Summary
In the Mayo Clinic Cohort Study of Oophorectomy and Aging, women who had both ovaries removed before reaching natural menopause experienced a long-term increased risk of parkinsonism, cognitive impairment or dementia, and depressive and anxiety symptoms. Here, we discuss five possible mechanistic interpretations of the observed associations: 1) the associations may be non-causal because they result from the confounding effect of genetic variants or of other risk factors; 2) the associations may be mediated by an abrupt reduction in levels of circulating estrogen; 3) the associations may be mediated by an abrupt reduction in levels of circulating progesterone or testosterone; 4) the associations may be mediated by an increased release of gonadotropins by the pituitary gland; and 5) genetic variants may modify the hormonal effects of bilateral oophorectomy through simple or more complex interactions. Results from other studies are cited as evidence for or against each possible mechanism. These putative causal mechanisms are probably intertwined, and their clarification is a research priority.
Keywords: oophorectomy, estrogen, progesterone, testosterone, gonadotropins, parkinsonism, dementia, anxiety, depression, menopause
Public health significance of bilateral oophorectomy
Every year, approximately 300,000 women face the decision to undergo prophylactic bilateral oophorectomy in conjunction with hysterectomy [1]. The practice of prophylactic bilateral oophorectomy has increased over time, more than doubling between 1965 and 1999 [2], but the risk-benefit balance of prophylactic oophorectomy versus ovarian preservation remains uncertain and controversial [1,3–7]. Based on a study of the incidence of oophorectomy in Olmsted County, MN, we project that an additional 300,000 U.S. women undergo bilateral oophorectomy for a benign ovarian condition every year [8]. In total, approximately 600,000 women undergo bilateral oophorectomy in the U.S. every year, many before reaching natural menopause. For all of these women, it remains unknown whether, and for how long, estrogen treatment is needed, or whether other hormonal replacement treatments are needed [6].
We previously reported from the Mayo Clinic Cohort Study of Oophorectomy and Aging that women who underwent early bilateral oophorectomy before the onset of menopause have an increased overall mortality, as well as increased mortality associated with cardiovascular disease and with neurological or psychiatric diseases, compared with referent women [3,9]. In addition, we reported that those women have an increased risk of parkinsonism, cognitive impairment or dementia, and depressive and anxiety symptoms compared with referent women (see executive summary) [7,10–13]. In this paper, we propose several possible mechanistic interpretations of the observed associations with aging-related neurological diseases, and we discuss their clinical and research implications. Results from other studies are cited as evidence for or against each possible mechanism.
Executive summary
Facts (associations)
Bilateral oophorectomy performed before the onset of menopause is associated with an increased risk of cognitive impairment or dementia. The association is stronger with younger age at oophorectomy, is independent of the indication for oophorectomy, and may be offset by estrogen treatment.
Bilateral oophorectomy performed before the onset of menopause is associated with an increased risk of parkinsonism and Parkinson’s disease. The association is stronger with younger age at oophorectomy and is independent of the indication for oophorectomy, but is not offset by estrogen treatment.
Bilateral oophorectomy performed before the onset of menopause is associated with an increased risk of long-term depressive and anxiety symptoms. The association is stronger with younger age at oophorectomy and is independent of the indication for oophorectomy, but is not offset by estrogen treatment.
Possible causal mechanisms
The associations may be due to a chain of causality prompted by reduced levels of circulating estrogen.
The associations may be due to a chain of causality prompted by reduced levels of circulating progesterone or testosterone.
The associations may be due to a chain of causality prompted by an increased release of gonadotropins by the pituitary gland.
The associations may involve the synergistic or antagonistic interaction of bilateral oophorectomy with genetic variants (e.g., APOE or ESR1 genes). The interactions may be complex and may also involve other non-genetic factors.
Clinical and research implications
The associations listed above need to be confirmed (or challenged) by other studies.
The four causal mechanisms listed above have implications for medical treatment in women who must undergo early bilateral oophorectomy.
Additional laboratory and clinical research is needed to clarify mechanisms and to guide treatment to restore normal physiology when oophorectomy is required.
The Mayo Clinic Cohort Study of Oophorectomy and Aging
We conducted a historical cohort study among all women residing in Olmsted County, Minnesota, who underwent unilateral or bilateral oophorectomy before the onset of menopause for a non-cancer indication from 1950 through 1987. Each member of the oophorectomy cohort was matched by age to a referent woman from the same population who had not undergone oophorectomy. In total, we studied 1,252 women with unilateral oophorectomy, 1,075 women with bilateral oophorectomy, and 2,368 referent women. Women were followed for a median of 25 to 30 years. Parkinsonism was assessed using screening and examination, through a medical records-linkage system, and through death certificates. By contrast, cognitive status or dementia, and depressive or anxiety symptoms were assessed using a structured questionnaire via a direct or proxy telephone interview. Our results are summarized in the executive summary, and were reported in detail elsewhere [3,7,9–13]. In this paper, we limit our discussion to the findings related to bilateral oophorectomy.
Endocrine consequences of early bilateral oophorectomy
The ovary is the primary source of estrogen and progesterone during reproductive life. In addition, the ovary produces testosterone both before and after menopause. This may be particularly important because testosterone is aromatized peripherally into estrone, the major circulating estrogen after menopause [1,14,15], and into estradiol, the most potent estrogen, in widespread tissues and organs including the brain [16–18].
The hormonal changes induced by premenopausal bilateral oophorectomy are different from those occurring during natural menopause or those induced by postmenopausal bilateral oophorectomy. In particular, bilateral oophorectomy may occur at ages much younger than natural menopause (median age of approximately 50 years) [19], and the resulting hormonal changes are abrupt. By contrast, the endocrine changes of natural menopause result from a progressive decline in ovarian function. Although hormone levels and menstrual cycles are quite irregular and unpredictable during the menopausal transition, the underlying physiologic process is a progressive decrease in ovarian follicle numbers [20,21]. Bilateral oophorectomy before menopause results not only in an abrupt drop in levels of circulating estrogen but also an abrupt drop in levels of circulating progesterone and testosterone and in a disruption of the hypothalamic-pituitary-ovarian axis [22]. Disruption of this axis is associated, in turn, with an increased release of the gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH) by the pituitary gland. By contrast, if the ovaries are removed long after a woman has experienced natural menopause, the hormonal changes related to estrogen, progesterone, and gonadotropins may be less dramatic because estrogen and progesterone levels are already naturally reduced. However, the postoperative drop in testosterone levels is abrupt and may have clinical consequences [15].
It is uncertain to what extent the harmful effects of premenopausal bilateral oophorectomy are uniquely mediated by estrogen deficiency, or whether other mechanisms are involved [22–26]. Indeed, in the Mayo Clinic Cohort Study of Oophorectomy and Aging, estrogen treatment through age 50 years in women with early bilateral oophorectomy did not offset the increased risks of parkinsonism, anxiety symptoms, or depressive symptoms [11,13]. However, estrogen treatment through age 50 years did offset the increased risk of overall mortality, of cardiovascular mortality, and of cognitive impairment and dementia (see executive summary) [3,9,10]. Thus, we need new mechanistic hypotheses and new research to address these unresolved issues.
Possible mechanisms linking bilateral oophorectomy with aging-related neurological diseases
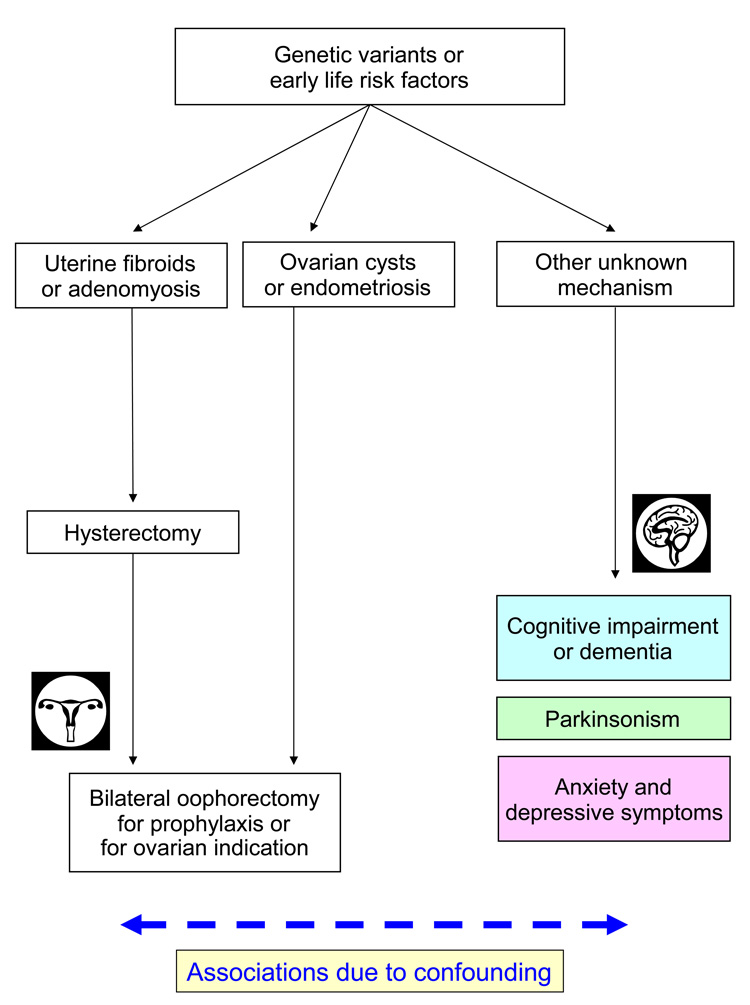
In figure 1 through figure 3 we depict five possible mechanistic interpretations of the observed associations between bilateral oophorectomy and aging-related neurological diseases; undoubtedly, other models could be devised. We first explore the possibility that these associations are non-causal, an artifact of confounding (figure 1). Following this line of reasoning, bilateral oophorectomy and aging-related neurological diseases may be independently linked to a third factor that explains the observed spurious associations. Thus, bilateral oophorectomy would be a marker of risk but not a causal factor.
Figure 1.
First possible mechanistic explanation of the associations between bilateral oophorectomy and brain outcomes. The associations are due to the confounding effect of genetic variants or other early life risk factors.
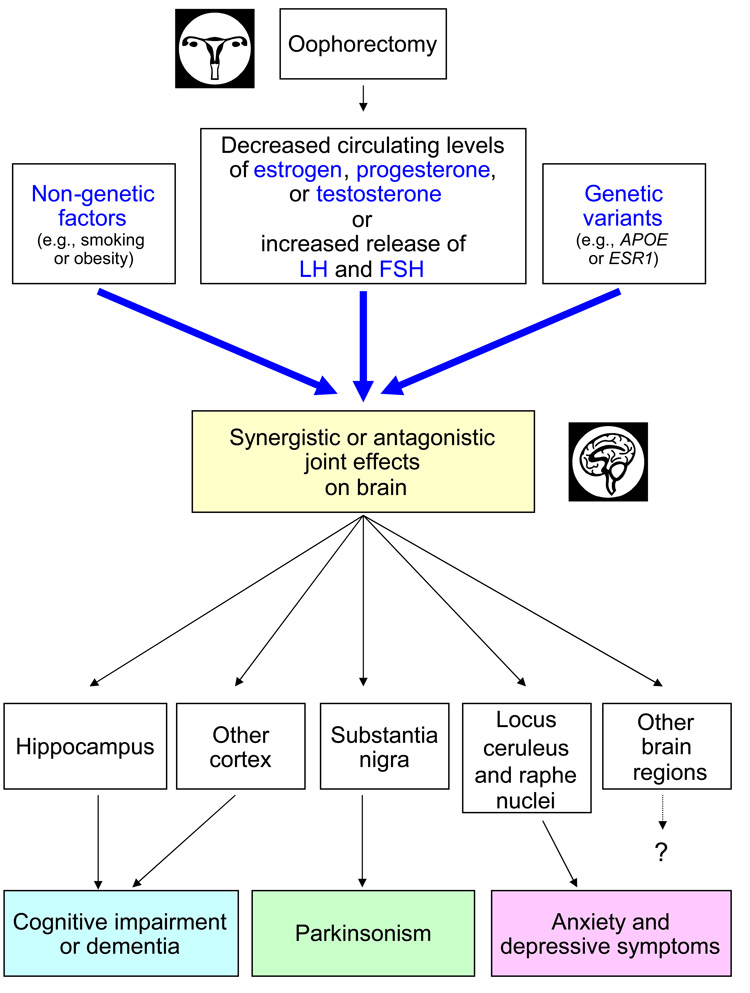
Figure 3.
Fifth possible mechanistic explanation of the associations between bilateral oophorectomy and brain outcomes. The associations involve the synergistic or antagonistic interaction of the hormonal effects of bilateral oophorectomy with genetic variants or with non-genetic factors. Some of the arrows and boxes are speculative and are depicted only as examples of possible causal relationships. For example, the brain regions mentioned and the corresponding diseases are given as preliminary examples of an uncertain causal map.
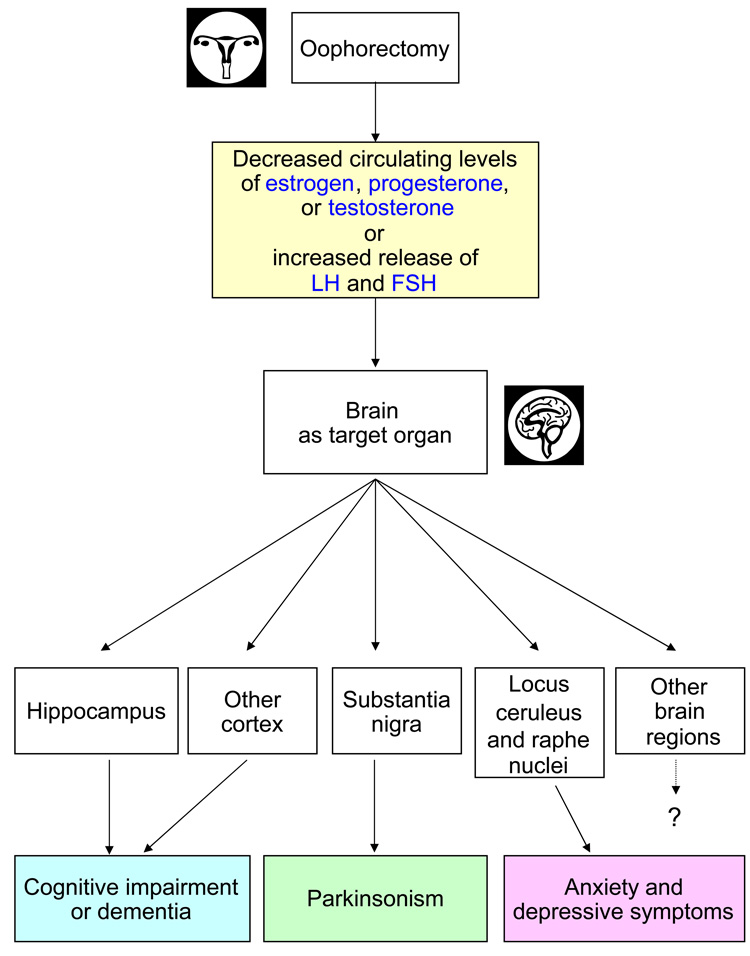
We then discuss four possible direct causal mechanisms that may act in isolation or in combination because they are not mutually exclusive (figure 2 and figure 3). Most likely, these mechanisms are intertwined and revolve around changes in the hypothalamic-pituitary-ovarian axis. Thus, multiple mechanisms may be responsible for an aging-related neurological disease in a particular woman (multifactorial etiology at the individual level), and multiple mechanisms may act differently across women (etiologic heterogeneity at the population level). In addition, the contribution of each mechanism may vary depending on the specific neurological disease considered (e.g., dementia versus parkinsonism).
Figure 2.
Second, third, and fourth possible mechanistic explanations of the associations between bilateral oophorectomy and brain outcomes. The associations are due to a chain of causality prompted by an abrupt decrease in circulating estrogen, progesterone, or testosterone, or by an increased release of gonadotropins (LH and FSH). Some of the arrows and boxes are speculative and are depicted only as examples of possible causal relationships. For example, the brain regions mentioned and the corresponding diseases are given as preliminary examples of an uncertain causal map.
Figure 1 through Figure 3 represent oversimplified and tentative conceptual schemes to guide our discussion. Some of the arrows and boxes are speculative and are depicted only as examples of possible causal relationships. For example, the brain regions mentioned and the corresponding diseases are given as preliminary examples of an uncertain causal map. Unfortunately, there is little evidence of the specific effects of bilateral oophorectomy on any of these brain regions.
First mechanism: Confounding by genetic variants or by early life risk factors
Under this hypothetical mechanism, the association between bilateral oophorectomy performed before the onset of menopause and aging-related neurological diseases is due to susceptibility genes that increase the risk of both outcomes independently (i.e., confounding by genetic predisposition; figure 1). For example, genetic variants in the estrogen synthesis or responsiveness pathway may predispose women to develop uterine abnormalities such as fibroids or adenomyosis leading to menorrhagia or metrorrhagia that, in turn, prompt the removal of the uterus. In approximately 50% of the cases, women and their physicians elect to perform a prophylactic bilateral oophorectomy in conjunction with the hysterectomy [6]. Similarly, genetic variants that predispose women to ovarian cysts or endometriosis may prompt a bilateral oophorectomy to treat the lesions, to prevent recurrences, or to prevent subsequent ovarian cancer. If the same genetic variants that increase the risk of benign uterine or ovarian lesions also increase the risk of neurological diseases (through unknown hormonal or non-hormonal mechanisms), the shared risk would cause confounding. In this situation, the bilateral oophorectomy has no causal role but becomes a marker of the underlying genetic predisposition (figure 1). Thus, changes in surgical practice favoring ovarian preservation in young women would not affect the risk of subsequent neurological diseases.
In support of this hypothesis, there is evidence that genetic factors predispose women to hysterectomy. A twin study showed 63% heritability for age at natural menopause and 59% heritability for hysterectomy prior to natural menopause. In addition, the heritability of the two major indications for hysterectomy was also high: 69% for fibroids and 55% for menorrhagia [27]. Heritability is likely due to the transmission of genetic variants. Indeed, another study showed that women with a variant in the estrogen receptor 1 gene (ESR1; SNP rs2234693) have an increased risk of surgical menopause [28]. Therefore, genetic variants could determine uterine diseases that prompt hysterectomy, in turn, prompting prophylactic bilateral oophorectomy. By contrast, the evidence for an association between genetic variants and ovarian cysts or endometriosis remains limited [29–31], and the contribution of genetic factors to the risk of benign ovarian conditions that may prompt bilateral oophorectomy remains unknown.
For the genetic variants to act as a confounder, they must be associated not only with the oophorectomy but also with the aging-related neurological diseases [32]. However, the evidence linking certain variants of the genes in the estrogen synthesis and responsiveness pathway with the risk of dementia, parkinsonism, or depressive and anxiety symptoms remains limited [33–37]. For example, in the first genome-wide association study of Parkinson’s disease involving a mixed sample of men and women, we found 11 single nucleotide polymorphisms (SNPs) associated with Parkinson’s disease. Of those SNPs, one was in the PR domain-containing protein 2 gene (PRDM2), which encodes an estrogen receptor co-activator protein [36]. In addition, we reanalyzed our data considering only women (172 cases; 229 controls), and found several additional associations with genetic variants in the ESR1, estrogen receptor 2 (ESR2), and PDRM2 genes [34]. However, these initial findings await replication.
Alternatively, confounding could be caused by another non-genetic risk factor. We can postulate that some early life events, such as the use or non-use of oral contraceptives, or the number and outcome of pregnancies, may predispose women to uterine or ovarian diseases leading to bilateral oophorectomy, and independently may predispose women to aging-related neurological diseases. Once again, in this case, bilateral oophorectomy would be only a marker of risk. However, we are not aware of any evidence in support of this hypothesis.
Against a confounding effect is our observation of a similar risk of neurological diseases among women who underwent bilateral oophorectomy for benign conditions or for prophylaxis of ovarian cancer because these findings suggest that the risk of neurological diseases was independent of the indication for the oophorectomy. By contrast, if confounding was present, we would expect a variation in risk across indications. Although the possibility that confounding is the explanation of the observed associations cannot be completely ruled out at this time, the evidence for a confounding mechanism is limited. Thus, we propose three potential causal mechanisms involving the hormonal changes induced by bilateral oophorectomy. In addition, we propose one mechanism combining hormonal changes with underlying genetic factors.
Second mechanism: Chain of causality prompted by decreased levels of circulating estrogen
Under this hypothetical mechanism, the abrupt reduction in circulating estrogen caused by bilateral oophorectomy is the initial step in a chain of causality leading to aging-related neurological diseases (figure 2). Premature estrogen deficiency (not compensated by a sufficient duration of estrogen replacement treatment) could affect several specific regions of the brain, in turn causing neurological symptoms and diseases. This hypothesis is partly supported by our analyses dividing women who underwent bilateral oophorectomy before age 49 years into two strata: women who received estrogen treatment through age 50 years or longer and those who did not. The increased risk of cognitive impairment or dementia was restricted to women who did not receive estrogen treatment [10]. By contrast, the increased risk of parkinsonism and of depressive and anxiety symptoms was not restricted to women who did not receive estrogen treatment [11,13]. Thus, estrogen treatment after bilateral oophorectomy may offset the risk of some but not all neurological outcomes.
The hypothesis of a neuroprotective effect of estrogen is corroborated by several observational studies that showed a reduced risk of dementia in women treated with estrogen started early in menopause compared with women not treated [38–42]. By contrast, the Women’s Health Initiative (WHI) clinical trials showed an increased risk of cognitive impairment or dementia in women aged 67 through 79 years treated with estrogen alone or in combination [43–46]. To reconcile the contrasting findings from observational studies and from clinical trials, it has been suggested that the effects of estrogen on the brain may vary with age (“timing hypothesis”or “window of opportunity hypothesis” [12,42,47–50]. However, several important questions remain unanswered about this hypothesis.
Third mechanism: Chain of causality prompted by decreased levels of circulating progesterone or testosterone
Under this hypothetical mechanism, the abrupt reduction in circulating progesterone or testosterone caused by bilateral oophorectomy is the initial step in a chain of causality leading to aging-related neurological diseases (figure 2). Premature progesterone and testosterone deficiency could affect several specific regions of the brain, in turn causing neurological symptoms and diseases. This hypothesis is supported by the failure of estrogen treatment to offset the increased risk of parkinsonism and of depressive and anxiety symptoms in the Mayo Clinic Cohort Study of Oophorectomy and Aging [11,13]. However, the analyses stratified by estrogen treatment were based on somewhat small samples, and the evidence is insufficient to completely exclude a protective effect of estrogen for these diseases [11,13]. For example, in the analyses for depressive symptoms, we compared 78 women who underwent oophorectomy before age 49 years and were given estrogen to age 50 years with 412 referent women [13]. Additional studies with a larger sample are needed to confirm the lack of protective effect of estrogen for some neurological outcomes.
The hypothesis of a neuroprotective effect of progesterone and testosterone is corroborated by several laboratory and clinical studies [23,25,26,44,51,52]. However, there is some contrasting evidence that progesterone could have harmful effects on the aging brain [53,54]. Further research is needed to clarify these issues.
Fourth mechanism: Chain of causality prompted by increased release of gonadotropins
Under this hypothetical mechanism, the abrupt reduction in circulating estrogen and progesterone caused by bilateral oophorectomy prompts a disruption of the hypothalamus-pituitary-ovarian axis resulting in increased release of the gonadotropins LH and FSH by the pituitary gland. The increased release of gonadotropins is then the initial step in a chain of causality leading to aging-related neurological diseases (figure 2). Thus, premature estrogen and progesterone deficiency results in increased release of gonadotropins which, in turn, affects several specific regions of the brain leading to neurological symptoms and diseases.
This hypothesis is supported by the protective effect of estrogen therapy (which lowers gonadotropin levels) on symptoms of dementia, but is refuted by the failure of estrogen treatment to offset the increased risk of parkinsonism and of depressive and anxiety symptoms in the Mayo Clinic Cohort Study of Oophorectomy and Aging [11,13]. However, the analyses stratified by estrogen treatment were based on small samples, and the evidence is insufficient to fully understand the possible protective effects of estrogen mediated by the modulation of gonadotropin levels.
The hypothesis of a harmful effect of LH on the brain is corroborated by several laboratory and clinical studies [22,24,55,56]. For example, a study in a transgenic mouse model of Alzheimer’s disease showed that drugs blocking the release of LH significantly attenuated cognitive decline and decreased β-amyloid deposition in treated animals compared with placebo-treated animals [57]. However, more clinical and epidemiologic research is needed to clarify the effects of LH and FSH on the brain.
Fifth mechanism: Interactions between genetic variants, non-genetic factors, and oophorectomy
Some genetic variants may modify the effect of bilateral oophorectomy on the brain causing a synergistic or an antagonistic joint effect (figure 3) [32]. Thus, some women may be more prone to experience aging-related neurological diseases following bilateral oophorectomy because, for example, they carry genetic variants that modify the effects of estrogen [37]. This mechanism is exemplified by the interaction between APOE genotype and estrogen in the pathogenesis of dementia. Women who carry the APOE genotypes ε3ε4 or ε4ε4 have an increased risk of Alzheimer’s disease and dementia [58,59], and approximately 27% of women in the general population carry one of these high risk genotypes [59].
It has been hypothesized that apolipoprotein E (apoE; the protein coded by the APOE gene) may be a critical factor in the neuroprotective actions of estrogen [56,57]. There is increasing evidence from both in vivo (mice) and in vitro studies (cell cultures) that estrogen may modulate the apoE protein and its receptor, namely, the low density lipoprotein receptor-related protein [60,61]. Results from numerous laboratory studies have demonstrated that: 1) nerve regeneration is severely delayed in APOE-gene knockout mice as compared to wild-type littermates; 2) estrogen replacement in ovariectomized mice resulted in a significant increase in levels of apoE protein and low density lipoprotein receptor-related protein in the olfactory bulb and other brain areas; 3) estrogen treatment increased apoE protein and increased neurite outgrowth in cortical and olfactory neuronal cultures; and 4) estrogen treatment had no effect on neurite outgrowth in cultures deprived of apoE protein or in cultures with the apoE4 protein.
These studies suggest that apoE protein is a critical intermediary for the beneficial effects of estrogen on neuronal protection and repair [61–64]. The hypothesis that the neuroprotective effects of estrogen may be modified by APOE genotype is also supported by some clinical and epidemiologic studies [65–68]; however, none of these studies focused on women with bilateral oophorectomy.
More complex etiologic interactions
The joint effects of oophorectomy and genes may be much more complex than the example provided, and likely involve the interactions of several genes with each other and with hormonal factors. For example, the effect of oophorectomy may be modified jointly by variants of the APOE gene and of the ESR1 gene with multiple levels of synergistic or antagonistic effects [33,66]. In addition, the genetic effects may involve several complex hormonal synthesis and responsiveness pathways rather than individual genes [69]. Genetic effects may also involve epigenetic mechanisms [70,71]. Finally, other non-genetic factors (e.g., smoking, obesity) may modify the effect of oophorectomy and of several genetic variants (figure 3).
Implications for research and clinical practice
The causal mechanisms discussed here have important implications for clinical practice and for research. If harmful long-term effects of bilateral oophorectomy on the brain are mediated primarily by decreased circulating levels of estrogen, estrogen replacement may be an adequate and important treatment for women who undergo oophorectomy for benign indications. By contrast, if some of the harmful effects of bilateral oophorectomy on the brain are caused by decreased circulating levels of progesterone or testosterone, additional hormonal treatments may be needed. Finally, if the increased release of LH or FSH is an important factor, drugs targeting gonadotropins may be useful. Our results from the Mayo Clinic Cohort Study of Oophorectomy and Aging suggest that the interplay of hormonal mechanisms may be complex and may vary depending on the specific neurological disease considered.
At present, it remains unknown if and for how long estrogen treatment should be continued, or whether other hormonal treatments are needed for the approximately 600,000 women who undergo bilateral oophorectomy in the U.S. every year, many before reaching natural menopause [6]. In general, regardless of the causal mechanism, the optimal practice is likely ovarian preservation in the majority of young women not known to be at increased risk of ovarian or breast cancer [1,3–5,7].
From a research perspective, additional clinical studies are needed to explore the specific effects of bilateral oophorectomy on brain aging in women. Although the Mayo Clinic Cohort Study of Oophorectomy and Aging provided new provocative evidence, the findings await replication, and raise many new questions. Studies with a long period of follow-up after oophorectomy are few and insufficient whereas studies with shorter follow-up may not be adequate to address the questions that remain unanswered. Thus, clinical trials are not likely to address the observed associations because their design is only adequate for testing short-term effects (5 to 10 years). In addition, the randomization of women to prophylactic bilateral oophorectomy, or the randomization of women who underwent bilateral oophorectomy to estrogen or other hormonal replacement treatment, may raise ethical concerns. In general, the practices of ovarian preservation, oophorectomy, and hormone replacement therapy tend to be individualized and the complexity of decision-making precludes a “one size fits all” approach.
In addition, new laboratory and clinical research studies are needed to clarify the hormonal mechanisms and the possible genetic interactions. Studies of the effects of estrogen, progesterone, testosterone, LH, and FSH on the brain may provide new strategies for the prevention and treatment of aging-related neurological diseases. However, these hormonal effects may vary greatly with age [12]. For example, estrogen effects on the brain may vary in premenopausal women, during the menopausal transition, and after menopause (“timing hypothesis” or “window of opportunity hypothesis”) [12,42,47–50].
Future perspective
We are facing a rapid aging of the population worldwide [72]. This rapid aging will increase dramatically the number of people projected to be affected by aging-related neurological diseases. This dramatic trend is well exemplified by the available statistics for dementia in general and Alzheimer’s disease in particular. In 2006, the number of people affected by Alzheimer’s disease was 26.6 million worldwide and the majority of patients were women. Moreover, it was estimated that $156 billion is spent annually to care for dementia patients worldwide [73]. By 2050, the prevalence is expected to quadruple, so that 1 in 85 persons will be living with the disease [74], and 43% of them are expected to need a high level of care (e.g., a nursing home).
Although the prevalence of dementia and its associated disability increases exponentially with age [75,76], the focus of research has recently shifted towards younger persons and very early stages of cognitive decline and mild cognitive impairment. The hope is to delay the conversion of cognitive decline and mild cognitive impairment to full dementia. Indeed, if interventions could delay disease onset or progression by as little as 1 year, we would expect nearly 9.2 million fewer Alzheimer’s disease patients by the year 2050 [74].
A better understanding of the long-term sequelae of bilateral oophorectomy and of the effects of ovarian hormones on brain aging may guide the development of interventions to delay disease onset or slow disease progression, leading to a reduced burden of neurological diseases. Thus, we suggest that this is an area of high research priority and of major significance for the health of aging women.
Financial disclosure/Acknowledgements
This research was supported by the National Institute of Neurological Disorders and Stroke (grant R01 NS33978) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant R01 AR30582).
Footnotes
The authors have no conflicts of interest to disclose.
Bibliography
Papers of special note have been highlighted as either of interest (•) of considerable interest (••) to readers.
- 1.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet. Gynecol. 2005;106:219–226. doi: 10.1097/01.AOG.0000167394.38215.56.Initial summary of the scientific evidence on the risks and benefits of prophylactic bilateral oophorectomy.
- 2.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance--United States, 1994–1999. MMWR. Surveill. Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 3.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5.Mortality findings from the Mayo Clinic Cohort Study of Oophorectomy and Aging.
- 4.Shoupe D, Parker WH, Broder MS, Liu Z, Farquhar C, Berek JS. Elective oophorectomy for benign gynecological disorders. Menopause. 2007;14:580–585. doi: 10.1097/gme.0b013e31803c56a4. [DOI] [PubMed] [Google Scholar]
- 5.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Clin. Obstet. Gynecol. 2007;50:354–361. doi: 10.1097/GRF.0b013e31804a838d. [DOI] [PubMed] [Google Scholar]
- 6.ACOG. ACOG Practice Bulletin No. 89. Elective and risk-reducing salpingo-oophorectomy. Obstet. Gynecol. 2008;111:231–241. doi: 10.1097/01.AOG.0000291580.39618.cb.Current practice recommendations for bilateral oophorectomy in the U.S.
- 7.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111–116. doi: 10.1258/mi.2008.008016.Summary of findings from the Mayo Clinic Cohort Study of Oophorectomy and Aging.
- 8.Melton LJ, 3rd, Bergstralh EJ, Malkasian GD, O'Fallon WM. Bilateral oophorectomy trends in Olmsted County, Minnesota, 1950–1987. Epidemiology. 1991;2:149–152. [PubMed] [Google Scholar]
- 9.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality following early bilateral oophorectomy. Menopause. 2008 doi: 10.1097/gme.0b013e31818888f7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6.Cognitive impairment and dementia findings from the Mayo Clinic Cohort Study of Oophorectomy and Aging.
- 11.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a.Parkinsonism and Parkinson's disease findings from the Mayo Clinic Cohort Study of Oophorectomy and Aging.
- 12.Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener. Dis. 2008;5:257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms following early bilateral oophorectomy. Menopause. 2008 doi: 10.1097/gme.0b013e318174f155. in press.Depressive and anxiety symptoms findings from the Mayo Clinic Cohort Study of Oophorectomy and Aging.
- 14.Hughes CL, Jr, Wall LL, Creasman WT. Reproductive hormone levels in gynecologic oncology patients undergoing surgical castration after spontaneous menopause. Gynecol. Oncol. 1991;40:42–45. doi: 10.1016/0090-8258(91)90083-h. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen DG. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 16.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis--some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 17.Simpson ER. Aromatization of androgens in women: current concepts and findings. Fertil. Steril. 2002;77:S6–S10. doi: 10.1016/s0015-0282(02)02984-9. [DOI] [PubMed] [Google Scholar]
- 18.Iivonen S, Corder E, Lehtovirta M, et al. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology. 2004;62:1170–1176. doi: 10.1212/01.wnl.0000118208.16939.60. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J. Clin. Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 20.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15:603–612. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- 21.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 22.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J. Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006.Comprehensive review on the basic neuroscience mechanisms involved in menopause.
- 23.Gibbs RG. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol. Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 24.Webber KM, Casadesus G, Marlatt MW, et al. Estrogen bows to a new master: the role of gonadotropins in Alzheimer pathogenesis. Ann. N. Y. Acad. Sci. 2005;1052:201–209. doi: 10.1196/annals.1347.020. [DOI] [PubMed] [Google Scholar]
- 25.Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29:271–274. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front. Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J. Clin. Endocrinol. Metab. 1998;83:1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 28.Weel AE, Uitterlinden AG, Westendorp IC, et al. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J. Clin. Endocrinol. Metab. 1999;84:3146–3150. doi: 10.1210/jcem.84.9.5981. [DOI] [PubMed] [Google Scholar]
- 29.Cramer DW, Petterson KS, Barbieri RL, Huhtaniemi IT. Reproductive hormones, cancers, and conditions in relation to a common genetic variant of luteinizing hormone. Hum. Reprod. 2000;15:2103–2107. doi: 10.1093/humrep/15.10.2103. [DOI] [PubMed] [Google Scholar]
- 30.Di W, Guo SW. The search for genetic variants predisposing women to endometriosis. Curr. Opin. Obstet. Gynecol. 2007;19:395–401. doi: 10.1097/GCO.0b013e328235a5b4. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery GW, Nyholt DR, Zhao ZZ, et al. The search for genes contributing to endometriosis risk. Hum. Reprod. Update. 2008;14:447–457. doi: 10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers; 2007. [Google Scholar]
- 33.Porrello E, Monti MC, Sinforiani E, et al. Estrogen receptor alpha and APOEepsilon4 polymorphisms interact to increase risk for sporadic AD in Italian females. Eur. J. Neurol. 2006;13:639–644. doi: 10.1111/j.1468-1331.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 34.Rocca WA, Grossardt BR, de Andrade M, Bower JH, Maraganore DM. Variants in genes in the estrogen synthesis and responsiveness pathway and risk of Parkinson's disease: a case-control study in women. Neurology. 2006;66:A64. [Google Scholar]
- 35.Luckhaus C, Sand PG. Estrogen Receptor 1 gene (ESR1) variants in Alzheimer's disease. Results of a meta-analysis. Aging Clin. Exp. Res. 2007;19:165–168. doi: 10.1007/BF03324684. [DOI] [PubMed] [Google Scholar]
- 36.Maraganore DM, de Andrade M, Lesnick TG, et al. High-resolution whole-genome association study of Parkinson disease. Am. J. Hum. Genet. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogervorst E, Bandelow S. Should surgical menopausal women be treated with estrogens to decrease the risk of dementia? Neurology. 2007;69:1070–1071. doi: 10.1212/01.wnl.0000279584.03800.3d. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 39.Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 40.LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 41.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N. Engl. J. Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 42.Harman SM, Naftolin F, Brinton EA, Judelson DR. Is the estrogen controversy over? Deconstructing the Women's Health Initiative Study: a critical evaluation of the evidence. Ann. N. Y. Acad. Sci. 2005;1052:43–56. doi: 10.1196/annals.1347.004. [DOI] [PubMed] [Google Scholar]
- 43.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 44.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 45.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 46.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 47.Manson JE, Bassuk SS, Harman SM, et al. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause. 2006;13:139–147. doi: 10.1097/01.gme.0000177906.94515.ff. [DOI] [PubMed] [Google Scholar]
- 48.Siegfried T. Neuroscience: it's all in the timing. Nature. 2007;445:359–361. doi: 10.1038/445359a. [DOI] [PubMed] [Google Scholar]
- 49.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J. Neurol. Neurosurg. Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. Am. J. Epidemiol. 2007;166:506–510. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- 51.Wierman ME, Basson R, Davis SR, et al. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J. Clin. Endocrinol. Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- 52.Yaffe K, Barnes D, Lindquist K, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol. Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Gouras GK, Greenfield JP, et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat. Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 54.Huang J, Guan H, Booze RM, Eckman CB, Hersh LB. Estrogen regulates neprilysin activity in rat brain. Neurosci. Lett. 2004;367:85–87. doi: 10.1016/j.neulet.2004.05.085. [DOI] [PubMed] [Google Scholar]
- 55.Casadesus G, Milliken EL, Webber KM, et al. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol. Cell. Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues MA, Verdile G, Foster JK, et al. Gonadotropins and cognition in older women. J. Alzheimers Dis. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- 57.Casadesus G, Webber KM, Atwood CS, et al. Luteinizing hormone modulates cognition and amyloid-[beta] deposition in Alzheimer APP transgenic mice. Biochim. Biophys. Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 59.Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Cheng X, McAsey ME, Li M, et al. Estradiol replacement increases the low-density lipoprotein receptor related protein (LRP) in the mouse brain. Neurosci. Lett. 2007;417:50–54. doi: 10.1016/j.neulet.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 61.Struble RG, Cady C, Nathan BP, McAsey M. Apolipoprotein E may be a critical factor in hormone therapy neuroprotection. Front. Biosci. 2008;13:5387–5405. doi: 10.2741/3088. [DOI] [PubMed] [Google Scholar]
- 62.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer's disease. J. Neurosci. 1998;18:3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horsburgh K, Macrae IM, Carswell H. Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J. Cereb. Blood Flow Metab. 2002;22:1189–1195. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- 64.Nathan BP, Barsukova AG, Shen F, McAsey M, Struble RG. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145:3065–3073. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- 65.Mattila KM, Axelman K, Rinne JO, et al. Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer's disease in women. Neurosci. Lett. 2000;282:45–48. doi: 10.1016/s0304-3940(00)00849-1. [DOI] [PubMed] [Google Scholar]
- 66.Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 67.Burkhardt MS, Foster JK, Laws SM, et al. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J. Alzheimers Dis. 2004;6:221–228. doi: 10.3233/jad-2004-6302. [DOI] [PubMed] [Google Scholar]
- 68.Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial Alzheimer disease in Latinos: interaction between APOE, stroke, and estrogen replacement. Neurology. 2006;66:35–40. doi: 10.1212/01.wnl.0000191300.38571.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanehisa M, Goto S, Hattori M, et al. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin. Pharmacol. Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 71.Grandjean P. Late insights into early origins of disease. Basic Clin. Pharmacol. Toxicol. 2008;102:94–99. doi: 10.1111/j.1742-7843.2007.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Day JC. Population projections of the United States, by age, sex, race, and Hispanic origin: 1993 to 2050. Washington, DC: U.S. Government Printing Office; Current population reports. 1993:25–1104.
- 73.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement. Geriatr. Cogn. Disord. 2006;21:175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 74.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 75.Rocca WA. Dementia, Parkinson's disease, and stroke in Europe: a commentary. Neurology. 2000;54:S38–S40. [PubMed] [Google Scholar]
- 76.Fratiglioni L, Rocca WA. Epidemiology of Dementia. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 2001. pp. 193–215. [Google Scholar]