Abstract
In an attempt to identify a sensitive and improved marker of mammalian copper status during neonatal development experiments compared two plasma cuproenzymes, peptidylglycine α-amidating monooxygenase (PAM ), an enzyme involved in peptide posttranslational activation, to ceruloplasmin (Cp), a ferroxidase involved in iron mobilization. Dietary Cu deficiency (Cu−) was studied in dams and offspring at postnatal age 3 (P3), P12, and P28. Rodent Cp activity rose during lactation whereas PAM activity fell. Reduction in Cp activity was more severe than reduction in PAM activity in Cu− offspring and dams. Cp activity was greater in rats than mice whereas PAM activity was similar in adults but greater in mouse than rat pups. Both cuproenzymes changed during neonatal development and when dietary copper was limiting. With proper controls, each enzyme can be used to assess copper status.
Keywords: PAM, Peptidylglycine α-amidating monooxygenase, Ceruloplasmin, Copper-deficient, Rats, Mice, Plasma, Age
1. Introduction
Mammals rely on many exogenous dietary factors to ensure proper development and homeostasis. One such factor is the essential transition metal, copper. North American recommendations set by the Food and Nutrition Board, Institute of Medicine, National Academy of Sciences suggest that adults consume 900 µg of copper a day to provide an adequate supply to support physiological functions of this metal. It is believed that the biochemical functions of copper are provided by approximately one dozen mammalian cuproenzymes, copper-binding proteins, that catalyze important biochemical reactions (Failla et al., 2001). Optimal copper homeostasis also depends on expression of a number of copper chaperone proteins and copper import and efflux transporters (Prohaska and Gybina, 2004).
Although adults consume approximately the recommended intake of copper daily, pregnant and lactating women, depending on which survey is evaluated, tend not to consume the extra 100–400 µg of copper to meet recommendations (Trumbo et al., 2001). One of the challenges is to evaluate copper status in this subset of the population and in infants. There is no widely accepted biochemical marker for evaluation of human copper status (Milne, 1994; Failla et al., 2001). Furthermore, pregnancy and lactation could have an impact on currently used markers.
Plasma copper has been and continues to be used as a marker of copper status (Milne, 1994). The vast majority of plasma copper is represented by the cuproenzyme ceruloplasmin (Cp) also known as ferroxidase. Even when dietary copper is limiting, liver is fully capable of synthesizing and secreting Cp protein into the plasma after it is fully glycosylated (Holtzman and Gaumnitz, 1970; Gitlin et al., 1992). However, following dietary copper restriction plasma Cp activity is reduced due to lack of the cofactor copper. Also Cp immunoreactive protein levels fall following dietary copper deficiency perhaps due to faster turnover of apo-Cp. Cp, an alpha-2 globulin, is an acute phase protein that is highly expressed during inflammation, which can be a common confounding variable using Cp to assess copper status. Furthermore Cp is induced by estrogen, thus activity of Cp is influenced by pregnancy and lactation.
Another potential copper status marker is erythrocyte Cu, Zn-superoxide dismutase (ESOD) activity (Uauy et al., 1985). ESOD activity may not be as sensitive as Cp because ESOD synthesis would depend on copper release from the liver and delivery to bone marrow. Furthermore, ESOD levels would reflect a more chronic intake of copper since red cell turnover is much slower than plasma proteins. Erythrocyte SOD protein level, as well as enzyme activity, is lower following dietary copper deficiency in rodents similar to plasma Cp (West and Prohaska, 2004).
An additional potential cuproenzyme marker is peptidylglycine-α-amidating monooxygenase (EC 1. 17. 14. 3) (PAM). PAM was discovered to be a cuproenzyme more than 20 years ago in seminal studies (Eipper et al., 1983). PAM is an enzyme with two catalytic domains. One domain binds copper and catalyzes the hydroxylation of the C-terminal glycine in a large number of inactive neuropeptides and is referred to as peptidylglycine-α-hydroxylating monooxygenase (PHM). The second catalytic domain is a lyase (PAL) that subsequently hydrolyzes the previous hydroxylated peptide releasing glyoxylate and the resulting amidated peptide product (Eipper et al., 1992). PAM is responsible for the posttranslational modification of many important neuropeptides including oxytocin, vasopressin, ACTH, αMSH, VIP, substance P, neuropeptide Y, cholecystokinin, gastrin, and a large number of other molecules (Eipper et al., 1992). PAM is normally a membrane bound or vesicular enzyme but is detected in plasma (Eipper et al., 1985).
Previous research has indicated that the in vitro biochemical activity of PAM is lower in tissues and plasma of copper deficient rats (Prohaska et al., 1995; Peterson and Prohaska, 1999). A preliminary study in humans with a variant of Menkes' disease, an inherited disorder with symptoms of copper deficiency, suggested that plasma PAM might be useful to assess copper status in humans (Prohaska et al., 1997). However, PAM has not been evaluated during pregnancy and lactation, nor in experimental mammals besides the albino rat. Furthermore, PAM activity has not been evaluated in neonatal mammals.
The purpose of the current studies was to compare Cp and PAM activity in two rodent models during lactation to determine if PAM might be a more suitable biochemical marker than Cp to evaluate copper status during this physiological state. Offspring from both rodent models were evaluated just after delivery, midway through lactation, and one week after weaning to evaluate early postnatal development. Dams were evaluated three weeks after delivery at the termination of lactation. Both Cp and PAM activity were influenced by age and dietary copper intake.
2. Materials and methods
2.1. Experimental animals and diets
Holtzman rats (Rattus norvegicus) and ICR mice (Mus musculus) were purchased commercially (Harlan Sprague Dawley, Indianapolis, IN, USA). Animals received one of two dietary treatments, copper-deficient (Cu−) or copper-adequate (Cu+), consisting of a copper-deficient purified diet (Teklad Laboratories, Madison, WI, USA) and either low copper drinking water or copper-supplemented drinking water, respectively. The purified diet was formulated according to the AIN-76A diet except that cupric carbonate was omitted from the AIN-76 mineral mix. The purified diet contained 0.35 mg Cu/kg by chemical analysis. Offspring and dams on the copper-deficient treatment drank deionized water, whereas copper adequate treatment groups drank water that contained 20 mg Cu/L by adding CuSO4 to the drinking water. Animals were given free access to diet and drinking water. All animals were maintained at 24 °C with 55% relative humidity on a 12-h light cycle (0700–1900-h). All protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Established models of perinatal copper deficiency were used with slight modifications (Prohaska and Bailey, 1993; Prohaska and Brokate, 2001). Samples taken from offspring and dams were representative of two–three independent replicate experiments. Male offspring, one per litter, were killed at postnatal age 3 days (P3), P12, and P28. Typically, a minimum of 8 litters (4 Cu+and 4 Cu−) were sampled in each of the rodent experiments. Dams were killed at day 20 of lactation when pups were weaned.
Pregnant rats were placed on the copper-deficient or copper-adequate treatment 7 days after mating (E7). Two days following parturition litter size for each dam was adjusted to ten pups. Pregnant mice were placed on treatment at E17. Gestation in both rats and mice is about 21 days. Therefore, rat dams were maintained on the copper-deficient protocols for 34 days (14 of gestation and 20 of lactation) compared to mice 24 days (4 days of gestation and 20 days of lactation). These times were designed to produce viable offspring but severe copper deficiency and were based on our previous models. Offspring were weaned when 20 days old and maintained on the same treatment as their respective dams for an additional 8 days.
2.2. Sample collection
After light ether anesthesia, and following decapitation, trunk blood was drawn from rats and mice into heparinized microhematocrit tubes. A small aliquot was also removed for hemoglobin analysis. Plasma was obtained by centrifugation. A portion of the liver was removed, rinsed with deionized water, weighed, and processed for metal analysis.
2.3. Chemical analyses
Portions of liver and 1-g samples of diets were wet-digested with 4 ml of concentrated HNO3 (Trace Metal Grade, Fischer Scientific, Pittsburgh, PA, USA), and the residue was brought to 4.0 ml with 0.1 mol/l HNO3. Samples were then analyzed for copper and iron by flame atomic absorption spectroscopy (Model 1100B, Perkin-Elmer, Norwalk, CT, USA). Hemoglobin was determined spectrophotometrically as cyanmethemoglobin as described previously (Prohaska, 1983).
2.4. Enzyme assays
Plasma ceruloplasmin diamine oxidase activity was determined spectrophotometrically by measuring o-dianisidine oxidation using acetate buffers optimized for both rats and mice (Prohaska, 1991). Activity of plasma PAM was assayed by measuring the conversion of labeled d-tyr–val–gly to d-tyr–val–NH2 by HPLC using basal conditions (no added copper) as described in detail elsewhere (Prohaska et al., 1995).
2.5. Gel filtration chromatography
A 3 mL pooled adult rat serum sample from control animals was fractionated on Sephadex G-150 with 20 mmol/L potassium phosphate (pH 7.0) buffer. Absorbance at 280 nm was measured and an aliquot of each fraction was used to determine Cp and PAM activity. The G-150 column was previously calibrated with blue dextran (void volume) and with protein standards: glucose oxidase, alkaline phosphatase, bovine albumin, ovalbumin, and myoglobin (Sigma Chemical Co.). Size of Cp and PAM was estimated by linear regression by comparing their elution volume (Ve) to void volume (Vo) ratio with standards.
2.6. Statistical analyses
Means and SEM were calculated. Student's unpaired two-tailed t-test was used when comparing data between the two diet treatments within species, α=0.05 (Statview 4.5, Abacus Concepts, Inc., Berkeley, CA, USA). Factorial ANOVA was used to evaluate species and age differences only in copper adequate pups and dams since dietary treatment times were different for rats and mice.
3. Results
3.1. Characteristics of plasma PAM
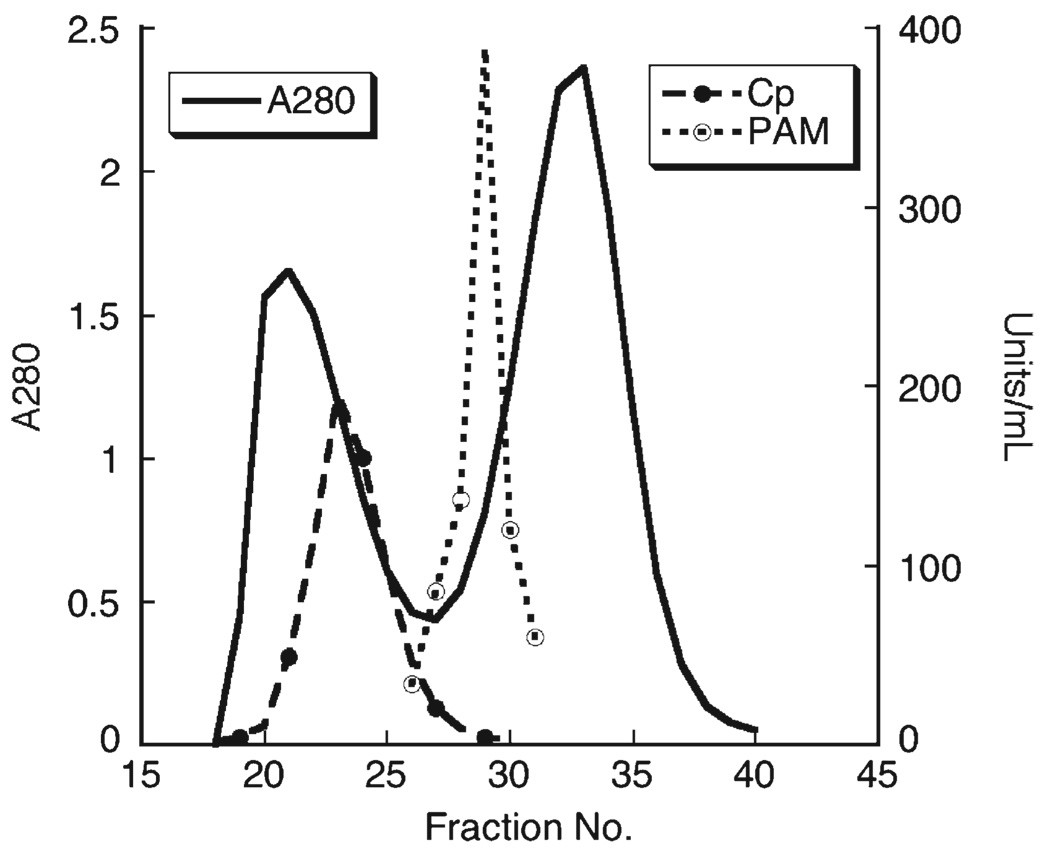
Plasma from adult control rats was subjected to gel permeation chromatography and fractions were assayed for protein content, and activity of two cuproenzymes PAM and Cp (Fig. 1). PAM eluted as a single symmetrical peak with an estimated size of 95 kDa compared to protein standards used to calibrate the column. Cp activity eluted as a larger protein, 140 kDA, as a shoulder on the void volume and two large protein peaks, the void volume and albumin, were detected. We repeated the G-150 experiments three times with similar size estimates for both PAM and Cp.
Fig. 1.
Gel filtration chromatography of rat plasma. Three milliliters of pooled adult copper adequate rat plasma was applied to a Sephadex G-150 column 1.6 × 83 cm equilibrated in 0.02 mol/L postassium phosphate (pH 7.0). Fractions were used to measure protein (absorbance at 280 nm), and the enzyme activity of Cp and PAM (U/mL). The void volume was detected with blue dextran and the column was calibrated with standards. Log molecular weight (y-axis) was plotted versus Ve/Vo (x-axis), y=6.24−0.91, R=0.991, to estimate size of Cp and PAM.
3.2. Copper status of rat and mouse offspring following perinatal copper deficiency
Data on copper status indicators (Table 1, Table 2 and Table 3) represent a single experiment. However each experiment was repeated at least once and often twice. When copper was restricted during the last two thirds of gestation and throughout lactation, male Cu− rat pups displayed signs consistent with severe copper deficiency, in a time dependent manner, such as lower body weight and cardiac hypertrophy (Table 1). Pups at postnatal day 3 (P3) exhibited mild cardiac hypertrophy without anemia. At age P12 and P28 anemia and hypertrophy were most evident. Liver copper levels were lower in Cu− rats at all ages compared to Cu+ rats but liver iron was only elevated at P28. In fact, at P3 and P12 liver iron was significantly lower in Cu− than Cu+rats (Table 1).
Table 1.
Copper status of Cu-adequate and Cu-deficient male rats following perinatal copper deficiency
| Characteristics | Cu+ | Cu− | Cu+ | Cu− | Cu+ | Cu− |
|---|---|---|---|---|---|---|
| Age, days | 3 | 3 | 12 | 12 | 28 | 28 |
| Body mass, g | 7.81±0.49 | 7.77±0.42 | 30.1±1.0 | 29.1±0.6 | 79.1±4.1 | 54.6±4.6* |
| Heart/Body, mg/g | 6.80±0.20 | 7.50±0.23* | 5.88±0.07 | 7.98±0.49* | 5.24±0.17 | 13.6±2.17* |
| Liver Cu, µg/g | 28.3±2.71 | 3.36±0.38* | 44.3±6.4 | 1.14±0.14* | 10.5±3.2 | 0.35±0.03* |
| Liver Fe, µg/g | 207±12.8 | 166±13.5* | 27.7±1.0 | 22.6±0.65* | 31.4±4.3 | 54.1±2.5* |
| Hemoglobin, g/L | 119±7.1 | 112±1.72 | 111±4.4 | 76.4±3.6* | 131±4.7 | 78.8±5.9* |
Values are means±SEM. Rats, n=(4 or 8 at P3), were born to and nursed by Cu-deficient or Cu-adequate dams. Treatment began two weeks prior to parturition. Liver metal concentrations were determined by flame atomic absorption following wet ashing and are based on fresh weight.
P<0.05 compared to Cu+ rats.
Table 2.
Copper status of Cu-adequate and Cu-deficient male mice following perinatal copper deficiency
| Characteristics | Cu+ | Cu− | Cu+ | Cu− | Cu+ | Cu− |
|---|---|---|---|---|---|---|
| Age, days | 3 | 3 | 12 | 12 | 28 | 28 |
| Body mass, g | 2.54±0.16 | 2.38±0.15 | 8.08±0.6 | 8.15±0.3 | 19.9±0.7 | 16.1±1.8 |
| Heart/Body, mg/g | 6.08±0.18 | 7.11±0.22* | 5.24±0.12 | 8.03±0.67* | ||
| Liver Cu, µg/g | 28.9±4.0 | 4.79±1.19* | 22.2±4.7 | 1.49±0.22* | 3.62±0.19 | 1.09±0.06* |
| Liver Fe, µg/g | 104±17.7 | 77.5±14.9 | 30.4±2.1 | 37.6±5.4* | 112±19 | 386±74* |
| Hemoglobin, g/L | 104±5.0 | 66.7±3.7* | 138±2.9 | 57.9±6.2* |
Values are means ± SEM. Mice, n=(4 or 5), were born to and nursed by Cu-deficient or Cu-adequate dams. Treatment began four days prior to parturition. Liver metal concentrations were determined by flame atomic absorption following wet ashing and are based on fresh weight.
P<0.05 compared to Cu+ mice.
Table 3.
Copper status of rat and mouse dams following dietary copper deficiency
| Characteristics | Rats | Mice | ||
|---|---|---|---|---|
| Cu-adequate | Cu-deficient | Cu-adequate | Cu-deficient | |
| Body mass, g | 344±22 | 355±18.7 | 31.2±1.5 | 30.5±0.38 |
| Liver Cu, µg/g | 4.60±0.70 | 0.79±0.10* | 4.28±0.33 | 2.26±0.11* |
| Liver Fe, µg/g | 68.8±2.5 | 109±13* | 73.9±11 | 316±37.9* |
| Hemoglobin, g/L | 132±9.4 | 104±12.8 | 151±3.5 | 62.8±11.4* |
Values are means±SEM (n=4). Dams were killed 20 days after lactation began. Treatment began two weeks (rats) or four days (mice) prior to parturition. Organ metal concentrations were determined by flame atomic absorption following wet ashing and are based on fresh weight.
P<0.05 compared to Cu+ dams.
Similar trends were observed for mouse pups following perinatal copper deficiency (Table 2). Treatment began four days prior to delivery in the mouse studies. Body weight was not impacted by diet of dams but cardiac hypertrophy was evident in Cu− mouse pups at both P12 and P28. Hearts were not removed from P3 pups in this study but in a recent experiment we were unable to detect hypertrophy in Cu− P3 pups (data not shown). Liver copper was lower at all age comparisons and liver iron higher at P12 and P28 in Cu− compared to Cu+mice. Frank anemia was evident in P12 and P28 Cu− mouse pups (Table 2). At P3 mean hematocrit values of the Cu− pups (34.1%) were not different than Cu+pups (33.3%). Collectively these data indicate that mice of two distinct copper status were evaluated.
3.3. Copper status of dams following dietary copper deficiency
The Cu− rat and mouse dams displayed signs of copper deficiency similar to their offspring (Table 3). Both Cu− rat and mouse dams had lower liver copper and higher liver iron concentrations. However, anemia (lower hemoglobin) was evident only in the Cu− mouse dams.
3.4. Comparison of plasma Cp and PAM in rat and mouse pups following copper deficiency
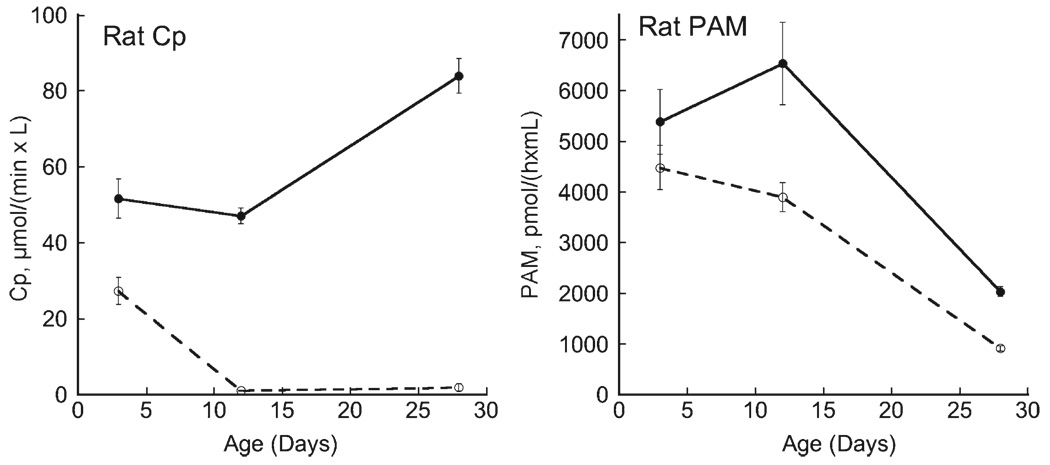
Plasma samples were obtained from individual pups from individual litters. Cp activity in rat plasma increased modestly between P3 and P28 (Fig. 2). However those pups delivered and nursed by Cu− rat dams rapidly lost detectable Cp activity. In contrast, rat plasma PAM activity fell markedly in pups during lactation. Dietary copper restriction also affected PAM activity as it was significantly lower in both P12 and P28 Cu− rats compared to Cu+samples (Fig. 2).
Fig. 2.
Comparison of rat plasma Cp and PAM activity during perinatal copper deficiency. Plasma was obtained from individual male pups, one per litter, during the suckling period (P3 and P12) and shortly after weaning (P28). Deficiency was started at gestational day 7. Activity of Cp and PAM was determined in copper-adequate (Cu+) (solid lines) and copper-deficient (Cu−) (dashed lines) pups. Means with SEM of 4 to 5 male offspring are shown. Cp activity was lower in Cu-samples at all ages, P<0.05. PAM activity was lower in Cu-samples at P12 and P28 compared to Cu+ samples, P<0.05.
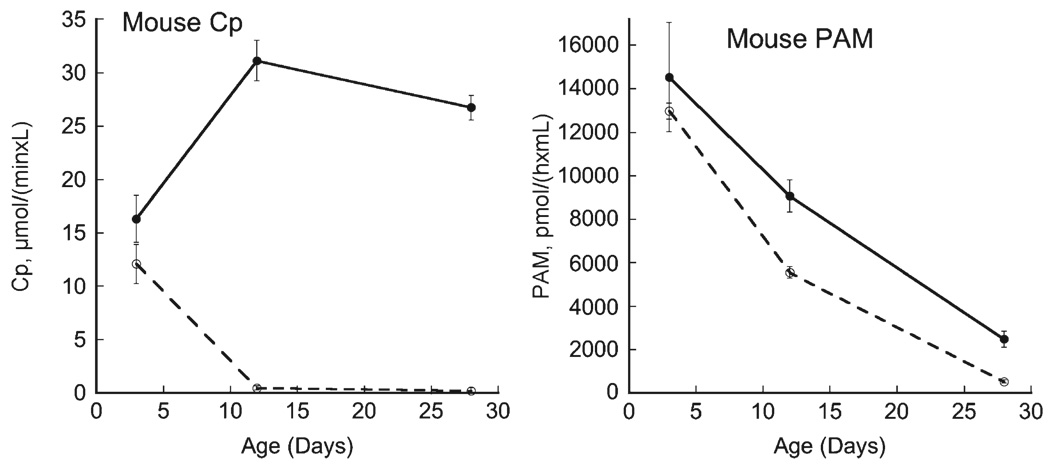
A similar pattern was observed for mouse pups (Fig. 3). Plasma Cp in mice is much lower than rats (P<0.01) and also was nearly undetectable following perinatal copper deficiency in P12 and P28 Cu− pups. There was no difference in Cp activity at P3 between Cu− and Cu+pups despite lower liver copper (Table 2). As for rats, plasma PAM activity in mouse pups also fell markedly during lactation (Fig. 3). Furthermore, at P12 and P28 plasma PAM activity in Cu− mice was lower than Cu+mice. Note that plasma PAM activity in Cu+ mouse pups was modestly higher than Cu+rats (P<0.01). This was opposite to the magnitude of Cp activity between Cu+ rats and mice (P<0.01).
Fig. 3.
Comparison of mouse plasma Cp and PAM activity during perinatal copper deficiency. Plasma was obtained from individual male pups, one per litter, during the suckling period (P3 and P12) and shortly after weaning (P28). Deficiency was started at gestational day 17. Activity of Cp and PAM were determined in copper-adequate (Cu+) (solid lines) and copper-deficient (Cu−) (dashed lines) pups. Means with SEM of 4 to 5 male offspring are shown. Cp and PAM activity were lower in Cu-samples at P12 and P28 compared to Cu+ samples, P<0.05.
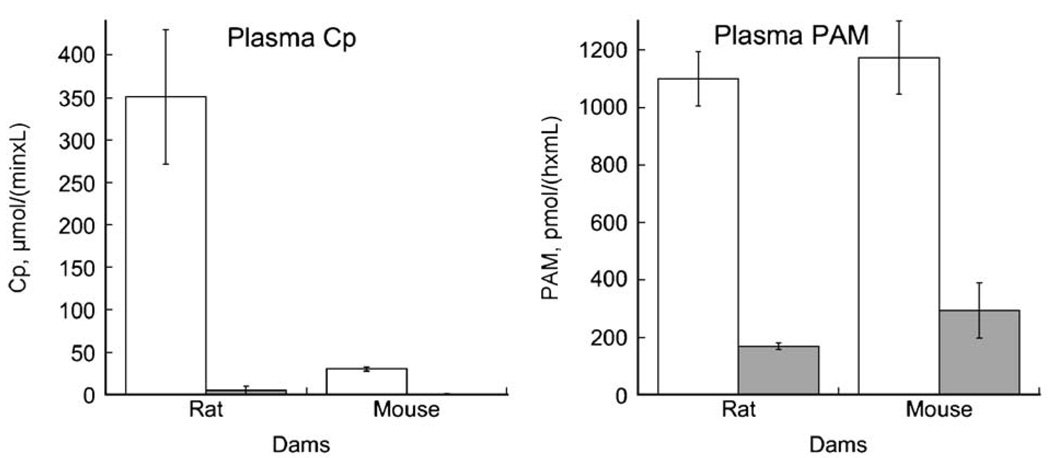
3.5. Comparison of plasma Cp and PAM in rat and mouse dams following copper deficiency
Cp activity of rat dams was very high compared to mouse dams similar to their offspring (P<0.01) (Fig. 4). Restriction of dietary copper resulted in lower Cp activity in both Cu− rodent dam groups. We did not measure Cp and PAM in plasma of age-matched non-lactating rats in these studies. However, prior work (Prohaska and Hoffman, 1996) on adult Sprague Dawley Cu+non-lactating rats reported approximately half the Cp activity, 184±3.4 U/L (n=4), observed in the Holtzman lactating rats in this study (Fig. 4). Holtzman rats are derived from Sprague Dawley. In contrast, a separate study using another outbred albino mouse strain (ND4 Swiss Webster), Cp activity of adult female non-lactating mice was 34.3±1.5 (n=4) U/L compared to 39.2±4.1 (n=3) in age-matched lactating mouse dams, P>0.05.
Fig. 4.
Plasma Cp and PAM activity in rat and mouse dams following perinatal copper deficiency. Plasma was obtained from dams at day P20 of lactation. Deficiency was started at gestational day 7 for rats and day 17 for mice. Bars represent means and error bars SEM (n=4). Compared to copper-adequate dams (open bars) activity of plasma enzymes in copper-deficient dams (shaded bars) was lower for both Cp and PAM, P<0.05.
Plasma PAM of lactating Cu+rat and mouse dams, in contrast to Cp, was similar in magnitude in both rodent species (P>0.05) (Fig. 4). Similar to Cp, copper deficiency resulted in significant and similar reductions in plasma PAM activity in Cu− dams compared to Cu+controls. PAM activity in mice, as for Cp, was also not impacted by lactation. Adult non-lactating mice averaged 670±10 (n=3) U/mL compared to 764±149 (n=3) in age-matched lactating mice, P>0.05.
4. Discussion
Identification of suitable markers for nutrient status has long been a challenge. Finding suitable markers during infancy is even more challenging since both nutrient and endocrine influences can impact these procedures. Assessment of copper status has long relied on measurement of serum copper. This copper pool reflects plasma Cp in mammals (Evans and Wiederanders, 1967). Cp is widely detected in vertebrate plasma by its amine oxidase activity (Seal, 1964). Our current studies in developing rodents indicate that both Cp and PAM may be useful markers to evaluate copper status. Plasma copper and plasma Cp activity reflect the same copper pool but enzyme assay would require less sample and would be less impacted by contamination.
Though Cp synthesis is not dependent on cellular copper the amount of holo-Cp secreted by liver is lower following copper deficiency (Gitlin et al., 1992). Thus measurement of the amine oxidase activity of Cp does reflect copper status. In the present studies, plasma Cp activity of lactating dams and their suckling offspring rapidly dropped to near non-detectable levels by P12. In rapidly growing weanling copper deficient mice and rats the drop in Cp activity is detectable in a few days (Prohaska and Lukasewycz, 1989; Prohaska, 1997). Thus lower Cp activity represents a good measure of copper deficiency when compared to copper adequate age-matched controls.
Increased Cp activity of rat plasma during the suckling period has been observed previously (Evans et al., 1970b). Our data confirm that process and extend it to another rodent model, the mouse. Similar increases in Cp during infancy occur in humans (Tessmer et al., 1973; Salmenpera et al., 1989). Thus, to use Cp activity during infancy age-matched controls are critical. Cp activity can also be impacted by inflammation since Cp is an acute phase protein and responds transcriptionally to several cytokines. Estrogen is known to result in higher Cp activity in rats (Evans et al., 1970a). Pregnancy and lactation impacts Cp levels as both result in higher activity in humans, rats, and most other mammals (Seal, 1964; Evans et al., 1970a; DiSilvestro, 1986). Interestingly, we did not detect significant elevation in Cp between young female adult and age-matched lactating mice in these studies; whereas, in rats lactation approximately doubled plasma Cp activity. Therefore, to use Cp activity to evaluate copper status requires consideration of physiological status as well as nutritional status.
These studies were designed to compare Cp with PAM, another cuproenzyme. PAM is present in plasma as 95 kDa protein, a smaller version of the full length membrane associated protein. The size of plasma PAM is consistent with rPAM-3 a secreted version of PAM lacking the transmembrane domain (Eipper et al., 1992). In rat models of dietary copper deficiency starting with weanling animals, plasma PAM activity has been shown to be lower due to copper restriction (Prohaska, 1997; Peterson and Prohaska, 1999). Current experiments extend those observations by showing that PAM activity drops following dietary copper restriction in lactation in both pups and dams. Furthermore, PAM activity in a second mammal, the mouse, behaves similarly. This suggests that plasma PAM may also be useful to assess copper status in human infants. One preliminary study in young patients with a form of Menkes' disease, an inherited copper deficiency, suggested that plasma PAM was impacted (Prohaska et al., 1997). In the mouse, plasma PAM was not altered by lactation. One very preliminary study in rats indicated that PAM activity did not change in response to an inflammatory stimulus but Cp did (data not shown). This suggests that PAM may not be as susceptible to endocrine changes compared to Cp.
Further research will be necessary to determine the value of plasma Cp and PAM activity as a biomarker of human copper status in infancy. The ease of assay and sensitivity of these plasma enzymes make them potential targets for clinical functionality.
Acknowledgements
This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2001-00998 and by National Institutes of Health grant HD 39708.
Abbreviations
- Cu
copper
- Cu−
copper-deficient
- Cu+
copper-adequate
- PAM
peptidylglycine α-amidating monooxygenase
- Cp
ceruloplasmin
References
- DiSilvestro RA. Plasma levels of immunoreactive ceruloplasmin and other acute phase proteins during lactation. Proc. Soc. Exp. Biol. Med. 1986;183:257–261. doi: 10.3181/00379727-183-42415. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Mains RE, Glembotski CC. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc. Natl. Acad. Sci. U. S. A. 1983;80:5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper BA, Myers AC, Mains RE. Peptidyl-glycine alpha-amidation activity in tissues and serum of the adult rat. Endocrinology. 1985;116:2497–2504. doi: 10.1210/endo-116-6-2497. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Evans GW, Wiederanders RE. Blood copper variation among species. Am. J. Physiol. 1967;213:1183–1185. doi: 10.1152/ajplegacy.1967.213.5.1183. [DOI] [PubMed] [Google Scholar]
- Evans GW, Cornatzer NF, Cornatzer WE. Mechanism for hormone-induced alterations in serum ceruloplasmin. Am. J. Physiol. 1970a;218:613–615. doi: 10.1152/ajplegacy.1970.218.3.613. [DOI] [PubMed] [Google Scholar]
- Evans GW, Myron DR, Cornatzer NF, Cornatzer WE. Age-dependent alterations in hepatic subcellular copper distribution and plasma ceruloplasmin. Am. J. Physiol. 1970b;218:298–300. doi: 10.1152/ajplegacy.1970.218.1.298. [DOI] [PubMed] [Google Scholar]
- Failla ML, Johnson MA, Prohaska JR. Copper. In: Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. Washington, DC: ILSI Press; 2001. pp. 373–383. [Google Scholar]
- Gitlin JD, Schroeder JJ, Lee-Ambrose LM, Cousins RJ. Mechanisms of caeruloplasmin biosynthesis in normal and copper-deficient rats. Biochem. J. 1992;282:835–839. doi: 10.1042/bj2820835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman NA, Gaumnitz BM. Studies on the rate of release and turnover of ceruloplasmin and apoceruloplasmin in rat plasma. J. Biol. Chem. 1970;245:2354–2358. [PubMed] [Google Scholar]
- Milne DB. Assessment of copper nutritional status. Clin. Chem. 1994;40:1479–1484. [PubMed] [Google Scholar]
- Peterson DJ, Prohaska JR. Evaluation of rat white blood cell and plasma petidylglycine α-amidating monooxygenase activity as indicators of copper status. Nutr. Res. 1999;19:1041–1047. [Google Scholar]
- Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J. Nutr. 1983;113:2048–2058. doi: 10.1093/jn/113.10.2048. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J. Nutr. 1991;121:355–363. doi: 10.1093/jn/121.3.355. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Responses of rat cuproenzymes to variable dietary copper. J. Nutr. Biochem. 1997;8:316–321. [Google Scholar]
- Prohaska JR, Bailey WR. Persistent regional changes in brain copper, cuproenzymes and catecholamines following perinatal copper deficiency in mice. J. Nutr. 1993;123:1226–1234. doi: 10.1093/jn/123.7.1226. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Brokate B. Lower copper, zinc-superoxide dismutase protein but not mRNA in organs of copper-deficient rats. Arch. Biochem. Biophys. 2001;393:170–176. doi: 10.1006/abbi.2001.2470. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J. Nutr. 2004;134:1003–1006. doi: 10.1093/jn/134.5.1003. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Hoffman RG. Auditory startle response is diminished in rats after recovery from perinatal copper deficiency. J. Nutr. 1996;126:618–627. doi: 10.1093/jn/126.3.618. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Lukasewycz OA. Biochemical and immunological changes in mice following postweaning copper deficiency. Biol. Trace Elem. Res. 1989;22:101–112. doi: 10.1007/BF02917420. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Bailey WR, Lear PM. Copper deficiency alters rat peptidylglycine α-amidating monooxygenase activity. J. Nutr. 1995;125:1447–1454. doi: 10.1093/jn/125.6.1447. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Tamura T, Percy AK, Turnlund JR. In vitro copper stimulation of plasma peptidylglycine alpha-amidating monooxygenase in Menkes disease variant with occipital horns. Pediatr. Res. 1997;42:862–865. doi: 10.1203/00006450-199712000-00023. [DOI] [PubMed] [Google Scholar]
- Salmenpera L, Siimes MA, Nanto V, Perheentupa J. Copper supplementation: failure to increase plasma copper and ceruloplasmin concentrations in healthy infants. Am. J. Clin. Nutr. 1989;50:843–847. doi: 10.1093/ajcn/50.4.843. [DOI] [PubMed] [Google Scholar]
- Seal US. Vertebrate distribution of serum ceruloplasmin and sialic acid and the effects of pregnancy. Comp. Biochem. Physiol. 1964;13:143–159. doi: 10.1016/0010-406x(64)90201-4. [DOI] [PubMed] [Google Scholar]
- Tessmer CF, Krohn W, Johnston D, Thomas FB, Hrgovcic M, Brown B. Serum copper in children (6–12 years old): an age-correction factor. Am. J. Clin. Pathol. 1973;60:870–878. doi: 10.1093/ajcp/60.6.870. [DOI] [PubMed] [Google Scholar]
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- Uauy R, Castillo-Duran C, Fisberg M, Fernandez N, Valenzuela A. Red cell superoxide dismutase activity as an index of human copper nutrition. J. Nutr. 1985;115:1650–1655. doi: 10.1093/jn/115.12.1650. [DOI] [PubMed] [Google Scholar]
- West EC, Prohaska JR. Cu,Zn-superoxide dismutase is lower and copper chaperone CCS is higher in erythrocytes of copper-deficient rats and mice. Exp. Biol. Med. 2004;229:756–764. doi: 10.1177/153537020422900807. [DOI] [PubMed] [Google Scholar]