PKCε regulate the exocytosis of integrins [10; 11]. Accordingly, Rab GTPases which control the targeting and tethering of transport vesicles have been shown to modulate integrin trafficking and cell motility [9; 12]. However, not much is known about the molecular mechanism of the final event of integrin exocytosis, i.e. fusion of integrin-containing vesicles with the plasma membrane.
On the other hand, research in the vesicle trafficking field has demonstrated that the interactions between SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins on vesicles (v-SNAREs) and SNARE proteins on target membranes (t-SNAREs) [13; 14; 15] drive intracellular vesicle fusion. Most SNAREs are C-terminally anchored transmembrane proteins, with their N-terminal functional domains facing the cytosol. These cytoplasmic domains of v- and t-SNAREs form an extremely stable four-helix bundle [16]. Energy made available from the assembly of the SNARE complex is used to bring the vesicular and target membranes into close proximity and drive membrane fusion [17; 18; 19]. What is not clear is exactly which SNARE proteins mediate the trafficking of integrins to the plasma membrane. Enriched in recycling endosomes and endosome-derived vesicles [20], the v-SNARE VAMP3 (cellubrevin) has been implicated in the exocytosis of α-granules in platelets [21] as well as the recycling of endocytosed transferrin receptors [22] to the cell surface. By the expression of a protease that cleaves VAMP3, recent studies suggest that VAMP3 is involved in integrin trafficking and cell migration [23; 24; 25].
In the current work, we further examined the roles of VAMP3 in cell migration and integrin trafficking using siRNA-induced gene silencing. We found that silencing of VAMP3 inhibited cell migration and diminished cell surface β1 integrin without altering total cellular β1. Furthermore, the effects of VAMP3 silencing on cell adhesion to laminin, fibronectin and collagen were determined.
Materials and methods
Cell culture
PANC-1 cells were obtained from the American Type Culture Collection, and cultured in Dulbecco Modified Eagle's Medium (DMEM) with 4.5g/l glucose and 10% Fetal Bovine Serum.
siRNA transfection
A 21 nucleotide siRNA oligo that targets VAMP3 sequence TCAAGCTTACCTACTGTTA was synthesized by Dharmacon Thermo Scientific. A predesigned siRNA oligo (Hs_ITGB1_9) that targets β1 integrin sequence ACAGATGAAGTTAACAGTGAA was obtained from QIAGEN. The day before transfection for transwell migration assay, cell adhesion assay or immunoblotting analysis, PANC-1 cells were seeded in 6-well plates at 2.5 × 105 cells per well. siRNAs were transfected into the cells at 10 nM using Lipofectamine RNAiMAX (Invitrogen). The AllStars Negative Control siRNA (QIAGEN) was used as a control.
Immunoblotting
After 48 h of siRNA transfection, PANC-1 cells were lysed in 2× SDS-PAGE sample buffer. The whole cell lysates were analyzed on SDS-PAGE and immunoblotted with a polyclonal antibody to VAMP3, a polyclonal antibody to SNAP-23 (both from Synaptic Systems GmbH), a monoclonal antibody to β-actin (Sigma) or a polyclonal antibody to β1 integrin (Santa Cruz Biotechnology), followed by HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Bound antibodies were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). The proteins bands visualized on the films were scanned and quantified using the ImageJ software.
Transwell migration assay
The transwell migration assay was performed as described [26] with modifications. DMEM serum-free medium containing growth factor-reduced Matrigel (BD Biosciences, 20 μg/ml) was added to the lower chambers of the 12-well format transwells (8 μm-pore, BD Biosciences) as chemoattractant. After 48 h of transfection, PANC-1 cells were harvested using trypsin/EDTA and added to the upper chambers at 8 × 104 cells per transwell. After 20 h at 37°C, the transwells were fixed in methanol, and stained with Giemsa Stain solution (Sigma). The unmigrated cells were removed from the top of the membranes using cotton swabs. To quantify the number of migrated cells, five to ten random images were taken at 10× on a light microscope for each transwell. The number of migrated cells per image was counted using the ImageJ software.
Cell proliferation assay
The day before transfection cells were seeded in 24-well plates at 3 × 104 cells per well. 0, 24, 48, and 72 h after siRNA transfection, cell culture medium was replaced with DMEM medium containing no phenol red, and the CellTiter 96 AQueous One Solution Reagent (Promega) was added to measure the number of living cells. After incubation at 37°C for 2 h, absorbance at 490 nm was measured in a 96-well ELISA plate reader. Absorbance from wells containing only the DMEM medium but no cells was taken as blank reading.
Immunocytochemistry
The day before transfection, 6 × 104 PANC-1 cells were seeded on sterile 12-mm glass coverslips contained in 24-well plates. 48 h after transfection, the cells were fixed with 4% paraformaldehyde in PBS++ (PBS supplemented with 0.1 g/l CaCl2 and 0.1 g/l MgCl2). To stain cell surface β1 integrin, the fixed cells were incubated in neat culture medium conditioned by the hybridoma cell line P5D2. P5D2 was developed by Dr. Wayner and was obtained from the Developmental Studies Hybridoma Bank maintained by The University of Iowa. FITC-conjugated secondary antibodies were used at a dilution of 1:500. Images were collected at 60× on an Olympus confocal microscope.
Cell adhesion assay
Each well of 24-well plates was coated with 20 μg of laminin, fibronectin (both from BD Biosciences) or collagen I (Sigma) for 1 h at 37°C. The plates were rinsed with PBS and blocked with 2% heat-inactivated BSA for 1 h at 37°C. 48 h after transfection, PANC-1 cells were harvested with trypsin/EDTA, added to the wells at 1 × 105 cells per well and allow to adhere for various time at 37°C. Nonadherent cells were removed by gentle washing and the number of attached cells measured using the CellTiter 96 AQueous One Solution Reagent as described in the cell proliferation assay.
Results
Silencing of VAMP3 inhibits cell migration
The exocytosis of integrins at the cell front contributes to the formation and stabilization of protrusions in cell migration. To examine the roles of VAMP3 in integrin trafficking and cell migration, we depleted VAMP3 expression by siRNA gene silencing. PANC-1, a human pancreatic cancer cell line widely used for studying cell migration and cancer biology [27; 28], was used as a model. As shown by immunoblotting analysis in Figs. 1 A and B, within 48 h after transfection of a siRNA against VAMP3, the expression of VAMP3 protein in PANC-1 cells was depleted by more than 95%. The large reduction of VAMP3 protein indicated high efficiency of siRNA transfection and interference. Transfection of VAMP3 siRNA did not modulate the expression of SNAP-23, a different SNARE protein, or the expression of β-actin (Fig. 1A). Altogether these data showed that the VAMP3 knockdown effect was specific.
Fig.1.
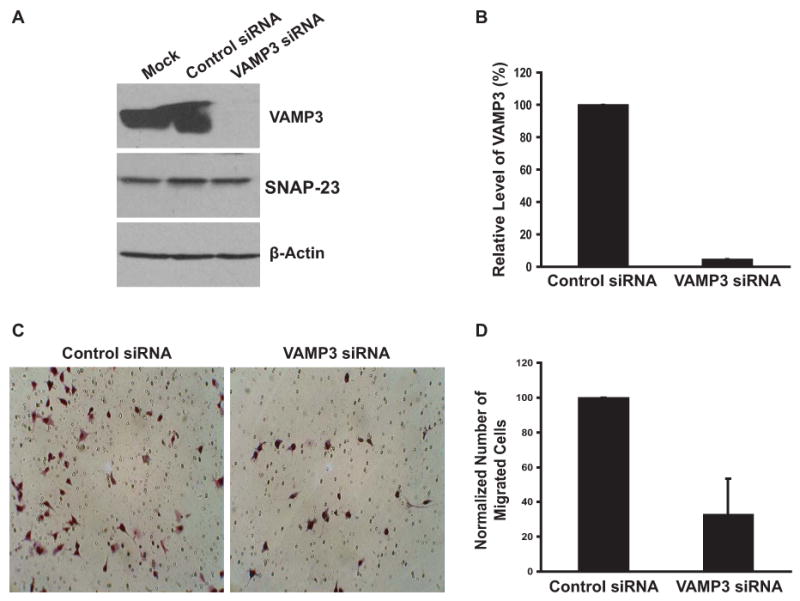
VAMP3 knockdown inhibits cell migration. (A) Representative immunoblotting analysis of VAMP3 expression. PANC-1 cells were mock transfected, transfected with a non-targeting control siRNA or a siRNA against VAMP3. 48 h after transfection whole cell lysates were immunoblotted with an antibody to VAMP3. The same membrane was blotted with an antibody to SNAP-23 or β-actin to examine the specificity of siRNA silencing. (B) The level of VAMP3 proteins in three independent immunoblotting experiments were quantified by densitometry and pooled. Error bar represents standard deviation of the three experiments. (C) 48 h after transfection with the control siRNA or the VAMP3 siRNA, PANC-1 cells were harvested and loaded to the top chambers of transwells. Matrigel (20 μg/ml) was included in the bottom chambers as chemoattractant. After 20 h at 37°C unmigrated cells were removed, and migrated cells were Giemsa stained. Representative images of migrated cells are shown. (D) Random images were taken for each transwell, and the number of migrated cells in each image was quantified using the ImageJ software then averaged for each experimental group. The number of migrated cells transfected with the VAMP3 siRNA was normalized to the number of migrated cells transfected with the control siRNA. Error bar represents standard deviation of six independent experiments in which two transwells were used for each transfection.
Motility of VAMP3 knockdown cells was analyzed using the transwell migration assay [26]. In initial experiments, we found that PANC-1 cells did not migrate through transwell membranes in the absence of chemoattractants and that among complete media, Collagen I and Matrigel, Matrigel was the strongest chemoattractant (data not shown). Matrigel was therefore used as the chemoattractant in further experiments. When loaded to the upper chambers of the transwells, PANC-1 cells migrated efficiently across the membranes in a relatively homogeneous fashion (Fig. 1C). Interestingly, cells transfected with VAMP3 siRNA had much reduced motility. Compared with the cells transfected with a non-targeting control siRNA, the number of migrated VAMP3 knockdown cells decreased by 67% (Figs. 1C and D), indicating that VAMP3 was required in cell migration.
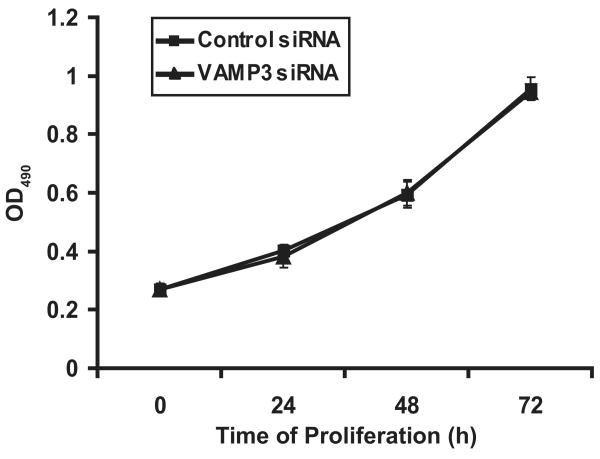
VAMP3 silencing has no effect on Cell Proliferation
To determine if the reduced number of migrated cells was due to inhibition of cell proliferation, we assessed the proliferation of VAMP3 knockdown cells using a colorimetric method. Clearly, the VAMP3 knockdown cells proliferated at the same rate as the cells transfected with the control siRNA (Fig. 2). This was an indication that VAMP3-mediated vesicle trafficking was not required for cell survival or proliferation, and ruled out the possibility that the observed reduction in the number of migrated cells (Figs. 1C and D) was a result of decreased cell viability.
Fig. 2.
VAMP3 silencing has no effect on cell proliferation. At 0, 24, 48, or 72 h after transfection with the control siRNA or the VAMP 3 siRNA, the number of living PANC-1 cells was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay by absorbance at 490nm. The experiment was done using three replicates for each transfection. The error bars represent standard deviation of three independent transfections.
VAMP3 is required for trafficking of β1 integrin to the cell surface
β1 integrin is a predominant β subunit that can pair with multiple α subunits to form heterodimeric integrin receptors [2]. It promotes the migration of various types of cells [29; 30]. When β1 integrin in PANC-1 cells was depleted by siRNA transfection (Fig. 3A), chemotactic cell migration was inhibited by more than 85% (Fig. 3B), indicating that β1 integrin was required for migration of PANC-1 cells. Since VAMP3 was also required for PANC-1 cell migration (Figs. 1C and D), we next tested if VAMP3 was involved in the trafficking of β1 integrin. PANC-1 cells transfected with the control siRNA or the VAMP3 siRNA were immunostained for cell surface β1. Indeed, silencing of VAMP3 effectively reduced the amount of β1 at the cell surface (Fig. 3C). In contrast, immunoblotting analysis showed that the levels of total cellular β1 were not altered in the VAMP3 knockdown cells (Fig. 3D). The reduction of cell surface β1 but not total cellular β1 suggested that VAMP3 knockdown disrupted the trafficking of β1 integrin to the plasma membrane. The decreased motility of VAMP3 knockdown cells (Fig. 1) probably resulted from reduced delivery of β1 integrin to the cell surface.
Fig.3.
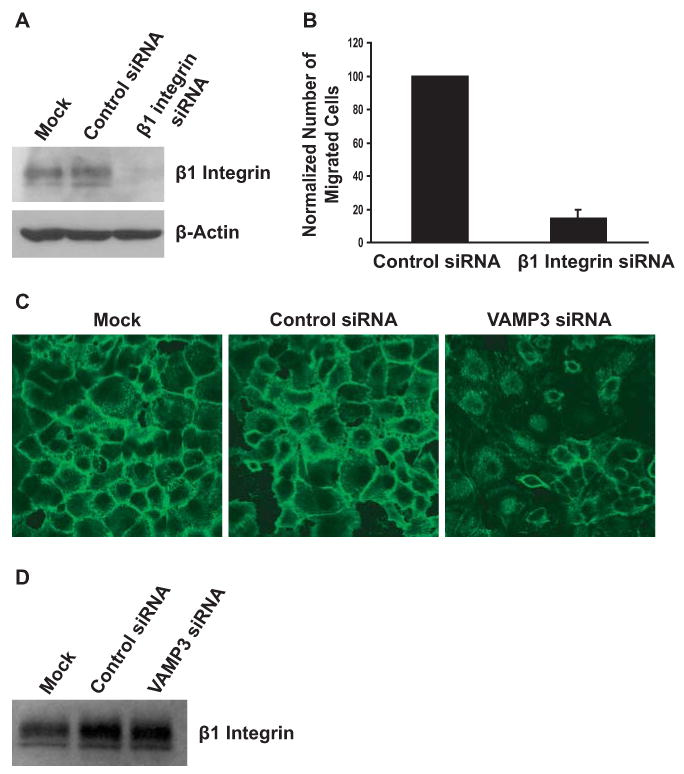
VAMP3 is required for the trafficking of β1 integrin to the cell surface. (A) 48 h after mock transfection, transfection with the control siRNA or a siRNA to β1 integrin, whole cell lysates of the PANC-1 cells were immunoblotted with an antibody to β1. (B) 48 h after transfection with the control siRNA or the siRNA to β1 integrin, motility of PANC-1 cells was determined as described in Fig. 2. Error bar represents standard deviation of three independent experiments. (C) Depletion of VAMP3 reduced cell surface β1 integrin. 48 h after mock transfection, transfection with the control siRNA or the VAMP3 siRNA, unpermeabilized PANC-1 cells were stained with an antibody to β1 integrin. Representative confocal images of five experiments are shown. (D) VAMP3 knockdown had no effect on total cellular β1 integrin. 48 h after mock transfection, transfection with the control siRNA or the VAMP3 siRNA, whole cell lysates of PANC-1 cells were immunoblotted with an antibody to β1 integrin to determine the amount of total cellular β1.
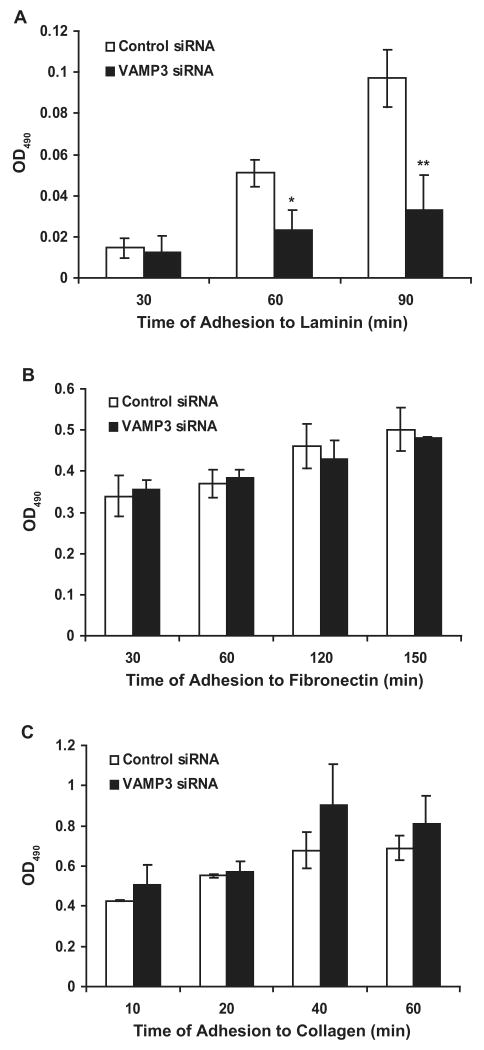
Silencing of VAMP3 inhibits cell adhesion to laminin
β1 integrin can pair with the α3, α6 or α7 integrin subunit to form heterodimeric laminin receptors, with α5, αV or α8 to form fibronectin receptors, or with α1, α2, α10 or α11 to form collagen receptors [2]. To further elucidate which types of integrin receptors are transported by VAMP3-dependent vesicle trafficking, we measured the effects of VAMP3 knockdown on cell adhesion to laminin, fibronectin or collagen. VAMP3 siRNA markedly suppressed adhesion of PANC-1 cells to laminin. At 60 min and 90 min, adhesion to laminin was attenuated by 55% and 66%, respectively (Fig. 4A). However, cell adhesion to fibronectin or collagen was not affected by VAMP3 silencing (Figs. 4B and C). Consistent with the observation that VAMP3 silencing reduced cell surface β1 (Fig. 3C), the cell adhesion data suggested that VAMP3 participated selectively in trafficking of the laminin receptors - α3β1 integrin, α6β1 integrin and α7β1 integrin.
Fig. 4.
Silencing of VAMP3 inhibits cell adhesion to laminin. 48 h after transfection with the control siRNA (white columns) or the VAMP3 siRNA (black columns), PANC-1 cells were added to laminin-coated plates (A), fibronectin-coated plates (B) or collagen-coated plates (C). At different time points, unattached cells were washed away, and the number of adherent cells was measured using a colorimetric assay by absorbance at 490 nm. Error bars represent standard deviation of three independent experiments. *P < 0.02 vs. cells transfected with the control siRNA. **P < 0.01 vs. cells transfected with the control siRNA.
Discussion
In migrating cells, integrins are transported in vesicles and exocytosed actively at the cell front [4]. Although it is well established that SNAREs mediate intracellular vesicle fusion [14; 15], it is not clear which SNARE proteins mediate the trafficking of integrins to the plasma membrane. Using RNA interference, we show here that silencing of the v-SNARE VAMP3 effectively inhibited cell migration without affecting proliferation. VAMP3 silencing reduced the amount of β1 integrin at the cell surface without altering total cellular β1, indicating that VAMP3 is required for trafficking of β1 to the plasma membrane. In addition, VAMP3 silencing inhibited cell adhesion to laminin but not to fibronectin or collagen. Together, these data suggest that VAMP3-mediated integrin trafficking is critical in cell migration and cell adhesion to laminin.
By expression of the catalytic light chain of tetanus toxin (TeTx-LC), a protease that specifically cleaves and inactivates VAMP1, VAMP2 and VAMP3, several studies show that inhibition of VAMP3 function interferes cell motility and integrin functions. In CHO cells, expression of TeTx-LC impairs cell migration, reduces cell surface α5β1 integrin, and inhibits cell spreading but not adhesion to fibronectin [23; 24]. In MDCK cells, TeTx-LC expression reduces cell motility and disrupts the recycling of β1 integrin [25]. Since among the three VAMP proteins, VAMP3 is the only one expressed in CHO cells and MDCK cells [24; 25], these results suggest that VAMP3 participates in integrin trafficking and cell migration. In the present study, we used siRNA to silence VAMP3 and found that VAMP3 silencing inhibited cell migration by more than 60% and effectively reduced the amount of β1 integrin at the cell surface. This work provides further evidence that VAMP3-dependent integrin trafficking plays an important role in cell migration. In addition, we showed that VAMP3 silencing inhibited cell adhesion to laminin but not to fibronectin or collagen. For the first time, these results indicated that VAMP3 participates selectively in trafficking of the integrins that function as laminin receptors.
Vesicle trafficking is an integral part of the cellular cascades that lead to concerted motions from different parts of migrating cells [3]. The small Rho GTPases are the central regulators of actin polymerization and cell migration. Interestingly, a few Rho GTPases (Cdc42, Rac1, RhoB, etc.) are associated with vesicular compartments [31]. In addition to actin polymerization, the Rho GTPases regulate vesicle trafficking in cell migration [31]. To extend protrusions, plasma membrane needs to be expanded at the cell front. In migrating cells, exocytosis of the cell surface receptors such as transferrin receptors, occurs mainly at the leading edge [32], indicating that new plasma membrane components are indeed added at the cell front. Matrix metalloproteinases, which degrade basement membrane to facilitate migration, are also delivered to the leading edge through polarized exocytosis [33]. Furthermore, integrins, which serve as the “feet” of a migrating cell, are endocytosed from the plasma membrane, transported and exocytosed at the cell front [3; 4]. Our current work showed that silencing of VAMP3, a SNARE protein that mediates the trafficking of β1 integrin to the cell surface, effectively reduced cell motility. These findings further indicate that vesicle trafficking is indispensable in cell migration.
Acknowledgments
We thank McAnthony Tarway for the initial work of setting up the transwell migration assay in the lab. This work was supported by a scientist development grant from the American Heart Association, startup funds from the University of Louisville School of Medicine and CA135123 from the National Institutes of Health (to C.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. doi: 10.1016/s0092-8674(00)81380-x. [DOI] [PubMed] [Google Scholar]
- 4.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 5.Lenter M, Vestweber D. The integrin chains beta 1 and alpha 6 associate with the chaperone calnexin prior to integrin assembly. J Biol Chem. 1994;269:12263–12268. [PubMed] [Google Scholar]
- 6.Rigot V, Andre F, Lehmann M, Lissitzky JC, Marvaldi J, Luis J. Biogenesis of alpha6beta4 integrin in a human colonic adenocarcinoma cell line involvement of calnexin. Eur J Biochem. 1999;261:659–666. doi: 10.1046/j.1432-1327.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- 7.Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 8.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivaska J, Whelan RD, Watson R, Parker PJ. PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002;21:3608–3619. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 14.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 15.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 16.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 17.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 18.McNew JA, Weber T, Parlati F, Johnston RJ, Melia TJ, Sollner TH, Rothman JE. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C, Ahmed M, Melia TJ, Sollner TH, Mayer T, Rothman JE. Fusion of cells by flipped SNAREs. Science. 2003;300:1745–1749. doi: 10.1126/science.1084909. [DOI] [PubMed] [Google Scholar]
- 20.McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Sudhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- 21.Polgar J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- 22.Galli T, Chilcote T, Mundigl O, Binz T, Niemann H, De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skalski M, Coppolino MG. SNARE-mediated trafficking of alpha5beta1 integrin is required for spreading in CHO cells. Biochem Biophys Res Commun. 2005;335:1199–1210. doi: 10.1016/j.bbrc.2005.07.195. [DOI] [PubMed] [Google Scholar]
- 24.Tayeb MA, Skalski M, Cha MC, Kean MJ, Scaife M, Coppolino MG. Inhibition of SNARE-mediated membrane traffic impairs cell migration. Exp Cell Res. 2005;305:63–73. doi: 10.1016/j.yexcr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Proux-Gillardeaux V, Gavard J, Irinopoulou T, Mege RM, Galli T. Tetanus neurotoxin-mediated cleavage of cellubrevin impairs epithelial cell migration and integrin-dependent cell adhesion. Proc Natl Acad Sci U S A. 2005;102:6362–6367. doi: 10.1073/pnas.0409613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 27.Stahle M, Veit C, Bachfischer U, Schierling K, Skripczynski B, Hall A, Gierschik P, Giehl K. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835–3846. doi: 10.1242/jcs.00679. [DOI] [PubMed] [Google Scholar]
- 28.Vogelmann R, Nguyen-Tat MD, Giehl K, Adler G, Wedlich D, Menke A. TGFbeta-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci. 2005;118:4901–4912. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- 29.Sakai T, Peyruchaud O, Fassler R, Mosher DF. Restoration of beta1A integrins is required for lysophosphatidic acid-induced migration of beta1-null mouse fibroblastic cells. J Biol Chem. 1998;273:19378–19382. doi: 10.1074/jbc.273.31.19378. [DOI] [PubMed] [Google Scholar]
- 30.Rigot V, Lehmann M, Andre F, Daemi N, Marvaldi J, Luis J. Integrin ligation and PKC activation are required for migration of colon carcinoma cells. J Cell Sci. 1998;111(Pt 20):3119–3127. doi: 10.1242/jcs.111.20.3119. [DOI] [PubMed] [Google Scholar]
- 31.Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- 32.Bretscher MS. Distribution of receptors for transferrin and low density lipoprotein on the surface of giant HeLa cells. Proc Natl Acad Sci U S A. 1983;80:454–458. doi: 10.1073/pnas.80.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]