Abstract
Aim
Deficiency of Alpha-1-antitrypsin (AAT) can be a genetic condition that increases the risk of developing liver, lung and possibly gastrointestinal disease. Since many autistic children also have gastrointestinal disorders, this study was designed to measure serum concentration of AAT and establish AAT genotypes in autistic children, age and gender matched non-autistic siblings, parents and controls.
Subjects and Methods
We used an indirect ELISA with monoclonal IgG to AAT to measure AAT serum concentrations in 71 members from 16 families of individuals with autism and 18 controls (no family history of autism). We used a duplex polymerase chain reaction to detect M, S and Z alleles for alpha-1 antitrypsin expression in 52 members of 12 of the above families.
Results
A significantly high number of autistic family members had lower than normal serum levels of AAT when compared to controls. Autistic children with regressive onset had significantly lower levels of AAT compared to controls, and a significant number of autistic children with low serum AAT also had hyperbilirubinemia, gastrointestinal disease and respiratory problems. We also found that a significantly high number of these individuals had the PiMZ genotype and correspondingly low levels of serum alpha-1 antitrypsin.
Discussion
Knowing that low levels of alpha-1 antitrypsin may be inherited, and that low levels of AAT may be associated with GI disease in autistic children, genotyping autistic children may help identify individuals susceptible to developing digestive problems.
Keywords: alpha-1 antitrypsin, autism, gastrointestinal disease
Introduction
Alpha-1-antitrypsin (AAT) is the most abundant circulating serine protease inhibitor with normal serum concentration of 85–250 mg/dL, but peak concentrations as great as fourfold that of normal may occur during inflammation.1–3 AAT is a 394 amino acid, 52 kDa glycoprotein synthesized in the liver and secreted into the circulation with a half-life of 4–5 days.3
AAT deficiency is a genetic condition that increases the risk of developing a variety of diseases including pulmonary emphysema, cirrhosis of the liver and possibly GI disease. It is caused by mutations in the AAT gene coding4–6 on the long arm of human chromosome 14 (locus 14q32.1).7 Over 100 allelic variants of this gene have been identified and 34 of them have been associated with a quantitative or functional deficiency of circulating AAT.8
The protease inhibitor or Pi system has been used to name the various mutations of the AAT gene.9 The normal allele is Pi M and the classic severe deficiency is associated with the Pi Z allele. Individuals with the recessive Pi ZZ genotype tend to have circulating levels of AAT, which are 10%–15% of individuals with the Pi MM genotype.10 Since the alleles are co-dominant, heterozygotes (PiMZ) have values approximately 35% of the normal concentration. Other genotypes associated with severe deficiency include Pi SZ, Pi Z/Null and Pi Null, as well as an array of much more rare Pi types.1–11 The population prevalence for the MM, MS, and MZ genotypes among whites are 86, 9, and 3%, respectively.12
Alpha-1 antitrypsin (AAT) is mostly secreted by hepatocytes and, to a lesser extent, by lung epithelial cells and phagocytes. It inhibits a variety of serine proteinases, but its preferred target is human neutrophil elastase (HNE), for which it demonstrates the highest affinity.13
The major function of AAT in the lungs is to protect the connective tissue from HNE released from triggered neutrophils, as supported by the development of pulmonary emphysema early in life in subjects affected by severe inherited deficiency of AAT.14 In the majority of humans, the lungs are defended from HNE attack by normal AAT plasma levels ranging from 85 to 250 mg/dl.15 Although AAT is a well-known acute phase reactant, this wide variability in its normal plasma level mostly reflects the marked pleomorphism of the glycoprotein. More than 100 genetic variants of AAT have been identified and these are strictly associated with specific AAT plasma levels in a co-dominantly inherited fashion.16–17
Alpha-1 antitrypsin production increases markedly after stresses such as surgery, injury, infection, or inflammation and with estrogen administration.3 Low values are associated with emphysema, liver disease and possibly gastrointestinal disease.18
Toxic chemicals found in cigarette smoke, air pollutants, oxidizing chemicals, and oxidizing agents released by activated neutrophils, all inactivate a critical portion of the alpha-1-antitrypsin molecule.19
If autistic children express low levels of alpha-1-antitrypsin.20 they may be more susceptible to proteolytic damage caused excess digestive enzymes released from white blood cells during inflammation or infection.21
Children with autism frequently have nongastro-intestinal symptoms suggestive of digestive diseased, such as reflux esophagitis.22 Infants and children with gastroesophageal reflux disease more frequently have sleep disturbance than the normal population.23 Autistic children also have functional gastrointestinal abnormalities. Low activities of disaccharidase enzymes (lactase, maltase, sucrase, palatinase, and glucoamylase) have been found in autistic children.22 Abnormal serum liver function tests have been described in children with AD.24–25 Wakefield et al.26 obtained ileocolonic biopsies from 60 consecutive children with developmental disorders, 83% of whom had autism. Fifty-nine had one or more GI symptoms (e.g. abdominal pain, constipation, diarrhea, changing stool consistency [constipation alternating with diarrhea], or bloating. All were well nourished with height and weight within the normal range. Colonic endoscopic findings included segmental swelling, hyperemia, superficial erosions, and nodularity. On histologic examination, mild to moderate ileal lymphoid nodular hyperplasia (LNH) was described in 93% of the developmentally delayed and autistic children examined.
Since some autistic children have GI problems which might be associated with inflammation, we hypothesized that low AAT levels may be associated with these conditions.
In this study, we determined AAT serum concentrations of 71 members from 16 families of individuals with autism, compared these to concentrations of AAT in 18 controls (parents with no family history of autism), and found that a significant number of family members had lower than normal AAT levels (less that 85 mg/dl). Using a duplex polymerase chain reaction, we detected M, S and Z alleles for alpha-1 antitrypsin expression in 52 family members from 12 of the above families. We found a significantly high number of family members (n= 37) with the MZ genotype. A significant number of these heterozygotes also had correspondingly low levels of serum alpha-1 antitrypsin.
Since AAT concentration may be lower than that of the normal population, detection of low serum AAT and establishment of AAT genotype, might be useful tools for assessing prognosis in autism. Also, a better understanding of the incidence of AAT deficiency in autistic families and its potential relationship to gastrointestinal, liver and lung diseases, may lead to better therapy.
Methods
We used an indirect ELISA and western blotting, as previously described27,28 to quantitate AAT concentration in serum of autistic and control individuals. We also used a duplex polymerase chain reaction to detect M, S and Z alleles for alpha-1 antitrypsin expression.29
Indirect ELISA to quantitate the concentration of serum AAT
Purified Alpha-1 antitrypsin (Sigma) at concentrations of 1 μg, 0.1μg, 0.01 μg and 0.001 μg per 100 μl of bicarbonate buffer (pH 9.6), and 100 μl serum protein at a dilution of 1:500 (PBS), were fixed to wells of 96 well polystyrene microculture plate (Corning) by incubation overnight at 4 degrees C. Excess AAT/ serum was dumped from wells, and all wells were blocked by washing 3X with 300 μl blocking solution (Superblock, Pierce). One hundred microliters of primary antibody (mouse Mab to AAT (Biomedia), diluted 1:500 with PBS, was added to all wells and plate was incubated for 2 hours at 37 degrees C. All wells were washed 3 times with PBS/tween. One hundred microliters of affinity purified alkaline phosphotase conjugated goat anti mouse IgG (Biorad), diluted 1:5000 with PBS, were added to all wells and incubated for 45 minutes at 37 degrees C. All wells were washed 5 times with PBS/tween. One hundred microliters of AP substrate (Biorad) were added to all wells and the plate was incubated at room temperature until significant color change in positive control (10–15 minutes). Optical density was measured using ELISA Reader (Biorad).
Western blot to test specificity of monoclonal anti-AAT IgG to purified AAT
Protein Preparation
Purified AAT (Sigma), and controls were incubated 1:1 with loading dye (with beta mercaptoethanol) at 95 C for 5 minutes. Protein was run on SDS-Page (18%) (BioRad), at 100 volts, 1 μg/lane.
Blot
Proteins were transferred to nitrocellulose at 30 volts, overnight at 4 C. Nitrocellulose was flooded with blocking solution (1% casein/PBS) and rocked gently overnight.
Immunoblot
Ten ml of primary antibody (mouse Mab to AAT diluted 1:500 with PBS; Negative control—PBS) was flooded over appropriate nitrocellulose sheets and incubated for 2 hours at room temperature with gentle rocking. Sheets were washed 3 times using PBS/tween. Five minutes for each wash, rocking gently at room temperature. Sheets were flooded with 10 ml of affinity purified goat anti-mouse IgG conjugated with Horse Radish Peroxidase (HrP) (Biorad), diluted 1:5000 with PBS. Sheets were washed 5 times with PBS/tween, five minutes each wash, rocking gently at room temperature. Sheets were flooded with 10 ml of HrP substrate at room temperature until significant color change.
Genotyping of autistic and nonautistic family members using pcr and restriction enzyme analysis
Polymerase chain reactions was performed with standard buffer (0.1 μ Tris-HCl, pH = 8.3, 0.5 μ KCl and 10 mμ MgCl2). in a total volume of 100 μl containing: 250 ng of genomic DNA, 0.25 pM each of the oligonucleotide primers, 0.2 mμ of dNTPs and 2.5 U of Taq polymerase. Primers used 11detectingtheSmutationare:5′-TGAGGGGAAAC-TACAGCACCTCG-3′ and 5′AGGTGT-GGGCAGCTTCTTGGTCA-3′; primers detecting the Z mutation are: 5′-ATAAGGCTGTGCTGAC-CATCGTC-3′ and 5′-TTGGGTGGGATTCAC-CACTTTTC-3′. Temperature cycling conditions were: initial 10 min denaturation at 94 °C, 30 cycles of 2 min at 94 °C, annealing for 2 min at 55 °C, extension for 3 min at 72 °C, and final extension for 10 min at 72 °C. Twenty microlitres of amplification product were digested for 3 h at 65 °C in a 50 μl volume containing 20 U of TaqI restriction enzyme, in the appropriate buffer. The digested DNA was electrophoresed in a 3% agarose minigel for 3 h at 90 volts, stained with ethidium bromide and visualized under 254 nμ uv trans-illumination.
Patient group
Seventy-one family members from 16 families of individuals with autism, obtained from the Autism Genetic Resource Exchange (AGRE, see below), and 18 controls (parents with no family history of autism), obtained from National Disease Research Interchange (NDRI, see below) were tested for their serum concentration of alpha-1 antitrypsin and AAT genotype.
Results
AAT concentration
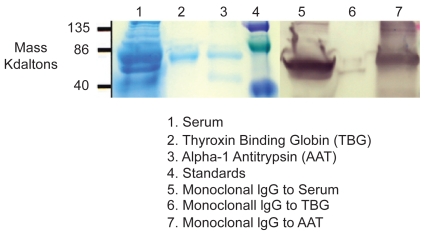
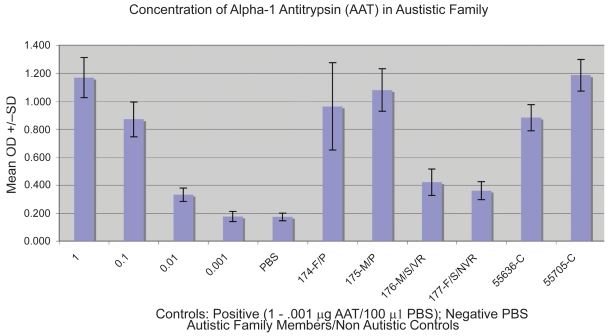
Using an indirect ELISA, serum from individuals was tested using anti-AAT monoclonal IgG and compared to varying concentrations (1 μg–0.001 μg/) of purified AAT and negative control (PBS). Specificity of monoclonal anti-AAT IgG was established using western blotting (Fig. 1). Results of a typical ELISA are shown on Figure 2.
Figure 1.
Figure 2.
Forty-five of the 71 individuals from the 16 families had AAT concentrations less than 85 mg/dL serum, making them low producing individuals (Table 1). Whereas none of the 18 non-autistic controls had levels of AAT less than 85 mg/dL (Fig. 3) (p < 0.05).
Table 1.
| Diagnosis | Sex | Twins | Relationship | AAT mg/dL | Genotype | |
|---|---|---|---|---|---|---|
| Family 1 | F | Parent | 95 | |||
| M | Parent | 54 | ||||
| Asp | M | Sibling | 58 | |||
| AR | M | IT | Sibling | 49 | ||
| A | M | IT | Sibling | 47 | ||
| Family 2 | F | Parent | 45 | |||
| M | Parent | |||||
| AR | M | Sibling | 51 | |||
| AR | M | Sibling | 54 | |||
| NA | F | Sibling | 44 | |||
| Family 3 | F | Parent | 22 | MZ | ||
| M | Parent | 24 | MZ | |||
| AR | F | FT | Sibling | 38 | MZ/Null | |
| PDD | M | FT | Sibling | 46 | MZ | |
| Family 4 | F | Parent | 54 | |||
| M | Parent | 65 | ||||
| NA | M | Sibling | 61 | |||
| A | F | Sibling | 73 | |||
| A | F | Sibling | 45 | |||
| Family 5 | PDD | M | Sibling | 176 | ||
| AR | M | Sibling | 100 | |||
| M | Parent | 72 | ||||
| M | Parent | 74 | ||||
| PDD | F | Sibling | 76 | |||
| Family 6 | M | Parent | 126 | MZ | ||
| A | M | Sibling | 123 | MM | ||
| PDD | F | Sibling | 86 | MZ | ||
| A | M | Sibling | 78 | MZ | ||
| F | Parent | 141 | MM | |||
| Family 7 | A | M | Sibling | 93 | ||
| A | M | Sibling | 133 | MM | ||
| F | Parent | 88 | MZ | |||
| M | Parent | 117 | MM | |||
| Family 8 | F | Parent | 124 | MZ | ||
| M | Parent | 104 | SZ | |||
| PDD | M | FT | Sibling | 78 | MZ | |
| A | F | FT | Sibling | 70 | SZ | |
| NA | M | Sibling | 146 | MZ | ||
| NA | M | Sibling | 68 | SZ | ||
| Family 9 | NA | F | Parent | 146 | MM | |
| NA | M | Parent | 214 | MM | ||
| PDD | M | Sibling | 89 | MM | ||
| NA | F | Sibling | 197 | MM | ||
| AR | M | Sibling | ||||
| Family 10 | NA | F | Parent | 97 | SZ | |
| NA | M | Parent | 94 | MZ | ||
| A | F | Sibling | 55 | MZ | ||
| A | M | IT | Sibling | 129 | MS | |
| A | M | IT | Sibling | 100 | MS | |
| Family 11 | NA | F | Parent | 46 | MZ | |
| NA | M | Parent | 51 | MZ | ||
| AR | F | Sibling | 29 | MZ | ||
| AR | M | Sibling | 18 | MZ | ||
| Family 12 | A | F | Sibling | 84 | MZ | |
| NA | F | Parent | 84 | MZ/ZZ | ||
| Asp | M | Sibling | 53 | MZ | ||
| NA | M | Parent | 88 | MZ | ||
| Family 13 | NA | F | Parent | 54 | MZ | |
| NA | M | Parent | 23 | MZ/ZZ | ||
| A | M | Sibling | 15 | MZ | ||
| A | M | Sibling | 35 | MZ | ||
| Family 14 | NA | F | Parent | 82 | MZ/SZ | |
| NA | M | Parent | 92 | MZ | ||
| AR | M | Sibling | 36 | MZ | ||
| AR | F | Sibling | 31 | MZ | ||
| Family 15 | NA | F | Parent | 50 | MZ | |
| NA | M | Parent | ||||
| NA | F | Sibling | 49 | MZ | ||
| AR | M | FT | Sibling | 51 | MZ | |
| A | M | FT | Sibling | 51 | MZ | |
| Family 16 | NA | F | Parent | 171 | MM | |
| NA | M | Parent | 93 | MZ | ||
| AR | M | Sibling | 51 | MZ | ||
| A | M | Sibling | 57 | MZ |
Figure 3.
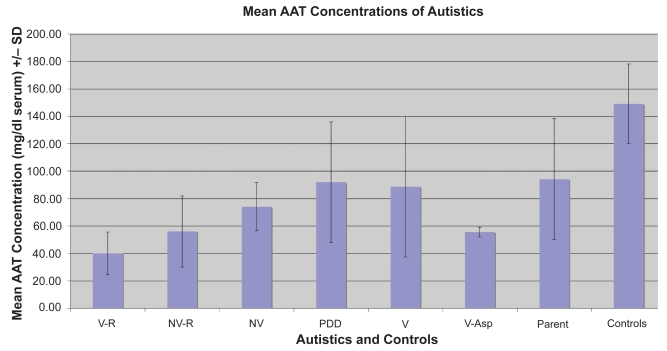
Comparing AAT levels in parents and autistic children with a variety of demographics (Fig. 4 and Table 2), a significantly high number of autistic family members (45/72) had lower than normal levels of AAT when compared to controls (0/18) (p < 0.01). Of the types of autistics tested, we found that non verbal (11) and non verbal regressive (8), autistic siblings had significantly lower levels of AAT compared to controls (13) (p < 0.05).
Figure 4.
Table 2.
| Diagnosis | Sex | Twins | Relationship | AAT | Genotype | HB | HB RX | RD | O2 | GI | GI T | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | mg/dL | |||||||||||
| 2 | AR | M | IT | Sibling | 49 | 0 | −1 | 0 | 0 | 0 | −1 | |
| 3 | A | M | IT | Sibling | 47 | 0 | −1 | 0 | 0 | 0 | −1 | |
| 4 | AR | F | FT | Sibling | 38 | MZ/Null | 1 | −1 | 0 | −1 | 0 | −1 |
| 5 | PDD | M | FT | Sibling | 46 | MZ | 1 | −1 | 0 | 0 | −1 | −1 |
| 6 | PDD | M | Sibling | 176 | 0 | −1 | 0 | 0 | 0 | −1 | ||
| 7 | AR | M | Sibling | 100 | 1 | −1 | 0 | 0 | 1 | 5,6 | ||
| 8 | PDD | F | Sibling | 76 | 0 | −1 | 0 | 0 | 0 | −1 | ||
| 9 | A | M | Sibling | 123 | MM | 0 | −1 | 0 | −1 | 0 | −1 | |
| 10 | PDD | F | Sibling | 86 | MZ | −1 | −1 | −1 | −1 | 0 | −1 | |
| 11 | A | M | Sibling | 78 | MZ | 1 | 1 | 0 | 0 | 0 | −−1 | |
| 12 | A | M | Sibling | 93 | MM/MZ | 0 | −1 | 0 | 0 | 0 | −1 | |
| 13 | A | M | Sibling | 133 | MM | 1 | 1 | 0 | 0 | 0 | −1 | |
| 14 | PDD | M | Sibling | 89 | MM | 0 | −1 | 0 | 0 | 0 | −1 | |
| 15 | A | F | Sibling | 55 | MZ | 0 | −1 | 1 | 1 | −1 | −1 | |
| 16 | A | M | IT | Sibling | 129 | MS | 0 | −1 | 1 | 1 | −1 | −1 |
| 17 | A | M | IT | Sibling | 100 | MS | −1 | −1 | −1 | −1 | −1 | −1 |
| 18 | AR | M | Sibling | 1 | PT | 0 | 0 | 1 | 5 | |||
| 19 | AR | F | Sibling | 29 | MZ | −1 | −1 | −1 | −1 | 1 | 887 | |
| 20 | AR | M | Sibling | 18 | MZ | −1 | −1 | −1 | −1 | 1 | 6 | |
| 21 | A | F | Sibling | 84 | MZ | 1 | PT | 0 | 0 | 1 | 5 | |
| 22 | Asp | M | Sibling | 53 | MZ | 0 | −1 | 0 | 0 | 1 | 6 | |
| 23 | A | M | Sibling | 15 | MZ | 1 | 1 | 0 | 0 | 0 | −1 | |
| 24 | A | M | Sibling | 35 | MZ | 0 | −1 | 0 | 0 | 0 | −1 | |
| 25 | AR | M | FT | Sibling | 51 | MZ | −1 | −1 | 1 | 1 | −1 | −1 |
| 25 | A | M | FT | Sibling | 51 | MZ | 1 | −1 | 0 | 0 | 0 | −1 |
| 27 | AR | M | Sibling | 51 | MZ | 0 | −1 | 0 | 0 | 1 | 5 | |
| 28 | A | M | Sibling | 57 | MZ | 0 | −1 | 0 | 0 | 0 | −1 | |
| Key | ||||||||||||
| Hyperbilirubinemia and Respiratory History | ||||||||||||
| Select Value | Stored Value | Answer Text | ||||||||||
| No | 0 | No | ||||||||||
| Yes | 1 | Yes | ||||||||||
| N/A | 9 | N/A | ||||||||||
| Blank | −1 | Indicates that no response was entered on the collection form/instrument for this question | ||||||||||
| HB—Hyperbilirubinemia | ||||||||||||
| HB-RX—Hyperbilirubinemia Therapy | ||||||||||||
| PT—Phototherapy | ||||||||||||
| RD—Respiratory Distress | ||||||||||||
| O2—Oxygen Suppliment | ||||||||||||
| GI History | ||||||||||||
| Gastroesophageal Reflux (Ger) | 1 | |||||||||||
| Peptic Ulcer Disease (Pud) | 2 | |||||||||||
| Irritable Bowel Syndrome (Ibs) | 3 | |||||||||||
| Inflammatory Bowel Dis. (Ibd) | 4 | |||||||||||
| Chronic Diarrhea | 5 | |||||||||||
| Constipation | 6 | |||||||||||
| Other | 887 | |||||||||||
| Unknown | 888 | |||||||||||
| Multiple | 889 | |||||||||||
| GI—Gastrointestinal Problem(s) | ||||||||||||
| GI T—Gastrointestinal Problem Type | ||||||||||||
| GI D—Gastrointestinal Problem Description | ||||||||||||
Using medical history reports (gathered by AGRE), we analyzed the phenotypic characterization of the probands of family members tested in this study (Table 2). Overall, twenty-seven autistic children were tested for AAT concentration. Questions associated with hyperbilirubinemia were not answered for five of these children. Of the 22 remaining, 8 had hyperbilirubinemia at birth (36%). This is significantly higher than the expected 6 percent of normal children who have the disorder. Six of the remaining 8 with hyperbilirubinemia had low levels of AAT (less than 85 mg/dL) (p < 0.05). Two of the 8 with hyperbilirubinemia required phototherapy.
Three of the 23 affected individuals who answered the related questions had respiratory distress syndrome (RDS) (p < 0.05). Two of these three were born after short gestation (29 weeks). All three required oxygen supplimentation. None of these individuals had hyperbilirubinemia. Two of these three had deficient levels of AAT.
Six other affected siblings (not including the three with RDS) reported respiratory problems later in life. Five of these 6 had deficient levels of AAT. Although none of the individuals had emphysema, two reported multiple respiratory problems—tracheomalacia and sleep apnea, stridor and laryngomalacia. One reported reactive airway disease. One reported multiple incidences of pneumonia and the other two did not report a specific disorder.
Seven of 22 affected siblings reported gastrointestinal problems. One of these did not answer the question. Five of the remaining six had deficient levels of AAT (p < 0.05). Three of the six reported chronic diarrhea, one reported constipation, one colic and the other, projectile vomiting.
Genotyping
If we predicted the genotype of all 71 members of the 16 autistic families based on their AAT serum concentrations (Table 1), most (43/71) would be heterozygous (30–85 mg/dL). This is significantly higher than the expected 3% in the general population. Two pairs of identical twins and three pairs of fraternal twins had very similar AAT concentrations and the same predicted genotypes. In five of six families, when parents had deficient levels of serum AAT (less than 85 mg/dL), all their children were deficient. When both parents were heterozygous, all their children were heterozygous or normal. These findings suggest an inheritance pattern of this trait in these autistic families.
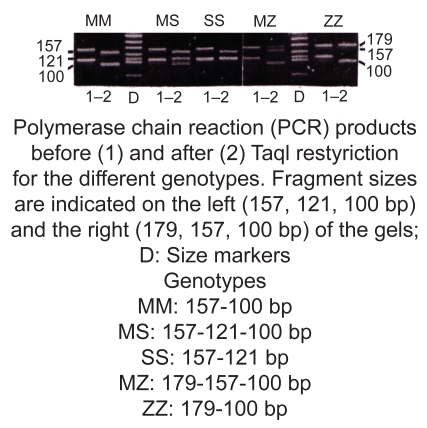
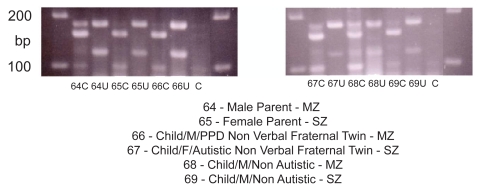
Using a PCR/Restriction Digest we genotyped 52 members from 12 of the 15 families who had been measured for serum AAT concentration (above) (DNA was not available for three of the families). Expected product demonstrating specific MM, MS, SS, MZ and ZZ genotypes for AAT expression is shown in Figure 5. The results of a typical assay (family 64–69) are shown in Figure 6. In this assay, the gel photo shows uncut and cut product for each family member and corresponding genotype.
Figure 5.
Figure 6.
A significantly high number of family members (37) had the MZ genotype. A significant number of these heterozygotes (35 of 37) had correspondingly low levels of serum alpha-1 antitrypsin (less than 100 mg/dL) (Table 1).
Discussion
Approximately 75 allelic variants have been described in the AAT gene locus resulting in a very complex genetic classification based upon the phenotypic features of the circulating AAT protein. The most common variant, PI M, is present in approximately 95% of the Caucasian U.S. population and is regarded as the normal variant associated with normal serum levels of functional AAT. The predominant deficiency alleles, such as PI Z and PI S, may result in decreased levels of circulating AAT but with completely normal functioning proteins. The MM phenotype is therefore designated as manifesting 100% concentration of circulating AAT (85–250 mg/dL). The heterozygous combinations MZ and MS yield 35% and the ZZ allele 10%–25% of this normal MM value. Approximately 95% of all AAT deficiency states leading to clinical manifestations are made up of PI ZZ homozygotes. Certain alleles, such as the S allele, either in the homozygous state or associated with the M allele, do not appear to be associated with the abnormally polymerized molecules within the endoplasmic reticulum and have not been incriminated in the development of liver disease unless combined with the Z allele.14,30
The association of AAT deficiency and liver disease in children was first described in 1969.31 Many subsequent clinical studies 32,18 have observed that liver disease occurrence in AAT deficiency is bimodal, affecting children in neonatal life or early infancy and, less commonly, adults in late middle life. However, only 10% of deficient newborns develop persistent cholestasis during the first year of life and many of these infants appear to undergo a spontaneous remission, with only about 3% of the originally diagnosed neonates progress to fibrosis or cirrhosis during childhood and teenage years. Nevertheless, careful surveillance revealed that many of these have persistently abnormal liver enzymes.30,31
Cederlund and Gillberg (2004),33 reported neonatal hyperbilirubinemia in 22 of 100 boys with Asperger syndrome. Bilirubin gets into neurons only if the blood-brain barrier is disrupted by some other factor.34,35 Some of the newborns with AAT deficiency, who develop jaundice, may suffer anoxia or sepsis in the perinatal period, and may therefore be at risk for bilirubin crossing the blood-brain barrier. Our data shows a significant relationship between low AAT levels and neonatal hyperbilirubinemia in autistic children.
Asperger first recorded the link between celiac disease and behavioral psychoses36 Walker-Smith and colleagues detected low concentrations of alpha-1 antitrypsin in a small group of children with typical autism,20 and D’Eufemia and colleagues identified abnormal intestinal permeability, a feature of small intestinal enteropathy, in 43% of a group of autistic children with no gastrointestinal symptoms, but not in matched controls.37 Some have also found a relationship between chronic enterocolitis and regressive developmental disorder.38 These studies and evidence of anemia and immune deficiency in some autistic children, suggest an association between intestinal dysfunction and autism spectrum disorders (ASD). However, increased incidence of gastrointestinal problems in autistic children is still under study because the exact relationship between GI symptoms and ASD is unclear.39 Our data, however, suggests that a high number of autistic children with low levels of AAT may also have gastrointestinal problems, especially diarrhea.
In inflammatory conditions, AAT levels are normally elevated. In this study, however, the results demonstrate low alpha-1-antitrypsin serum levels, which are probably due to genetic factors and not excess protein loss in stool. This might contradict the theory that autistic patients have inflammatory bowel disease because if there was chronic inflammation due to enterocolitis, the alpha-1-antitrypsin level should be elevated. However, there is evidence that increased inflammation is associated with AAT deficiency, such as GI inflammation in patients with ulcerative colitis, associated with the PiZ carriers,40 and neutrophil associated inflammation seen in the lungs of AAT deficient patients.41 Also, PiMZ subjects without airflow obstruction may have an IL-8 related neutrophilic inflammation in the pulmonary airways 42 and they are more likely to develop both liver (cirrhosis or fibrosis) and lung disease (emphysema).43
In this preliminary study, a significant number of autistic family members have low serum levels of AAT, which is, at least in part, caused by the PiMZ genotype. Low serum AAT in autistic children supports the findings of Walker-Smith and colleagues, and strongly suggests an association between autism and AAT deficiency.
A high number of autistic children with low levels of AAT in our study, also had neonatal hyperbilirubinemia (8/22), respiratory problems (9/23) and digestive disorders (7/22). Since AAT deficiency may be associated with these problems, this also supports our hypothesis of an association between AAT deficiency and autism.
Our study also suggests an association between autistic children with regressive disease and AAT deficiency. This suggests that, besides genetic predisposition, environmental factors may be influencing levels of serum AAT in these individuals.
Our observation, however, that some non-autistic siblings inherit AAT deficiency, and parents of autistic children who also have low levels of serum AAT, suggests that AAT deficiency alone is not a causative agent for ASD, but may make a subset of autistics susceptible to inflammatory disease.
Knowing that low levels of alpha-1 antitrypsin is inherited, and that low levels of AAT may be associated with GI disease, genotyping autistic children may help identify those autistics susceptible to developing digestive problems. And a better understanding of the incidence of AAT deficiency in autistic families and its potential relationship to liver, lung and bowel diseases, may lead to better therapeutic strategies.
Acknowledgments
We gratefully acknowledge that all autism family serums were provided by the Autism Genetic Resource Exchange (AGRE) Consortium and the participating AGRE families. The Autism Genetic Resource Exchange is a program of Cure Autism Now and is supported, in part, by grant MH64547 from the National Institute of Mental Health to Daniel H. Geschwind (PI)
All control serums were provided by the National Disease Research Interchange, Philadelphia, PA.
Footnotes
Disclosure
The author reports no conflicts of interest.
References
- 1.Blank CA, Brantley M. Clinical features and molecular characteristics of 1-antitrypsin deficiency. Ann Allergy. 1994;72:105–120. [PubMed] [Google Scholar]
- 2.Massi G, Chiarelli C. Alpha1-antitrypsin: molecular structure and the Pi system. Acta Paediatr Suppl. 1994;393:1–4. doi: 10.1111/j.1651-2227.1994.tb13197.x. [DOI] [PubMed] [Google Scholar]
- 3.Carrell RW. Alpha1-antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986;78:1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1998;84(Suppl 6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- 5.Kurachi K, Chandra T, Degen SJ, et al. Cloning and sequence of cDNA coding for alpha 1-antitrypsin. Proc Natl Acad Sci U S A. 1981;78:6826–30. doi: 10.1073/pnas.78.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman GA, Bird PI, Carrell RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–6. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 7.Billingsley GD, Walter MA, Hammond GL, et al. Physical mapping of four serpin genes: alpha1-antitrypsin, alpha1-antichymotrypsin, corticosteroid-binding globulin, and protein C inhibitor, within a 280-kb region on chromosome I4q32.1. Am J Hum Genet. 1993;52:343–53. [PMC free article] [PubMed] [Google Scholar]
- 8.Alpha 1-antitrypsin deficiency: memorandum from a WHO meeting. Bull WHO. 1997;75:397–415. [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerhol MK, Laurell CB. The Pi system-inherited variants of serum alpha1-antitrypsin. Prog Med Genet. 1970;7:6–111. [PubMed] [Google Scholar]
- 10.Lomas DA, Evans DL, Finch JT, et al. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–7. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Brantly M. Molecular mechanisms of alpha1-antitrypsin null alleles. Respir Med. 2000;94(Suppl C):S7–11. doi: 10.1053/rmed.2000.0851. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison DCS. Epidemiology of alpha1-protease inhibitor deficiency. Eur Respir J. 1990;3(Suppl 9):29s–34S. [PubMed] [Google Scholar]
- 13.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 14.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 15.Steiner SJ, Gupta SK, Croffie JM, Fitzgerald JF. Serum levels of alpha1-antitrypsin predict phenotypic expression of the alpha1-antitrypsin gene. Dig Dis Sci. 2003;48:1793–6. doi: 10.1023/a:1025411515683. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society/European Respiratory Society Statement. Standards for the diagnosis and management of individuals with alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 17.Brantly M. Alpha1-antitrypsin genotypes and phenotypes. In: Crystal RG, editor. Alpha1-antitrypsin deficiency. New York: Marcel Dekker; 1996. [Google Scholar]
- 18.Perlmutter DH. Clinical manifestations of alpha1-antitrypsin deficiency. Gastroenterol Clin North Am. 1995;24:27–43. [PubMed] [Google Scholar]
- 19.Janoff A, George-Nascimento C, Rosenberg S. A genetically engineered, mutant human alpha-1-proteinase inhibitor is more resistant than the normal inhibitor to oxidative inactivation by chemicals, enzymes, cells, and cigarette smoke. Am Rev Respir Dis. 1996;133(3):353–6. doi: 10.1164/arrd.1986.133.3.353. [DOI] [PubMed] [Google Scholar]
- 20.Walker-Smith J, Andrews J. Alpha-1-antitrypsin, autism, and coeliac disease. The Lancet II. 1972:883–884. doi: 10.1016/s0140-6736(72)92258-1. [DOI] [PubMed] [Google Scholar]
- 21.Engstrom G, Hedblad B, Stavenow L, et al. Incidence of Obesity-Associated Cardiovascular Disease Is Related to Inflammation-Sensitive Plasma Proteins. A Population-Based Cohort Study. Arterioscler Thromb Vasc Biol. 2004 Jun 3; doi: 10.1161/01.ATV.0000134293.31512.be. [DOI] [PubMed] [Google Scholar]
- 22.Horvath K, Papadimitriou JC, Rabsztyn A, et al. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135:559–563. doi: 10.1016/s0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- 23.Ghaem M, Armstrong KL, Trocki O, et al. The sleep patterns of infants and young children with gastro-oesophageal reflux. J Paediatr Child Health. 1998;34:160–163. doi: 10.1046/j.1440-1754.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 24.Alberti A, Pirrone P, Elia M, et al. Sulfation deficit in “low-functioning” autistic children: a pilot study. Biol Psychiatry. 1999;46:420–424. doi: 10.1016/s0006-3223(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 25.O’Reilly B, Waring R. Enzyme and sulfur oxidation deficiencies in autistic children with known food/chemical intolerances. Journal of Orthomolecular Medicine. 1993;4:198–200. [Google Scholar]
- 26.Wakefield AJ, Anthony A, Murch SH, et al. Enterocolitis in children with developmental disorders. Am J Gastroenterol. 2000;95:2285–2295. doi: 10.1111/j.1572-0241.2000.03248.x. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Lane D. Antibodies: A Laboratory Manual . Cold Spring Harbor Laboratory Press; New York: 1988. [Google Scholar]
- 28.Ferencik M. Handbook of Immunochemistry. Chapman and Hall; New York: 1993. [Google Scholar]
- 29.Lucotte G, Sesbou R. Polymerase chain reaction detection of S and Z alpha-1-antitrypsin variants by duplex PCR assay. Molecular and Cellular Probes. 1999;13:389–391. doi: 10.1006/mcpr.1999.0264. [DOI] [PubMed] [Google Scholar]
- 30.Rosen HR. Liver disease associated with alpha 1-antitrypsin deficiency. In: Rosen HR, Martin P, editors. Metabolic Liver Disease Clinics in Liver Disease. Vol. 2. Philadelphia, PA: WB Saunders; 1998. [Google Scholar]
- 31.Sharp HL, Bridges RA, Krivit W, Freier EF. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med. 1969;1969;73:934–939. [PubMed] [Google Scholar]
- 32.Berg NO, Eriksson S. Liver disease in adults with alpha-1-antitrypsin deficiency. N Engl J Med. 1972;287:1264–1267. doi: 10.1056/NEJM197212212872502. [DOI] [PubMed] [Google Scholar]
- 33.Cederlund and Gillberg One hundred males with Asperger syndrome: a clinical study of background and associated factors. Developmental Medicine and Child Neurology. 2004;46:652–60. doi: 10.1017/s0012162204001100. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman HM, Yannet H. Kernicterus: jaundice of the nuclear masses of the brain. American Journal of Diseases of Children. 1933;45:740–759. [Google Scholar]
- 35.Lucey JF, Hibbard E, Behrman RE, Esquival FO, Windle WF. Kernicterus in asphyxiated newborn monkeys. Experimental Neurology. 1964;9:43–58. [Google Scholar]
- 36.Asperger H. Die Psychopathologie des coeliakakranken kindes. Ann Paediatr. 1961;197:146–51. [Google Scholar]
- 37.D’Eufemia P, Celli M, Finocchiaro R, et al. Abnormal intestinal permeability in children with autism. Acta Paediatrica. 1996;85:1076–79. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
- 38.Furlano RI, Anthony A, Day R, et al. Lymphocytic colitis, with CD8 and T cell infiltration and epithelial damage, in children with autism. J Pediatr. 2001;138:366–372. doi: 10.1067/mpd.2001.111323. [DOI] [PubMed] [Google Scholar]
- 39.Erickson CA, Stigler KA, et al. Gastrointestinal factors in autistic disorder: a critical review. J Autism Dev Disord. 2005;35(6):713–27. doi: 10.1007/s10803-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 40.Elzouki AN, Eriksson S, Lofberg R, Nassberger L, Wieslander J, Lindgren S. The prevalence and clinical significance of alpha1-antitrypsin deficiency (PiZ) and ANCA specificities (proteinase 3, BPI) in patients with ulcerative colitis. Inflamm Bowel Dis. 1999;5(4):246–52. doi: 10.1097/00054725-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Respir Res; Woolhouse Leukocyte adhesion and recruitment, and alpha-1-antitrypsin deficiency: a report from ATS; 2001, May 18–23; San Francisco. 2001. p. E004. [Google Scholar]
- 42.Malerba M, et al. Neutrophilic inflammation and IL-8 levels in induced sputum of alpha-1-antitrypsin PiMZ subjects. Thorax. 2006;61(2):129–33. doi: 10.1136/thx.2005.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eriksson S, Moestrup T, Hägerstrand I. Liver, lung and malignant disease in heterozygous (Pi MZ) alpha1-antitrypsin deficiency. Acta Med Scand. 1975;198(4):243–7. doi: 10.1111/j.0954-6820.1975.tb19535.x. [DOI] [PubMed] [Google Scholar]