synopsis
Long considered a niche technique, SPI is now enjoying more visibility. Hanley of the University of Illinois Chicago and Zimmermann of the University of Rostock (Germany) explain how recent advances in light sources coupled with the inherent strengths of SPI have been expanding the range of this technique.
Thanks to recent technological advances and SPI’s ability to detect all organics, the technique could become the long-sought universal soft ionization method. (To listen to a podcast about this feature, please go to the Analytical Chemistry website at pubs.acs.org/ac.)
Because the human eye does not detect IR radiation, we cannot see a warm object in the dark without an IR camera or other “transformation method”. MS has the same problem: it cannot directly detect the objects of interest—the analyte molecules—but only their ionized counterparts. Therefore, the transformation methods that ionize the analyte molecules are of particular importance to MS. The invention of new ionization methods such as MALDI or ESI for MS detection of biomolecules has had an enormous influence on the development of MS and its applications in analytical chemistry.
However, many ionization methods result in the formation not only of intact molecular ions but also of ion fragments. This is particularly true for electron impact (EI) ionization, the classical ionization method for smaller molecules ranging in mass up to ~500 Da. EI uses electrons of 70-eV kinetic energy, near where maximal ionization efficiency occurs (1), which makes it a “hard” ionization technique because the analytes are heavily fragmented upon ionization. The molecular peak is often barely visible in the mass spectra of labile compounds, although the fragment patterns do supply information about the presence of distinct functional groups in the investigated molecules. Furthermore, the fragment patterns of unknown substances can be used for a statistically based identification by comparison with EI-MS library data. The benefits of the latter feature made MS with EI the routine spectrometric detection method for GC. However, the frequent absence of information on the molecular weight of labile compounds is a severe disadvantage of EI, hampering the identification of unknowns. Furthermore, for complex mixtures of organic compounds, EI-MS requires a preseparation method such as GC; otherwise, the overlapping complex fragment patterns cannot be deconvoluted into mass spectra of individual compounds.
These shortcomings of EI have led to a continuing demand for robust fragmentation-free or “soft” ionization methods for MS. Several soft ionization techniques have been developed, including methods based on chemical ionization (CI), field ionization (FI), and photoionization (PI). More recently, MALDI and ESI emerged and became the dominant soft ionization methods for polar molecules. Although these soft ionization techniques became quite successful in various applications, a universal “standard” soft ionization method is still lacking. Such a method would cover both nonpolar and polar small molecules without matrix effects as well as higher-mass, nonpolar analytes. Recent developments in high-intensity vacuum UV (VUV) light sources and optical technologies support the hypothesis that PI may become a standard soft ionization technology in the future.
A closer look at PI requires consideration of at least two distinct strategies. On the one hand, laser-based resonance-enhanced multiphoton ionization (REMPI) has achieved some popularity for the analysis of certain compound classes (2, 3). REMPI is highly selective and sensitive for aromatic hydrocarbons using UV wavelengths that are readily accessible with standard pulsed lasers. Most applications of REMPI-MS involve direct-inlet MS, but successful couplings of REMPI-MS to GC and LC as well as laser desorption also have been reported (2, 4–7). For on-line detection of aromatics by REMPI-MS, the parts-per-trillion concentration region is readily available. Recently, the detection of polyaromatic hydrocarbons on individual aerosol particles in the 5 × 10−20 mol range using laser desorption REMPI-MS has been described (8). The high selectivity of REMPI for soft ionization of specific compound classes, however, has so far prevented REMPI from becoming a standard soft ionization method for MS.
On the other hand, single-photon ionization (SPI) with VUV radiation is capable of soft ionization of all organic compound classes, including the saturated alkanes (5). This article focuses on SPI because its ability to detect all organics makes it a candidate to become the long-sought universal soft ionization method. SPI has been used for decades for fundamental studies and increasingly is used for applied work (2, 9–17). More recently, the simplicity of SPI and the increasing availability of reliable, high-intensity VUV light sources have been motivating researchers to use this method for many new applications.
SPI fundamentals
The performance of VUV light sources is crucial for successful SPI-MS applications because the typical cross section of SPI, σspi, is relatively small: 2–20 megabarn (Mb; i.e., 10−18 cm2 ), which is only ~1/100 of the average cross section obtained with 70-eV EI ionization for organic compounds (18). In addition, the typical electron flux in an EI ion source is significantly higher than the VUV photon flux achievable with current light sources, so one could assume a comparably low sensitivity of SPI-MS.
Nevertheless, relatively low LODs (mid to low parts-per-billion range) have been demonstrated for organic compounds in direct-inlet MS experiments conducted with laser-generated VUV radiation (3, 19, 20) or novel VUV lamps known as electron-beam-pumped rare-gas excimer lamps (EBELs; 21, 22). For example, an SPI-MS LOD of 35 ppb was achieved for toluene with a measurement time of 0.65 seconds (s) by a TOF mass spectrometer equipped with an EBEL VUV light source (23). In a laser SPI-MS experiment (frequency-multiplied Nd:YAG laser at 118 nm), a LOD of 2 ppb was achieved for toluene for an average of 100 laser shots at 10-Hz repetition rate (measurement time 10 s). Table 1 shows SPI-MS LODs for several organic compounds obtained using different instruments and conditions.
Table 1.
Typical LODs for aromatic and aliphatic organic compounds obtained by direct-inlet MS with SPI-MS in several experimental setups.
| Compound | LOD1 | VUV light source/repetition rate | Type of mass spectrometer | Ref |
|---|---|---|---|---|
| Aromatics | ||||
| Benzene | 1.5ppb/10s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| 27 pph/1 s | EBELVUV lamp,126nm/50Hz | TOFMS | 21 | |
| 34 ppb/1 s(SIM) | EBEL, 126 nm/CW | QMS | 22 | |
| Toluene | 2 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| 35 ppb/650 ms | EBEL VUV lamp, 126 nm/CW | oa-TOFMS | 23 | |
| 30 ppb/l s | EBEL VUV lamp, 126 nm/50 Hz | TOFMS | 21 | |
| 37 ppb/1 s (SIM) | EBEL VUV lamp, 126 nm/CW | QMS | 22 | |
| Naphthalene | 4 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| Benzonitrile | 600 ppt/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| Chlorobenzene | 7 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| 14 ppb/20 s | Laser, 121,6 nm/10 Hz | TOFMS | 19 | |
| Nitrobenzene | 24 ppb/30 s | Laser, 118 nm/10 Hz | TOFMS | 20 |
| 2,4-Dinitrotoluene | 40 ppb/30 s | Laser, 118 nm/10 Hz | TOFMS | 20 |
| Aliphatics | ||||
| Acetone | 149 ppb/1 s (SIM) | EBEL VUV lamp, 126 nm/CW | QMS | 22 |
| 14 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 18 | |
| 1-Pentene | 149 ppb/l s (SIM) | EBEL VUV lamp, 126 nm/CW | QMS | 22 |
| 1,3-Butariiene | 137 ppb/l s (SIM) | EBEL VUV lamp, 126 nm/CW | QMS | 22 |
| 8 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 18 | |
| Decane | 27 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| Acetsldehyde | 14 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
| Isoprene | 11 ppb/10 s | Laser, 118 nm/10 Hz | TOFMS | 3 |
SIM, selected ion monitoring; QMS, quadrupole mass spectrometer; oa-TOFMS, orthogonal acceleration TOFMS.
S/N = 2/measurement time
Most SPI-MS LODs are similar to those achieved in MS instruments that utilize EI. This surprising result derives from two facts: first, organic compounds are heavily fragmented under EI, so that the most intense ion often carries <10% of the total ion current. In SPI all intensity is shifted to the one and only molecular peak, which can gain an order of magnitude in sensitivity this way. The second and more pronounced effect, however, is the high chemical noise associated with EI. Multiple analyte fragment ions, some of which are metastable, are generated with EI. Furthermore, the bulk gas matrix (carrier gas, air, etc.) is ionized in EI. Thus, the vast majority of ions in an EI ion source are actually matrix gas ions, the majority of which are excluded from detection by the mass analyzer. Nevertheless, the scattering of the matrix ions off ion optical components leads to a significant increase in noise and background signals, resulting in a relative increase in LODs for EI-MS. By contrast, SPI does not ionize these matrix ions, as discussed further below.
One practical advantage of SPI is its simplicity: exposure to VUV radiation will cause an atom or molecule to undergo SPI if its ionization energy (IE) is less than the VUV photon energy. The IE of most organic molecules is <~10 eV (24). The IEs of selected organic compounds (homologous series) are depicted in Figure 1a as a function of the molecular weight; the photon energies of common VUV sources are also shown. With increasing molecular weight in a given homologous series, the IE becomes smaller, and for large molecular weights (not shown), the IE approaches asymptotically the value of the work function of the bulk solid-state material. A noteworthy exception is that some low-molecular-weight matrix/background gases common in MS analysis (i.e., H2, N2, O2, CO2, and H2O) have higher IEs and thus are not ionized at the most commonly used VUV photon energies.
Figure 1.
Versatility of VUV SPI-MS as a soft ionization technique. (a) IEs of some organic compounds as a function of the molecular weight (homologous series), as well as the photon energy distribution of some lamp- and laser-based VUV light sources. (Adapted with permission from Ref. 18.) (b) Mass spectra of diesel fuel, recorded by SPI (top; EBEL-Ar lamp emitting 9.8-eV photons; the red stars indicate n-alkanes) and EI ionization (bottom; 70-eV kinetic energy). The homologous series of the alkanes in the SPI-MS is marked by red stars. The spectra are recorded by GC/SPI-EI-MS. All mass spectra of the respective runs have been added. (Adapted with permission from Ref. 5.)
The analysis of SPI-MS results is eased by the relatively similar efficiency of SPI for different species. The yield Y of cations from SPI of a gaseous atom or molecule is given by Y = σspiINgas, where I is the VUV radiation intensity and Ngas is the density of gaseous neutrals (2, 14). The efficiency is determined by σspi and varies by only ~1 order of magnitude with different species. Aromatic species such as benzene, benzene derivatives, and alkynes exhibit SPI cross sections of ~20 Mb; alkanes exhibit only ~1/10 of this value (~3 Mb), whereas alkenes (~8 Mb), dienes (~15 Mb), and aldehydes/ketones (~4 Mb) lie in between (18). This renders SPI more readily quantifiable than most other MS ionization methods, of which the ionization efficiencies can vary by many orders of magnitude and which are vulnerable to matrix effects.
The simplicity of SPI has yet another advantage in that it allows preparation of ions with known internal energy. Internal energy is considered the essential property determining the fragmentation energetics and timescales for molecular ions (1). SPI of a neutral molecule initially forms an intact radical cation and an electron, with the photon energy in excess of the IE being deposited into either the internal energy of the cation or the kinetic energy of the electron. The probability that this cation will remain intact or dissociate within the timescale of the experiment can be determined, but only if its internal energy is quantified. Threshold photoelectron photoionization coincidence (TPEPICO) experiments use SPI to prepare plots of internal energy versus fragmentation (25). TPEPICO data are extremely valuable because they can be used to calibrate other ionization techniques, which allow only the relative increase or decrease of internal energy and cannot deposit a known, absolute value of internal energy into a molecular ion (1).
If SPI is such a powerful ionization method and has been around for decades, then why is it not more widely used? First, prior difficulties in the optical technologies for VUV light generation and transmission led to a lack of sensitivity and stability, especially with the common, incoherent, low-intensity VUV light sources, such as discharge lamps. Another drawback of SPI is that the molecular ions it forms are radical cations; these tend to be less stable than the even electron ions most often formed by MALDI, ESI, atmospheric-pressure CI (APCI), and related methods. Yet another complicating event in SPI is proton transfer, which dominates in AP photoionization (APPI; 26). Finally, the relative experimental complexity and resultant shortage of commercial instruments are additional barriers to the application of SPI. However, as these issues are discussed later, it will become apparent that many of these barriers are falling.
Light sources
SPI requires sources and optics for VUV radiation in the photon energy range of 7.5–11.8 eV, which corresponds to wavelengths of ~165–105 nm (16). The VUV region requires the use of calcium fluoride optics for 7.5–8.0-eV radiation and magnesium or lithium fluoride optics for higher-energy photons. Furthermore, water and oxygen absorb strongly across the VUV, so optical path lengths must be under vacuum or purged with nitrogen or noble gases. Finally, even trace adventitious hydrocarbons (e.g., pump oil) adsorbed on lenses or remaining as trace contaminants in purge gases strongly absorb and thereby attenuate VUV radiation.
Several lamp-based VUV sources are available. Deuterium discharge lamps provide a continuum of continuous wave (CW) VUV radiation that can be energy-selected by a monochromator, but these lamps are relatively low intensity and, except for APPI, rarely used for SPI in applied MS. Discharge lamps filled with various noble gases emit relatively low intensity CW radiation at broad spectral lines centered at 8.4–11.8 eV. Such PI lamps have long been used as GC detectors, but they have also been applied to APPI (26) and other MS experiments in which averaging can compensate for their low intensity. Higher intensities are achieved by microwave discharge lamps using, for example, a He/H2 filling to generate hydrogen Lyman-α atomic radiation (121.5 nm) (27). A recently developed PI ion source based on an EBEL of high brilliance and flux is particularly well suited for analytical PI-MS (21, 28). The EBEL uses an ~12-keV electron beam that penetrates a 300-nm-thin silicon nitride foil into a dense rare-gas reservoir, where the electrons are quickly decelerated by impacts with the rare-gas atoms. Diatomic rare-gas excimers form from the excited rare-gas atoms in the small volume in front of the penetration foil, and these excimers emit VUV light upon their decay.
Pulsed-laser-based sources also can emit VUV radiation for SPI; the most popular example is ninth harmonic generation by passing the 355-nm third harmonic of an Nd:YAG laser into xenon or a xenon/argon mixture (9). This source has been widely used because its 10.5-eV photon energy is higher than the IE of most organic species and because its ~5-ns pulse length is readily coupled to TOFMS (9, 11, 17). Other nonlinear optical generation schemes have been developed, but they require complex tunable pump laser systems, which further complicate the experimental configuration (16). Laser plasma and femtosecond laser sources of VUV radiation also have been demonstrated (29, 30). Advances and cost reductions in commercial pump lasers could make any of these VUV sources more widely available in the future. In addition to frequency-multiplied lasers, direct VUV-generating laser processes also exist.
A particularly convenient VUV source is the molecular fluorine laser, a variety of excimer laser that emits ~10-ns-long pulses at 7.87 eV (16). Fluorine lasers are commercially available with repetition rates up to 1 kHz and powers up to tens of millijoules per pulse, more than sufficient to saturate SPI within even an unfocused laser beam. A homebuilt hydrogen laser, which produces photons at 7.75 eV, also has been used for SPI-MS (31). However, the low (~7.8-eV) photon energy of the fluorine or hydrogen laser is below the IEs of most molecular species. This apparent disadvantage can be favorably exploited for selective detection of low-IE analytes in a mixture of higher IE species (31). Furthermore, chemical derivatization of a high-IE species with a low-IE chromophore allows its detection by SPI with the fluorine laser (32). This “ionization tag” derivatization strategy is analogous to the use of fluorescent tags in optical detection, and it expands the realm of analytes that can be targeted with the fixed-wavelength fluorine laser.
Finally, VUV synchrotron sources such as the Advanced Light Source at Lawrence Berkeley National Laboratory or the Berliner Elektronenspeicherring-Gesellschaft für Synchrotronstrahlung (BESSY; Germany) produce tunable, quasi-CW, intense radiation that is perfect for fundamental and applied research on SPI (33, 34). The ability to continuously tune the photon energy at a synchrotron is a particularly useful feature not commonly available with laboratory sources. However, the need for researchers to locate at one of the world’s few facilities with a VUV synchrotron light source poses obvious limitations.
Real-world applications
In Figure 1a, the ionization energies of a homologous series of different organic compound classes are shown as a function of their molecular mass, along with the VUV emission profiles of common light sources. Compounds with IEs higher than the photon energy are excluded from ionization. The relatively small excess internal energy imparted by ionization with VUV photons causes the softness of ionization, as shown in the mass spectra of a diesel sample ionized by either EI or SPI (Figure 1b). These data were collected during a GC/SPI-TOFMS experiment using the EBEL light source for ionization and represent a summation of all the mass spectra obtained during the GC/MS run (5). Although the summed EI-MS spectrum depicts solely fragments, and thus provides very limited chemical information, the SPI mass spectrum of diesel shows the distribution of molecular masses very clearly. The homologous series of intact alkanes is indicated by asterisks, demonstrating that SPI-MS is well suited for profiling of highly complex mixtures such as mineral oil and petroleum-based products.
Most real-world applications of SPI-MS have been in the field of direct on-line gas analysis. More recently, hyphenated techniques such as GC/SPI-MS (5) and thermogravimetry-SPI-MS (28, 35, 36) also have been reported. Because SPI is fragmentation-free, the distribution of mass signals in the mass spectrum directly reflects the molecular composition. Nominally isobaric compounds with different elemental compositions are separable if the mass resolution is sufficiently high. Isomeric compounds can even be separated by MS by tuning the SPI wavelength.
A relatively large number of SPI-MS applications deal with on-line monitoring of pyrolysis and combustion off-gases; especially interesting are the highly dynamic processes involving exhaust gases of internal combustion engines. SPI-MS can study dynamic concentration changes of compounds such as butadiene, 2-propenal (acrolein), or benzene that are due to variation in load or acceleration, engine misfiring, or changes in the activity of exhaust gas treatment catalyst (15, 18). The time-resolved chemical profiles of several other combustion and pyrolysis processes of industrial importance have also been studied, including waste incineration (3, 27), biomass combustion, and coffee roasting (37). Furthermore, several fundamental studies on pyrolysis and combustion have been performed (5, 28, 35, 38). The common figure of merit for SPI-MS in the abovementioned applications is that a multitude of organic trace compounds (parts-per-billion to parts-per-million range) can be detected on-line in a complex gaseous matrix. Although some compounds, such as benzene, can also be detected by other MS methods, this is not the case for the aliphatic and substituted aromatics. Thus, SPI-MS allows a unique, rapid chemical profiling of complex organic mixtures (37, 39).
Figure 2 shows a variety of typical direct-inlet SPI-MS applications. A mass spectrum obtained from a prototype coupling of a thermobalance and an SPI-quadrupole mass spectrometer (i.e., thermogravimetry-SPI-QMS) is depicted in Figure 2a. A rather simple plastic, polyethylene, was investigated. The fragment-free SPI mass spectrum is recorded at the temperature of the maximal weight loss (~500 °C, nitrogen atmosphere) and depicts the homologous series of alkenes, dienes/cycloalkenes, and trienes/cyclodienes. In the corresponding EI mass spectrum of that fraction, only fragments with negligible chemical information are observed (data not shown) (28).
Figure 2.
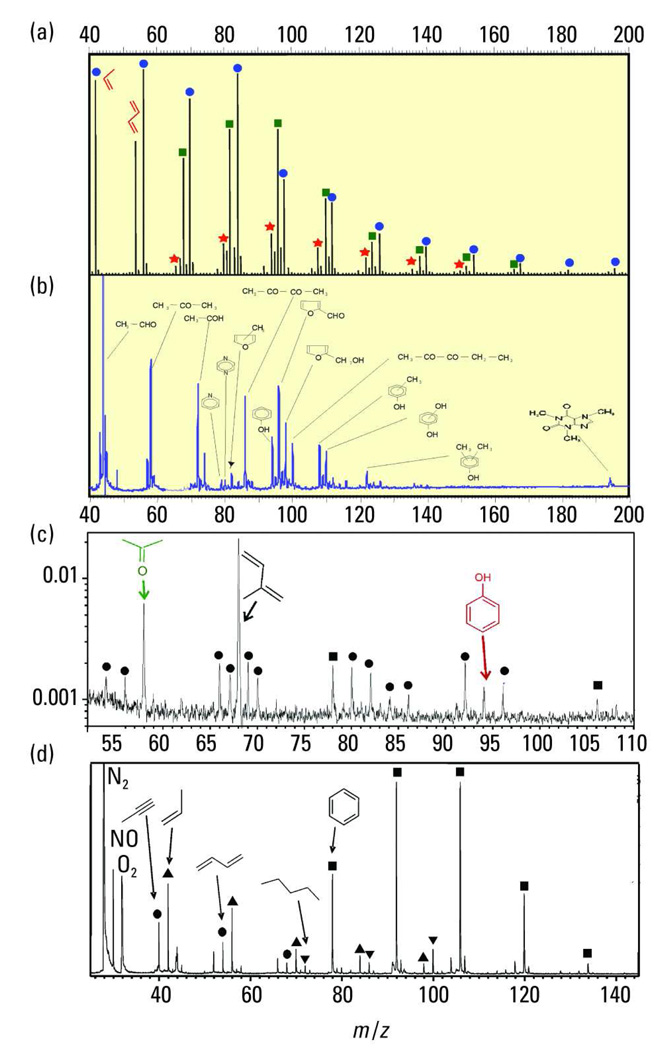
Typical direct-inlet SPI-MS applications. The y axis for all parts of this figure is the SPI signal in relative units. (a) Thermogravimetry-MS of gas evolved from polyethylene pyrolysis (9.8-eV EBEL-Ar lamp, quadrupole mass spectrometer; adapted from Ref. 28). Blue circles, homologous series of alkenes; green squares, homologous series of dienes; and red stars, homologous series of trienes. (b) Analysis of arabica coffee headspace (10.5-eV, frequency-multiplied Nd:YAG laser, TOF mass spectrometer). (c) Analysis of the breath of a smoker (9.8-eV, EBEL-Ar lamp, TOF mass spectrometer; adapted from Ref. 21). (d) Analysis of automotive exhaust (10.5-eV, frequency-multiplied Nd:YAG laser, TOF mass spectrometer; adapted with permission from Ref. 18). Triangles, homologous series of alkenes; circles, homologous series of dienes; inverted triangles, homologous series of alkanes; and squares, homologous series of benzenes.
The SPI coffee powder headspace mass spectrum (arabica coffee powder, 50 °C) depicted in Figure 2b shows the volatile fraction of the coffee odor. In addition to a tiny caffeine peak at m/z 194, carboxylic compounds (i.e., acetaldehyde, acetone, and butadiene), sulfur-containing compounds (methane thiol at m/z 48), phenolic compounds (i.e., phenol and cresols), and heterocyclic species (i.e., pyridine, furfural, and furfuryl alcohol) are visible. Other key flavor-active compounds, such as 4-vinylguaiacol, are not visible in the headspace mass spectrum but can be recorded in the SPI mass spectra of the coffee roasting off-gas (37). Note that without MS/MS or ultrahigh-mass-resolution MS detection, the assignation of mass peaks in the abovementioned application may require some chemical knowledge of the investigated system. However, both chromatographic separation and increased MS resolution have been implemented for improved analyses of multiple unknown compounds (4, 5, 15, 27).
Figure 2c depicts the application of SPI-MS to the on-line analysis of exhaled human breath from a cigarette smoker who had not smoked for an hour before the analysis was performed. The prominent indigenous metabolic markers acetone, isoprene, and phenol are observed. These compounds are present in the breath of smokers and nonsmokers at comparable concentrations. However, SPI-MS of the smoker’s breath also revealed the presence of several partly toxic tobacco-smoke-specific compounds, including alkenes, dienes, and benzene derivatives, even hours after smoking.
Finally, an SPI mass spectrum of automotive exhaust is depicted in Figure 2d. Here, the homologous series of aromatic compounds including benzene, toluene, and xylenes/ethylbenzene as well as C3-substituted benzene dominate the mass spectrum. NO and unsaturated aliphatic compounds also can be detected. Alkanes from unburned fuel are sometimes detectable, but their signals are usually weak because of their low thermal stability compared with aromatics and other compounds.
The analysis of cigarette smoke demonstrates a particular strength of SPI-MS, namely the possibility of performing high-speed on-line real-time analysis (39). Using fast TOF mass spectrometers and high-repetition-rate pulsed laser sources, such as that at 10.5-eV photon energy, measurement repetition rates in the 10–100-Hz region are possible. Recently, a comprehensive SPI-MS on-line study of the dynamic composition of tobacco smoke during the smoking process was performed with 100-ms time resolution. The tobacco smoking process is very dynamic, and within a single puff, which typically lasts ~2 s, the concentration of the organic tobacco smoke constituents can vary by several orders of magnitude. Furthermore, the chemical signatures are changing as a function of the puff number, necessitating fast recording of multiple organic species that are possible with this method.
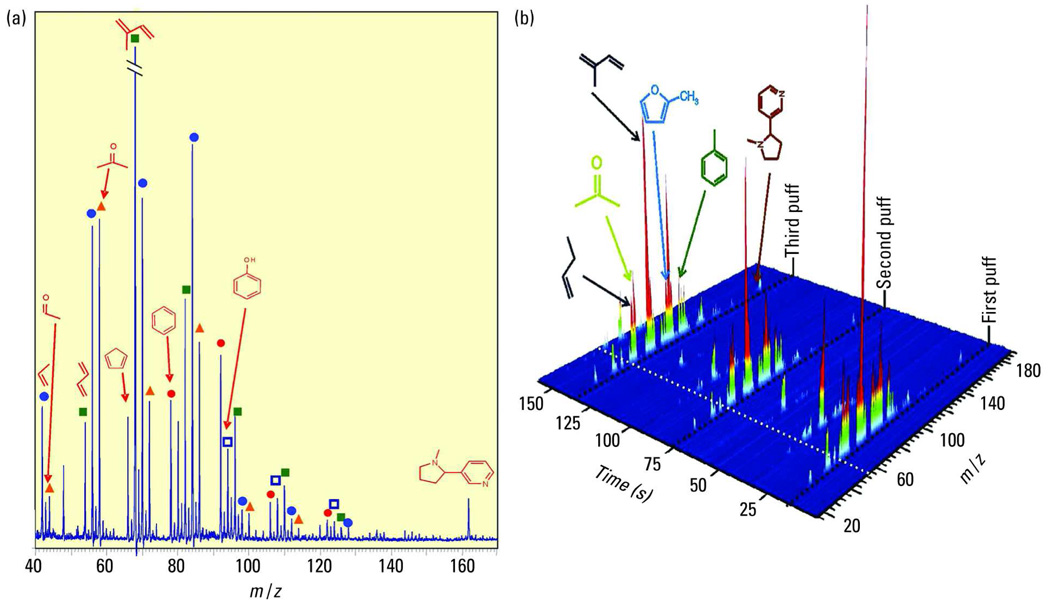
MS experiments with EI ionization of cigarette smoke found almost no information on the molecular organic composition because of the heavy fragmentation, although information on the small inorganic molecules such as H2O, O2, and CO2 is obtained. By contrast, SPI-MS reveals the main organic constituents of cigarette smoke. Figure 3a depicts an SPI direct-inlet mass spectrum of cigarette mainstream smoke (MSS; the smoke as it is inhaled by the smoker) recorded during a single smoking puff. In addition to nicotine and the typical intense isoprene peaks, a variety of gaseous tobacco smoke constituents are visible in the mass spectrum. This includes the homologous series of the dienes, starting with butadiene, which is considered one of the most health-relevant compounds in tobacco smoke. Furthermore, several benzene and phenol derivatives are visible as well as the homologous series of aldehydes/ketones, starting with acetaldehyde and acetone.
Figure 3.
Time-resolved analysis of cigarette smoke. (a) Direct-inlet SPI mass spectrum (9.8-eV, EBEL-Ar lamp, TOF mass spectrometer) of mainstream cigarette smoke, recorded from a single 2-s puff (third puff) by a smoking machine using SPI-TOFMS coupling (adapted from Ref. 39). Blue circles, homologous series of alkenes; green squares, homologous series of dienes; orange triangles, homologous series of ketones/aldehydes; red circles, homologous series of alkyl benzenes; and white squares with blue borders, homologous series of alkyl phenols. (b) 3D representation of an SPI-MS on-line cigarette smoking sequence (the first three puffs), recorded by a smoking machine using SPI-TOFMS coupling.
Figure 3b displays the 3D SPI-MS representations of a time-resolved measurement of tobacco smoke for the first three puffs of a MSS measurement. The MSS is generated by a standard single-port smoking machine, which simulates the smoking process in a standardized, reproducible way for research purposes. During the puff, high signals for a variety of volatile tobacco smoke constituents can be detected in the SPI mass spectra. This includes compounds such as ammonia, acetaldehyde, butadiene, isoprene, benzene, phenol, and nicotine (Figure 3a). Because the duration of a single puff is only 2 s, the high time resolution is needed for accurate on-line analysis of the transient concentrations during individual puffs. The chemical profile of the smoke constituents changes as a function of the puff number. For example, a “first-puff high” might be explained by this data: unsaturated compounds such as the highly toxic butadiene show significantly increased concentrations in the first puff because of the unstable combustion conditions present when the cold cigarette is lit.
Note that dynamic results such as these cannot be achieved by EI-MS or by other soft ionization techniques such as CI (because of chemical interferences and matrix effects among analytes) or FI (because of oxidation of carbon needle emitters). Current studies suggest that SPI-MS also has potential for sensitive and selective detection of explosives, chemical warfare agents, and illegal drugs (20, 34). SPI-MS has the advantages of higher sensitivity than FTIR spectroscopy (Table 1) and, if applied in an ion trap mass spectrometer, a lower false-positive rate than ion mobility spectrometry.
In addition, SPI-MS has great potential as a soft ionization MS detection technique for chromatography. The identification capability of GC/SPI-MS is significantly enhanced by the presence of molecular ion signals for fragile molecules such as trimethylsilyl-derivatized biomolecular analytes. As in soft ionization MS, molecules are “separated” solely according to their molecular mass; SPI-MS also can be used as a separation dimension in a comprehensive multidimensional separation approach. In fact, the separation characteristics of compounds in SPI-MS very much resemble a “boiling-point-type” gas chromatographic separation obtained for a GC column with a nonpolar stationary phase and the use of a temperature program (i.e., typical homologous series are detected by both approaches). Thus, if the GC/SPI-MS data set is displayed in a 2D plot of retention time versus m/z, the distribution of peaks on the 2D separation plane is similar to that obtained in a comprehensive GC × GC analysis (4, 5).
SPI for surface analysis
Surface, materials, and aerosol analyses can be performed with SPI by coupling with ion or laser desorption (14). The method using ion desorption has been referred to as secondary neutral MS. The method using laser desorption has been called LDPI-MS, where LD stands for laser desorption and PI may stand for single-photon ionization, photoionization, or postionization; another name is two-step laser MS (L2MS). These experiments utilize SPI (14), but similar strategies have also used REMPI (7). Focusing and moving the desorption laser or a focused ion beam across the surface permits the collection of chemical images from surfaces in what is commonly known as microprobe mode. Intact polymer films can be detected by these methods (12, 14), and the spectra usually show primarily the monomer from the depolymerized film. Careful desorption with a laser probe sometimes reduces fragmentation, compared with the use of an ion beam (14), because the latter can impart more internal energy into the neutral before SPI (1). A variant of LDPI-MS has also been applied to the chemical analysis of aerosols (40, 41), rendering mass spectra that are more representative of the aerosol molecular composition than can be obtained by the more common direct laser ablation technique.
Some of the most successful surface analysis work with SPI has used LDPI-MS to study self-assembled monolayers (SAMs) that are used as sensors, to guide tissue growth, and for combinatorial arrays (14). Alkanethiolate SAMs on gold surfaces with a wide range of head groups were detected by LDPI-MS using 10.5-eV VUV photons, with the spectra dominated by alkanedisulfide dimers that formed from the alkanethiolates during a gentle, thermal desorption event (14). Furthermore, 10.5-eV LDPI-MS of alkanethiolates showed less fragmentation, and fewer ions contained substrate atoms than appeared in their secondary ion mass spectra. In addition, 10.5-eV LDPI-MS has shown the ability to image photopatterned SAMs (14).
The same technique has also been used to examine siloxane SAMs and to distinguish the competitive events of adsorption, surface coupling, and self-polymerization of siloxanes on oxide surfaces (42). These disparate processes are difficult to distinguish by the traditional techniques of secondary ion MS or X-ray photoelectron spectroscopy. A methacrylate bisphenol A derivative covalently coupled to the siloxane overlayer also could be detected by using 10.5-eV LDPI-MS. Even better, the use of 7.87-eV LDPI-MS with a low-IE tag (dimethyl amino benzene) coupled to the bisphenol A derivative led to strong signal enhancement (43). Tagging a covalently bound surface analyte with a low-IE species also permitted detection of small peptides covalently bound to an oxide via a siloxane linker (32).
Biological applications
Some of the earliest work in SPI was focused on detecting biomolecules. Small peptides were successfully detected by laser desorption of neutrals from either frozen or dried aqueous solutions; the peptides were then detected after 10.5-eV SPI (11). At the time, researchers argued that there was a fundamental upper mass limit of ~1 kDa to species that can be detected by SPI, but later work instead attributed this apparent mass limit to the instability of radical cations of peptides (13). Recent 7.87-eV SPI experiments have extended the upper mass limit beyond 6.5 kDa (44). The true upper mass limit of SPI, if one exists, remains to be determined.
If a gaseous neutral has sufficient internal energy before ionization, then dissociation of its molecular ion formed by SPI is enhanced. This was demonstrated by a comparison of MALDI, ESI, fast atom bombardment, and SPI of laser-desorbed porphyrin oligomers (45): all of these soft ionization methods could provide similar low-fragmentation mass spectra displaying similar oligomer distributions. Furthermore, the SPI experiment that also cooled the neutrals via a supersonic jet displayed less fragmentation than SPI of internally hot neutral porphyrin oligomers. The above comments indicate that the internal energy of the neutral before SPI, the inherent stability of the radical cation formed, and the excess photon energy above the IE will all affect the extent of dissociation of the parent ion after SPI (9, 13, 14, 17, 33, 46).
Recent work has shown that SPI is beginning to establish a foothold in biological MS among the standard techniques, especially for the detection of compounds below ~2 kDa. For example, 10.5-eV LDPI-MS was used to detect organoselenium and organic acid metabolites that ranged up to 500 Da in human urine (47). Detection limits were in the range of 20 ng/mL for several of the organoselenium compounds. Furthermore, the urine samples were only lyophilized, centrifuged, and filtered before analysis; no chromatographic separation was performed. Compound identification in this work was aided by the unique natural isotopic signature of selenium, but no fundamental limitation exists to coupling the technique to high-resolution or tandem MS (14) for the analysis of a wider range of metabolites. The absence of matrix molecule ionization is a major reason why SPI is superior for such analyses, similar to the situation discussed above for combustion gas monitoring.
In biological MS, SPI with low photon energies allows selective detection of only low-IE analytes from complex biological fluids or tissues. This method was demonstrated using ~7.8-eV photon energies from the hydrogen laser (see above) to detect drugs of abuse and other pharmaceutical compounds (31). The analytes were all fused-ring, secondary amine, or tertiary amine compounds that typically display IEs below the ~7.8-eV photon energies used.
Furthermore, these drugs were detected from prescription tablets and urine samples after only a simple solid-phase extraction, again avoiding a chromatographic separation step.
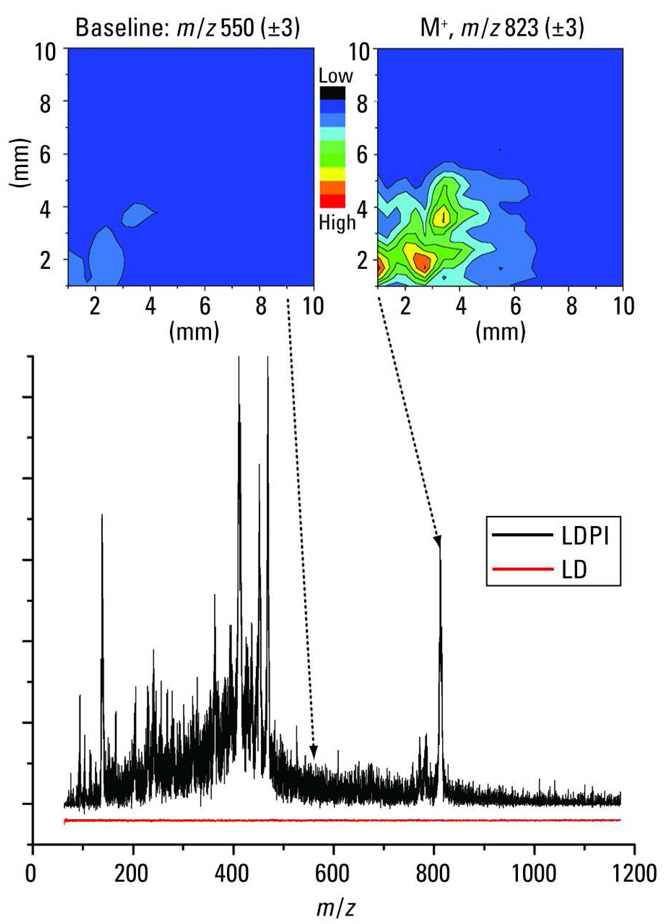
Low-photon-energy SPI can also be used in MS imaging of intact biological tissue and other samples. The fluorine laser’s selectivity with 7.8-eV photon energies makes it particularly convenient for such experiments (46). One specific example of an MS imaging analysis with SPI is the detection of small-molecule analytes within intact bacterial biofilms (48). Bacteria growing as biofilms are particularly resistant to treatment by antibiotics. Figure 4 displays a 7.87-eV LDPI mass spectrum of the antibiotic rifampicin (black curve) detected within a Staphylococcus epidermidis biofilm: M+ at m/z 823 is prominent along with several large fragments of the antibiotic near m/z 400. The LD-only mass spectrum (red curve) displays no signal: direct ion signal was suppressed by the use of low-desorption laser power. Within intact S. epidermidis biofilms, 7.87-eV LDPI-MS has been used to detect sulfadiazine and tetracycline antibiotics, the latter at a near-clinical concentration (49). This technique is being used to examine the fate of these antibiotics within intact biofilms to examine mechanisms of antibiotic resistance.
Figure 4.
LDPI-MS with VUV radiation for the analysis of bacterial biofilms. The 7.87-eV LDPI mass spectrum (black curve) is shown for the antibiotic rifampicin detected within an intact, dried S. epidermidis biofilm: the rifampicin molecular ion appears at m/z 823. The LD-only mass spectrum (red curve) displays no signal. (Top right) m/z 820–826 image of the rifampicin molecular ion from a biofilm attached to a piece of carbon tape. The rifampicin signal image roughly corresponds to the location of the biofilm; the region lacking signal is the carbon tape. (Top left) The background signal image at m/z 547–553 displays no signal in either region.
Bacterial biofilms use various biochemical signaling species to direct communal responses to environmental challenges (48). For example, Gram-positive bacteria use various peptides for signaling in a process known as quorum sensing, which mimics mammalian cell signaling. However, most peptides (except those containing tryptophan) and many other analytes have an IE too high to be detected by 7.87-eV photons. This disadvantage can be turned into a very favorable detection scheme, using molecular PI tags that can be bound in a specific chemical derivatization reaction to the molecules of interest (50, 51). Chemical derivatization of peptides with low-IE tags, such as anthracenic compounds, permits their detection via 7.87-eV LDPI-MS (46). This technique permitted detection and imaging of a quorum-sensing peptide within an intact Bacillus subtilis biofilm with orders of magnitude higher LOD than is possible with traditional LC/MS techniques (48). This derivatization strategy should work for many analytes within any biological sample, provided they can be derivatized without undue degradation of the sample.
Imaging with LDPI-MS can achieve resolution of a few micrometers (12, 14), depending on the focus of the desorption laser and the concentration of analyte species within the desorption spot. Submonolayer sensitivity is also possible with the method (14). Figure 4 (top right) shows the m/z 820–826 image of the rifampicin molecular ion from a biofilm attached to a piece of carbon tape. The rifampicin signal image roughly corresponds to the location of the biofilm, whereas the region lacking signal is the carbon tape. A background signal image at m/z 547–553 displays no signal in either the region of the biofilm or the carbon tape. The spatial resolution here is in the range of tens of micrometers, similar to that typical of imaging MALDI-MS. Ongoing work is comparing LDPI-MS with MS imaging using MALDI.
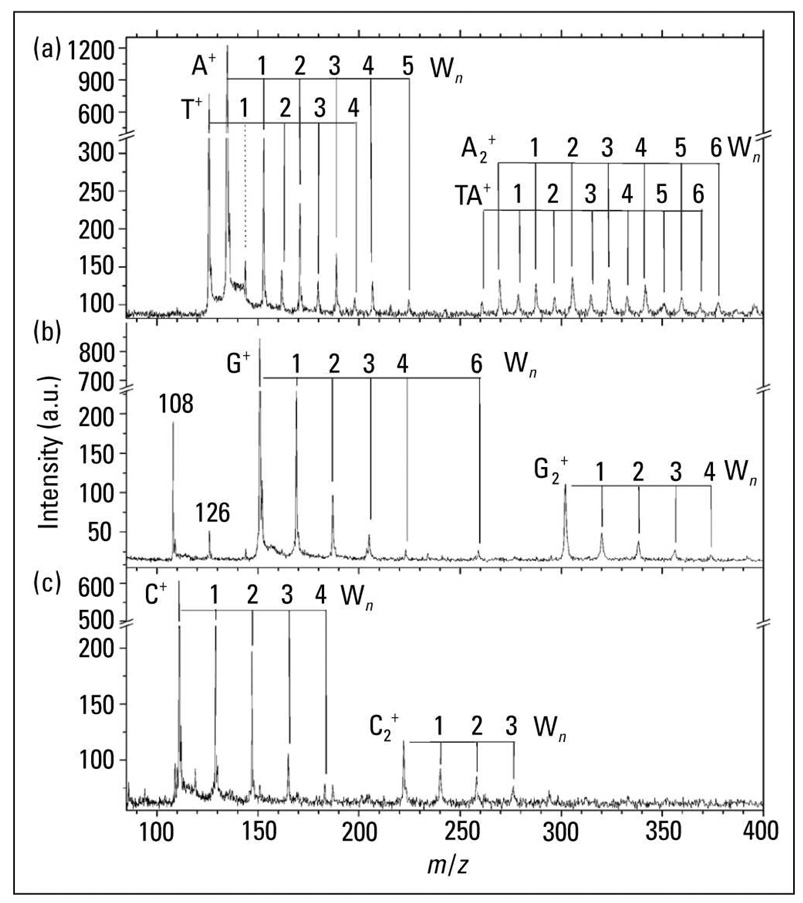
Another interesting field of research in biological MS is the study of hydrated biomolecules by SPI-MS. Most MS probes isolate molecules in the gas phase, yet biomolecules naturally exist in a hydrated environment, surrounded by a solvation shell of water molecules. SPI coupled with sources of biological aerosols can examine solvated biomolecules, directly forming cations of a biomolecule bound to one or more water molecules. An example of the results of such an experiment is shown in Figure 5, which displays the 10-eV SPI-MS of monomers and dimers of the four bases of DNA (52). Each base displays ions corresponding to adducts formed by binding to a distribution of water molecules. These spectra were recorded at the Advanced Light Source, and additional experiments tuned the photon energy to show that the IEs of these hydrated complexes decreased as water molecules were added sequentially (data not shown). For example, thymine displays an appearance energy of 8.90 ± 0.05 eV, which drops by 0.15 eV for (thymine)(H2O) and 0.3 eV for (thymine)(H2O)2 and (thymine)(H2O)3 (note that appearance energies are closely related to IEs, which should follow similar trends). Other work showed IE decreases of ~1 eV for water-solvated analytes (53).
Figure 5.
SPI for analysis of solvated biomolecules. SPI coupled with sources of biological aerosols can examine solvated biomolecules, directly forming cations of a biomolecule bound to one or more water molecules. 10.0-eV SPI-MS of hydrated monomers and dimers of the four bases of DNA are shown: (a) adenine, A, and thymine, T; (b) guanine, G; and (c) cytosine, C. The numbers correspond to how many water molecules (Wn) are bound to each DNA base. (Adapted from Ref. 52.)
APPI induces ionization via 10.0/10.6-eV VUV radiation from a discharge lamp (26). However, APPI occurs at high pressures, and the low penetration depth of VUV radiation into most gases at atmospheric pressure permits direct SPI of only the species physically closest to the discharge lamp window. For the most part, these ionized species are not the analyte, but their formation initiates proton transfer events that eventually lead to CI of the analyte. Thus, APPI behaves more like traditional or APCI. Similar CI events occur in ion mobility spectrometers that use VUV discharge lamps as sources. Proton transfer events should therefore become more prevalent as pressures of species with IEs below the photon energy increase within the ionization region.
Furthermore, many desorption events can form cluster species such as the (thymine)(H2O)n discussed above; in that case, the IEs may be lower than that of the isolated molecule. For example, pure water clusters (54) can dissociate to form protonated species upon SPI. Such a cluster dissociation mechanism may have contributed to the formation of protonated peptides during LDPI-MS (46). These proton transfer events can have different energetics than direct SPI and could become more significant in the high-pressure trapping schemes used to obtain high mass resolution in some modern mass spectrometers. However, although proton transfer events increase the number of ionization pathways, they do not necessarily hinder the efficacy of SPI-MS, given the proven utility of APPI (26).
Next steps
Further development of SPI-MS is strongly connected to further progress in the development and improvement of affordable and rugged incoherent and coherent VUV light sources. Such advances even raise the promise of commercialization of SPI-MS. The applications described here were performed with customized instrumentation, but the introduction of commercial instruments would open the floodgates to new applications in chemical identification of combustion products, foodstuffs, drugs, metabolites, and other burgeoning areas of research. In addition, more direct comparisons between SPI and other MS methods will help elucidate the unique capabilities of SPI-MS.
Acknowledgments
L. H. acknowledges funding from the National Institutes of Health (grant EB006532). R. Z. acknowledges funding from the German Research Foundation, the German Federal Ministry of Education and Research, and the Bavarian Research Foundation. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the aforementioned funding agencies.
Biography
Luke Hanley is a professor of chemistry and bioengineering at the University of Illinois Chicago. His research focuses on MS imaging with SPI and photoelectron spectroscopy for surface analysis. He applies these methods to the study of bacterial biofilms, biofilm–biomaterial interfaces, and organic–inorganic nanocomposite materials for use in biomaterials and optoelectronic devices. Ralf Zimmermann is a professor and chair of analytical chemistry at the Mass Spectrometry Center of the University of Rostock and has appointments at the German Research Center for Environmental Health and the Bavarian Institute for Applied Environmental Research and Technology (all in Germany). His research on SPI-MS is used in a wide range of applications in combustion, foodstuffs, homeland security, and other problems. Address correspondence about this article to Hanley at lhanley@uic.edu.
Contributor Information
Luke Hanley, University of Illinois Chicago.
Ralf Zimmermann, University of Rostock (Germany).
References
- 1.Vekey KJ. Mass Spectrom. 1996;31:445–463. [Google Scholar]
- 2.Boesl UJ. Phys. Chem. 1991;95:2949–2962. [Google Scholar]
- 3.Mühlberger F, et al. Anal. Chem. 2004;76:6753–6764. doi: 10.1021/ac049535r. [DOI] [PubMed] [Google Scholar]
- 4.Mitschke S, Welthagen W, Zimmermann R. Anal. Chem. 2006;78:6364–6375. doi: 10.1021/ac060531r. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann R, Welthagen W, Groger TJ. Chromatogr., A. 2008;1184:296–308. doi: 10.1016/j.chroma.2007.08.081. [DOI] [PubMed] [Google Scholar]
- 6.Constapel M, et al. Rapid Commun. Mass Spectrom. 2005;19:326–336. doi: 10.1002/rcm.1789. [DOI] [PubMed] [Google Scholar]
- 7.Pomerantz AE, et al. J. Am. Chem. Soc. 2008;130:7216–7217. doi: 10.1021/ja801927v. [DOI] [PubMed] [Google Scholar]
- 8.Bente M, et al. Anal. Chem. 2008;80:8991–9004. doi: 10.1021/ac801295f. [DOI] [PubMed] [Google Scholar]
- 9.Van Bramer SE, Johnston MV. J. Am. Soc. Mass Spectrom. 1990;1:419–426. doi: 10.1016/1044-0305(90)85024-G. [DOI] [PubMed] [Google Scholar]
- 10.Becker CH, Jusinski LE, Moro L. Int. J. Mass Spectrom. 1990;95:R1–R4. [Google Scholar]
- 11.Schlag EW, Grotemeyer J, Levine RD. Chem. Phys. Lett. 1992;190:521–527. [Google Scholar]
- 12.Lykke KR, et al. Appl. Opt. 1993;32:857–866. doi: 10.1364/AO.32.000857. [DOI] [PubMed] [Google Scholar]
- 13.Becker CH, Wu KJ. J. Am. Soc. Mass Spectrom. 1995;6:883–888. doi: 10.1016/1044-0305(95)00472-P. [DOI] [PubMed] [Google Scholar]
- 14.Hanley LJ, et al. Mass Spectrom. 1999;34:705–723. doi: 10.1002/(SICI)1096-9888(199907)34:7<705::AID-JMS845>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Butcher DJ. Microchem. J. 1999;62:354–362. [Google Scholar]
- 16.Misra P, Dubinski MA. UV Spectroscopy and UV Lasers. New York: Marcel Dekker; 2002. [Google Scholar]
- 17.Shi YJ, Lipson RH. Can. J. Chem. 2005;83:1891–1902. [Google Scholar]
- 18.Adam T, Zimmermann R. Anal. Bioanal. Chem. 2007;389:1941–1951. doi: 10.1007/s00216-007-1571-x. [DOI] [PubMed] [Google Scholar]
- 19.Tonokura K, Nakamura T, Koshi M. Anal. Sci. 2003;19:1109–1113. doi: 10.2116/analsci.19.1109. [DOI] [PubMed] [Google Scholar]
- 20.Mullen C, et al. Anal. Chem. 2006;78:3807–3814. doi: 10.1021/ac060190h. [DOI] [PubMed] [Google Scholar]
- 21.Mühlberger F, et al. Anal. Chem. 2005;77:7408–7414. doi: 10.1021/ac051194+. [DOI] [PubMed] [Google Scholar]
- 22.Mühlberger F, et al. Anal. Chem. 2005;77:2218–2226. doi: 10.1021/ac048319f. [DOI] [PubMed] [Google Scholar]
- 23.Mühlberger F, et al. Anal. Chem. 2007;79:8118–8124. doi: 10.1021/ac071217f. [DOI] [PubMed] [Google Scholar]
- 24.King BV, et al. Appl. Surf. Sci. 2003;203/204:244–247. [Google Scholar]
- 25.Ng CY. Int. J. Mass Spectrom. 2000;200:357–386. [Google Scholar]
- 26.Syage JA. J. Am. Soc. Mass Spectrom. 2004;15:1521–1533. doi: 10.1016/j.jasms.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Kuribayashi S, et al. Anal. Chem. 2005;77:1007–1012. doi: 10.1021/ac048761y. [DOI] [PubMed] [Google Scholar]
- 28.Saraj-Bozorgzad M, et al. Anal. Chem. 2008;80:3393–3403. doi: 10.1021/ac702599y. [DOI] [PubMed] [Google Scholar]
- 29.Borghese A, Di Palma TM. Appl. Opt. 2007;46:4948–4953. doi: 10.1364/ao.46.004948. [DOI] [PubMed] [Google Scholar]
- 30.Riedel D, et al. Rev. Sci. Instrum. 2001;72:1977–1983. [Google Scholar]
- 31.Finch JW, et al. Rapid Commun. Mass Spectrom. 2005;19:15–22. doi: 10.1002/rcm.1742. [DOI] [PubMed] [Google Scholar]
- 32.Edirisinghe PD, et al. Anal. Chem. 2004;76:4267–4270. doi: 10.1021/ac049434t. [DOI] [PubMed] [Google Scholar]
- 33.Wilson KR, et al. J. Phys. Chem. A. 2006;110:2106–2113. doi: 10.1021/jp0543734. [DOI] [PubMed] [Google Scholar]
- 34.Schramm E, et al. Appl. Spectrosc. 2008;62:238–247. doi: 10.1366/000370208783575483. [DOI] [PubMed] [Google Scholar]
- 35.Zoller DL, et al. Energy Fuels. 1999;13:1097–1104. [Google Scholar]
- 36.Arii T, Otake S. J. Therm. Anal. Calorim. 2008;91:419–426. [Google Scholar]
- 37.Dorfner R, et al. Anal. Chem. 2004;76:1386–1402. doi: 10.1021/ac034758n. [DOI] [PubMed] [Google Scholar]
- 38.Zoller D, Johnston MV. Anal. Chem. 1997;69:3791–3795. [Google Scholar]
- 39.Mitschke S, et al. Anal. Chem. 2005;77:2288–2296. doi: 10.1021/ac050075r. [DOI] [PubMed] [Google Scholar]
- 40.Oktem B, Tolocka MB, Johnston MV. Anal. Chem. 2004;76:253–261. doi: 10.1021/ac0350559. [DOI] [PubMed] [Google Scholar]
- 41.Nash DG, et al. Int. J. Mass Spectrom. 2005;241:89–97. [Google Scholar]
- 42.Zhou M, et al. J. Biomed. Mater. Res., Part A. 2006;77:1–10. doi: 10.1002/jbm.a.30591. [DOI] [PubMed] [Google Scholar]
- 43.Zhou M, et al. J. Am. Soc. Mass Spectrom. 2007;18:1097–1108. doi: 10.1016/j.jasms.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marksteiner M, et al. J. Am. Soc. Mass Spectrom. 2008;19:1021–1026. doi: 10.1016/j.jasms.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Siegal MMJ, et al. J. Mass Spectrom. 1999;34:661–669. doi: 10.1002/(SICI)1096-9888(199906)34:6<661::AID-JMS818>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 46.Edirisinghe PD, et al. Anal. Chem. 2006;78:5876–5883. doi: 10.1021/ac0605997. [DOI] [PubMed] [Google Scholar]
- 47.Chen YF, et al. Anal. Chem. 2006;78:8386–8394. doi: 10.1021/ac060827x. [DOI] [PubMed] [Google Scholar]
- 48.Edirisinghe PD, et al. Anal. Chem. 2007;79:508–514. doi: 10.1021/ac0615605. [DOI] [PubMed] [Google Scholar]
- 49.Gasper GL, et al. Proteomics. 2008;8:3816–3821. doi: 10.1002/pmic.200701142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes-Whaley M, et al. Anal. Chem. 2005;77:1–10. doi: 10.1021/ac040151a. [DOI] [PubMed] [Google Scholar]
- 51.de Vries MS, Hunziker HE. J. Photochem. Photobiol., A. 1997;106:31–36. [Google Scholar]
- 52.Belau L, et al. J. Phys. Chem. A. 2007;111:7562–7568. doi: 10.1021/jp0705929. [DOI] [PubMed] [Google Scholar]
- 53.Kinsel GR, et al. J. Mass Spectrom. 2002;37:1131–1140. doi: 10.1002/jms.374. [DOI] [PubMed] [Google Scholar]
- 54.Belau L, et al. J. Phys. Chem. A. 2007;111:10,075–10,083. doi: 10.1021/jp0705929. [DOI] [PubMed] [Google Scholar]