Abstract
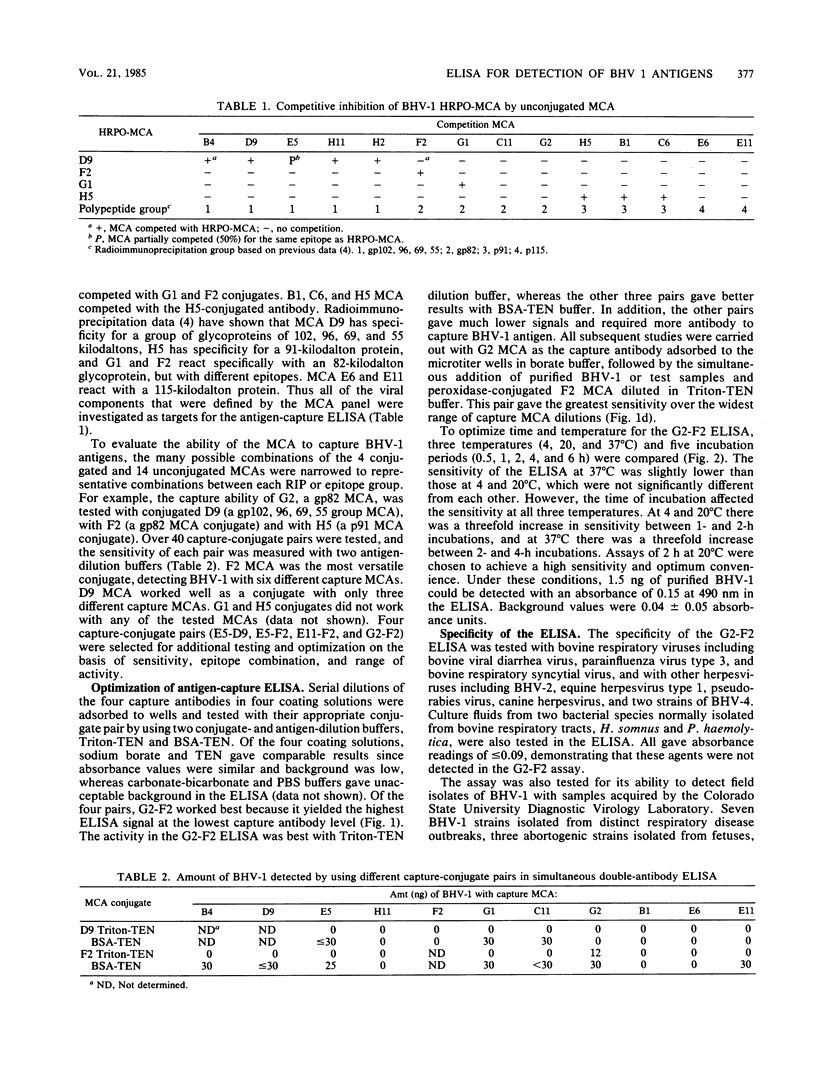
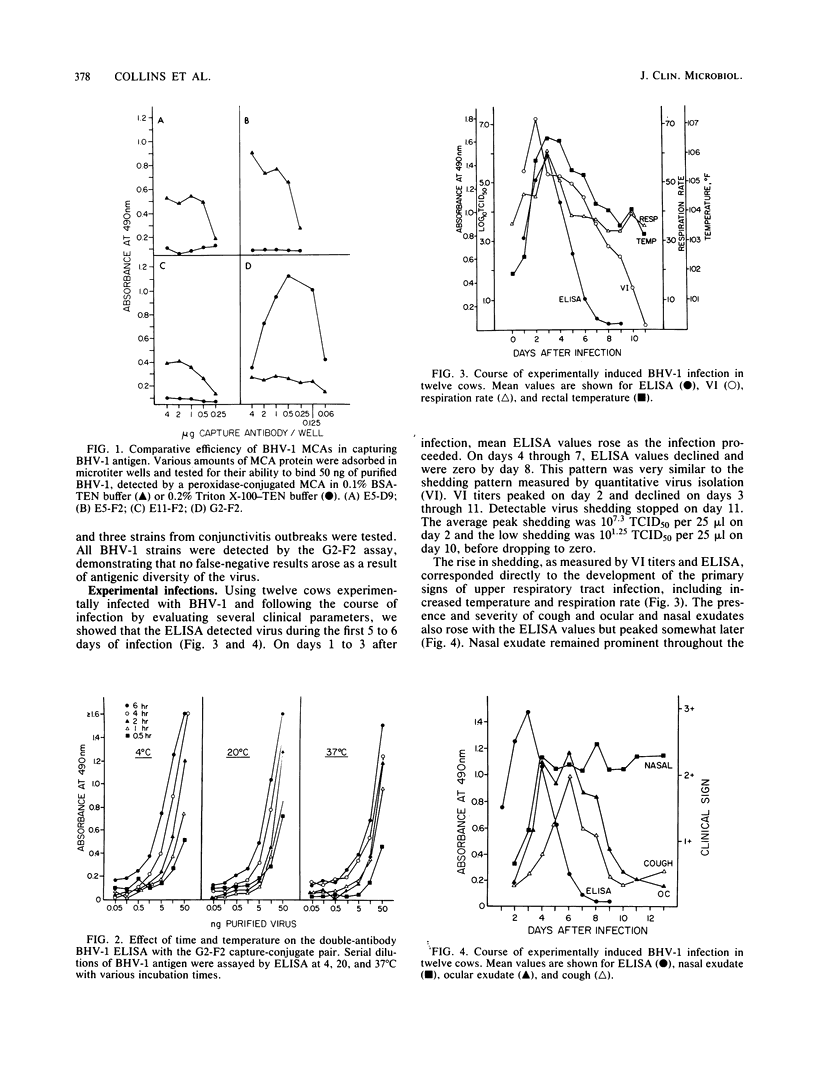
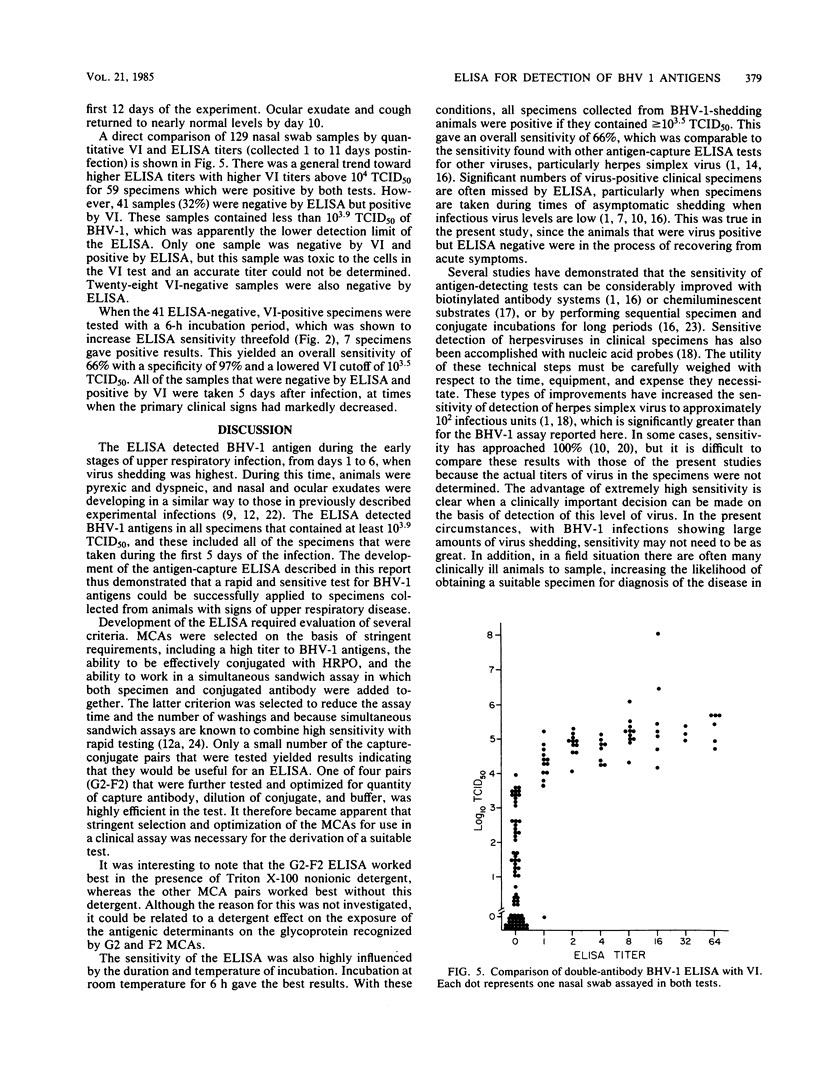
An enzyme-linked immunosorbent assay (ELISA) was developed for the detection of bovine herpesvirus type 1 (BHV-1) antigens in nasal swab specimens collected from infected animals. Development of the ELISA involved screening and selection of BHV-1-specific monoclonal antibodies for their ability to capture BHV-1 antigens and for their stability and activity after conjugation to horseradish peroxidase. Forty combinations of capture-conjugate monoclonal antibody pairs were screened for detection of nanogram amounts of purified BHV-1 by using a double-antibody-sandwich ELISA in which antigen and conjugated antibody were simultaneously added to antibody-coated wells. Of the 40 monoclonal antibody pairs, 4 were analyzed further and 1 was selected for routine application to clinical specimens. Of 129 nasal swab specimens collected during the first 10 days after experimental infection with BHV-1, 66 were found to be positive by both virus isolation and ELISA and 34 were positive for infectious virus but negative by ELISA. One specimen was positive by ELISA but negative by virus isolation, and the remaining 28 specimens were negative by both tests. Quantitation of the virus-containing specimens showed that the ELISA had a lower detection limit of 10(3.5) median tissue culture infective doses. The ELISA was judged to be highly useful for diagnosis of BHV-1 infections, since all of the nasal swab specimens that were collected from 12 animals during the first 5 days of the infection, when the clinical signs were the most apparent, were positive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler-Storthz K., Kendall C., Kennedy R. C., Henkel R. D., Dreesman G. R. Biotin-avidin-amplified enzyme immunoassay for detection of herpes simplex virus antigen in clinical specimens. J Clin Microbiol. 1983 Dec;18(6):1329–1334. doi: 10.1128/jcm.18.6.1329-1334.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Bryden A. S. Evaluation of a new enzyme-linked immunosorbent assay test for rotavirus antigen in faeces. J Clin Pathol. 1981 Dec;34(12):1388–1391. doi: 10.1136/jcp.34.12.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Butcher A. C., Riegel C. A., McGrane V., Blair C. D., Teramoto Y. A., Winston S. Neutralizing determinants defined by monoclonal antibodies on polypeptides specified by bovine herpesvirus 1. J Virol. 1984 Nov;52(2):403–409. doi: 10.1128/jvi.52.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S., Chasey D., White H. Experimental infectious bovine rhinotracheitis: comparison of four antigen detection methods. Res Vet Sci. 1983 Jan;34(1):42–45. [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Hendry R. M., Collins M. F. Factors involved in enzyme-linked immunoassay of viruses and evaluation of the method for identification of enteroviruses. J Clin Microbiol. 1979 Aug;10(2):210–217. doi: 10.1128/jcm.10.2.210-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrs R. F. Infectious bovine rhinotracheitis: a review and update. J Am Vet Med Assoc. 1977 Nov 15;171(10):1055–1064. [PubMed] [Google Scholar]
- Land S. A., Skurrie I. J., Gilbert G. L. Rapid diagnosis of herpes simplex virus infections by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Jun;19(6):865–869. doi: 10.1128/jcm.19.6.865-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton H. W., Reed D. E. Clearance and shedding of infectious bovine rhinotracheitis virus from the nasal mucosa of immune and nonimmune calves. Am J Vet Res. 1980 Jan;41(1):117–119. [PubMed] [Google Scholar]
- Mildbrand M. M., Teramoto Y. A., Collins J. K., Mathys A., Winston S. Rapid detection of canine parvovirus in feces using monoclonal antibodies and enzyme-linked immunosorbent assay. Am J Vet Res. 1984 Nov;45(11):2281–2284. [PubMed] [Google Scholar]
- Miller M. J., Howell C. L. Rapid detection and identification of herpes simplex virus in cell culture by a direct immunoperoxidase staining procedure. J Clin Microbiol. 1983 Sep;18(3):550–553. doi: 10.1128/jcm.18.3.550-553.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Smith T. F. Evaluation of an enzyme-linked immunosorbent assay for the detection of herpes simplex virus antigen. J Clin Microbiol. 1984 Jun;19(6):730–732. doi: 10.1128/jcm.19.6.730-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L. S., Jacob A. J., Madden D. L., Sever J. L. Detection of genital herpes simplex infections by a tissue culture-fluorescent-antibody technique with biotin-avidin. J Clin Microbiol. 1983 Jan;17(1):149–154. doi: 10.1128/jcm.17.1.149-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L. S., Namba M., Brashears G., Jacob A. J., Lee Y. J., Sever J. L. Rapid detection of herpes simplex virus in clinical specimens by use of a capture biotin-streptavidin enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Jul;20(1):109–114. doi: 10.1128/jcm.20.1.109-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronovost A. D., Baumgarten A., Hsiung G. D. Sensitive chemiluminescent enzyme-linked immunosorbent assay for quantification of human immunoglobulin G and detection of herpes simplex virus. J Clin Microbiol. 1981 Jan;13(1):97–101. doi: 10.1128/jcm.13.1.97-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield D. C., Richman D. D., Albanil S., Oxman M. N., Wahl G. M. Detection of herpes simplex virus in clinical specimens by DNA hybridization. Diagn Microbiol Infect Dis. 1983 Jun;1(2):117–128. doi: 10.1016/0732-8893(83)90041-x. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Devlin V., Gallo D., Mills J. Comparison of direct immunofluorescence and direct immunoperoxidase procedures for detection of herpes simplex virus antigen in lesion specimens. J Clin Microbiol. 1983 Aug;18(2):445–448. doi: 10.1128/jcm.18.2.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates W. D. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982 Jul;46(3):225–263. [PMC free article] [PubMed] [Google Scholar]
- Yates W. D., Babiuk L. A., Jericho K. W. Viral-bacterial pneumonia in calves: duration of the interaction between bovine herpesvirus 1 and Pasteurella haemolytica. Can J Comp Med. 1983 Jul;47(3):257–264. [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Investigation of enzyme immunoassay time courses: development of rapid assay systems. J Clin Microbiol. 1981 Apr;13(4):738–741. doi: 10.1128/jcm.13.4.738-741.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


