Abstract
Background
Long-term use of cocaine (⩾15 years) and antiretroviral therapy (ART) have been implicated in cardiovascular complications. Nevertheless, the individual and combined effects of ART and cocaine use on silent coronary artery disease have not been fully investigated.
Methods
Computed tomography coronary angiography was performed for 165 human immunodeficiency virus (HIV)–infected African American study participants aged 25–54 years in Baltimore, Maryland, with contrast-enhanced 64-slice multidetector computed tomography imaging.
Results
Significant (⩾50%) coronary stenosis was detected in 24 (15%) of 165 participants. The prevalence of significant stenosis among those who had used cocaine for ⩾15 years and had received ART for ⩾6 months was 42%. Exact logistic regression analysis revealed that long-term cocaine use (adjusted odds ratio, 7.75; 95% confidence interval, 2.26–31.2) and exposure to ART for ⩾6 months (adjusted odds ratio, 4.35; 95% confidence interval, 1.30–16.4) were independently associated with the presence of significant coronary stenosis. In addition, after controlling for confounding factors, both stavudine use for ⩾6 months or combivir use for ⩾6 months were independently associated with the presence of significant coronary stenosis.
Conclusions
Long-term exposure to ART may be associated with silent coronary artery disease; however, the magnitude of increased risk associated with ART was much lower than the risk associated with cocaine use or traditional risk factors. Cardiovascular monitoring and aggressive modification of cardiovascular risk factors are essential for reducing the risk of coronary artery disease in HIV-infected individuals. Extensive efforts should also be made to develop effective cocaine use cessation programs for HIV-infected cocaine users.
Since the introduction of HAART, HIV infection has become a chronic, manageable disease. With the dramatic improvement in survival of HIV-infected persons treated with HAART, coronary artery disease (CAD) has emerged as a key health concern in persons with HIV infection, especially because treatment-associated metabolic adverse effects of HAART have been observed [1–7]. Nevertheless, reports of cardiovascular adverse effects related to HAART have been inconsistent [7, 8]. HAART-associated toxicities generally fall into 2 categories: (1) worsening of traditional risk factors for CAD, such as glucose intolerance and dysli-pidemia [1–6], and (2) increase in cardiovascular events, such as myocardial infarction, and cardiovascular or cerebrovascular mortality [7, 8]. However, few studies have investigated whether antiretroviral therapy (ART), including HAART, is associated with silent CAD in persons with HIV infection who have no cardiovascular symptoms.
Cocaine use is not only strongly associated with HIV infection, but it also adversely affects the level of adherence that is necessary to maintain viral suppression [9–12]. However, only a few studies have examined the effect of cocaine use alone or its combined effect with ART on silent CAD.
The primary objectives of this study were (1) to estimate the prevalence of significant coronary stenosis among HIV-infected persons using contrast-enhanced CT coronary angiography and (2) to examine the individual and combined effects of ART and associated factors, such as cocaine use, on silent CAD in African Americans with HIV infection.
METHODS
Study participants
From August 2003 through June 2007, 165 HIV-infected African American study participants were consecutively enrolled at the HIV clinic at Johns Hopkins Hospital in Baltimore, Maryland. Interviews about sociodemographic characteristics and drug-use behaviors, clinical examinations, lipid profiles, high-sensitivity C-reactive protein tests, and contrast-enhanced 64-slice multidetector (MD) CT for coronary artery calcium and coronary angiography were performed. Information about medical history and medications was obtained by interview and was confirmed by medical chart reviews. Information about cocaine (powered cocaine and crack cocaine) and other illegal drug (mainly heroin) use was collected, including frequency of use, form of drug, administration mode (e.g., injected or smoked), and duration of drug use.
Inclusion criteria were age 25–54 years, HIV seropositivity, and African American ethnicity (self-designated). Exclusion criteria were (1) any evidence of hypertension or ischemic heart disease, (2) any symptoms believed to be related to cardiovascular disease, and (3) pregnancy. Information about sociodemographic characteristics, medical history, medication use, and cocaine-use behaviors was obtained by interviewer-administered questionnaires. The Committee on Human Research at the Johns Hopkins School of Medicine approved the study protocol, and all study participants provided written informed consent. All procedures used in this study were in accordance with institutional guidelines.
Coronary CT angiography with 64-slice MDCT
Non-contrast MDCT was performed on a Sensation 64 Cardiac Medical Solutions scanner (Siemens) to determine the coronary artery calcium score with a sequential scan of 3-mm slices with prospective electrocardiographic triggering, 30 mm × 0.6 mm detector collimation, and tube current of 135 mAs at 120 kV. Subsequently, coronary CT angiography was performed on the same equipment using 80 mL of isosmolar contrast agent (320 mg iodine/mL) injected at 4–5 mL/s. Imaging was performed with retrospective electrocardiograph gating, 32 mm × 0.6 mm detector collimation with flying focal spot to give effective detector collimation of 64 mm × 0.6 mm, 330 ms gantry rotation, 850 mAs, and 120 kV. Subsequently, 0.75-mm–thick axial slices were reconstructed at 0.4-mm intervals with B25 kernel using a half-scan reconstruction algorithm, with resulting temporal resolution of 165–185 ms. Ten reconstructions were done through the cardiac cycle at 10% increments in the R-R interval. If needed, patients were given metoprolol prior to the scan to achieve a heart rate of <65 beats per min.
Coronary artery calcium score, volume, and mass were measured on a Leonardo workstation (Siemens Medical Solutions USA). Regions of interest were placed over each of the coronary arteries with a threshold for pixels >130 HU for determining calcified plaque. Coronary vessels were assessed for patency and stenosis using 3D visualization tools after the axial images were reviewed for determination of anatomy, quality of the study, and appearance of the vessels.
Two reviewers (H.P. and E.K.F.; both were blinded to the risk profile of participants) independently evaluated the contrast-enhanced MDCT images by examining the axial slices, curved multiplanar reformations, and thin-slab maximum-intensity projections. The coronary artery tree was segmented according to the modified American Heart Association classification [13], and the segments were investigated for plaque and lumenal narrowing. The coronary arteries were divided into proximal, mid, and distal segments, with each segment investigated for lumenal narrowing. Plaques were classified as calcified or noncalcified, and the degree of stenosis was classified as a diameter of stenosis of <50% or ⩾50%. Diameter stenosis ⩾50% was defined as significant coronary stenosis.
Statistical analysis
Statistical analysis was performed using SAS Software (SAS Institute). All continuous parameters were summarized as medians and interquartile ranges, and all categorical parameters were summarized as proportions. To compare between-group differences in demographic and clinical characteristics, lipid profiles, drug-use behaviors, and other factors, the nonparametric Wilcoxon 2-sample test was used for continuous variables and the Fisher’s exact test was used for categorical variables.
The Clopper-Pearson approach was used to calculate 95% exact binomial CIs [14]. Because the number of participants with significant coronary stenosis was relatively small, an exact logistic regression model was used to examine associations between factors and significant coronary stenosis [15]. Univariate logistic regression models were first fitted to evaluate the crude association between the presence of significant coronary stenosis and each of the following factors individually: age, sex, total serum cholesterol level, high-density lipoprotein cholesterol level, low-density lipoprotein cholesterol level, serum triglyceride level, high-sensitivity C-reactive protein level, cigarette smoking, alcohol use, glucose level, blood pressure, body mass index (calculated as the weight in kilograms divided by the square of the height in meters), current CD4 cell count, current HIV RNA quantification, ART status, and cocaine or other illicit drug (heroin) use. Because the median duration of cocaine use was 13 years, a dichotomous variable, duration of cocaine use (⩾15 years vs. <15 years), was created to examine long-term effects of cocaine use. ART was categorized on the basis of exposure to 3 classes (nucleoside reverse-transcriptase inhibitors, nonnucleoside reverse-transcriptase inhibitors, and protease inhibitors) and the duration of ART. The factors that were significantly significant at P ⩾ .10 in the univariate models were put into the multiple logistic regression models to identify the factors that were independently associated with the presence of significant coronary stenosis. The variables that ceased to make statistically significant contributions to the models were deleted in a stagewise manner, yielding the final models. To explore whether short-term (up to 6 months) use of ART increased or decreased the risk of CAD, duration of ART was categorized as both trichotomous (ART naive, use for <6 months, or use for ⩾6 months) and dichotomous (ART naive or use for <6 months or use for ⩾6 months). The Framingham risk score was calculated to estimate the CAD risk [16].
To evaluate the magnitude of potential risk associated with ART, the multiple correlation coefficient R2 proposed for the logistic regression model was used to measure the proportion of variation explained by potential risk factors [17]. The P values reported are 2-sided. A P value <.05 indicated statistical significance.
RESULTS
Patient Characteristics
The general and clinical characteristics of the study participants by the presence of significant coronary stenosis are presented in table 1. According to the Framingham risk score algorithm, 100 (60.6%) of the 165 participants (60 of the 106 men and 40 of the 59 women) had a low risk of CAD, and 11 (45.8%) of the 24 participants (10 of the 21 men and 1 of the 3 women) with significant coronary stenosis had a low risk of CAD [16].
Table 1.
Characteristics of study participants by the presence of ⩾50% coronary stenosis.
| ⩾50% Coronary stenosis
|
||||
|---|---|---|---|---|
| Characteristic | All patients (n = 165) | No (n = 141) | Yes (n = 24) | P |
| Age, median years (IQR) | 44 (39.9–46.8) | 43 (39.5–46.6) | 47 (43.0–49.7) | <.001 |
| Female sex, % | 35.8 | 39.7 | 12.5 | .01 |
| Family history of CAD, % | 23.6 | 22.0 | 33.3 | .30 |
| CD4 cell count, median cells/mm3 (IQR) | 346 (197–498) | 343 (177–487) | 379 (247–569) | .61 |
| Plasma viral load, median copies/mL (IQR) | 441 (30–13,503) | 609 (30–22,771) | 300 (30–1809) | .36 |
| Cocaine use, % | 90.9 | 90.1 | 95.8 | .70 |
| Cocaine use for ⩾15 years, % | 34.6 | 29.1 | 66.7 | <.001 |
| Cigarette smoking, % | 86.1 | 85.1 | 91.7 | .53 |
| Cigarette smoking for ⩾15 years, % | 78.2 | 76.6 | 87.5 | .29 |
| Alcohol use, % | 90.9 | 91.5 | 87.5 | .46 |
| hsCRP level ⩾2 mg/dL, % | 42.1 | 43.6 | 33.3 | .38 |
| hsCRP level, median mg/dL (IQR) | 1.5 (0.6–4.2) | 1.5 (0.6–4.4) | 1.3 (0.6–2.7) | .51 |
| Systolic BP, median mm Hg (IQR) | 111 (103–119) | 111 (103–118) | 113 (108–122) | .18 |
| Diastolic BP, median mm Hg (IQR) | 74 (69–81) | 73 (68–80) | 80 (70–87) | .02 |
| Fasting glucose level, median mg/dL (IQR) | 84 (78–90) | 84 (78–90) | 84 (78–88) | .78 |
| BMI | 24 (21.4–26.9) | 24 (21.5–26.9) | 24 (21.4–26.4) | .50 |
| Cholesterol level, median mg/dL (IQR) | ||||
| Total | 160 (142–186) | 158 (137–180) | 187 (165–209) | <.001 |
| LDL | 86 (70–109) | 81 (65–106) | 102 (87–122) | .002 |
| HDL | 49 (38–60) | 49 (38–59) | 52 (37–63) | .44 |
| Triglyceride level, median mg/dL (IQR) | 106 (73–143) | 103 (72–141) | 137 (83–183) | .046 |
| Duration of cocaine use, median no. of years (IQR) | 13 (5–20) | 12 (5–18) | 20 (11–22) | .01 |
| Current ART use status, % | ||||
| Naïve | 40.0 | 44.0 | 16.7 | 0.01 |
| Currently no ART | 15.8 | 14.9 | 20.8 | .54 |
| NRTIs, no PI | 6.7 | 6.4 | 8.3 | .66 |
| NNRTIs, no PI | 8.5 | 7.8 | 12.5 | .43 |
| Regimen including PI | 33.9 | 31.2 | 50.0 | .10 |
| Regimen excluding PI | 10.3 | 9.9 | 12.5 | .71 |
| Exposure to ART, % | ||||
| Naïve | 40.0 | 44.0 | 16.7 | .01 |
| PI | 49.7 | 46.1 | 70.8 | .028 |
| NRTIs, no PI | 7.3 | 7.1 | 8.3 | .69 |
| NNRTIs, no PI | 8.5 | 7.8 | 12.5 | .43 |
| Duration of ART, median no. of months (IQR) | ||||
| NRTIs | 0 (0–12) | 0 (0–12) | 0 (0–40) | .08 |
| NNRTIs | 0 (0–0) | 0 (0–0) | 0 (0–24) | .047 |
| Pis | 0 (0–12) | 0 (0–9) | 6 (0–24) | .058 |
| All | 2 (0–24) | 1 (0–24) | 18.5 (6–40.5) | .004 |
| Coronary calcification, % | 23.6 | 14.2 | 79.2 | <.001 |
| Median Framingham risk score (IQR) | 3 (2–5) | 3 (2–5) | 6 (4–8) | <.001 |
| Framingham risk score ⩾3.0, % | 68.1 | 63.3 | 95.8 | <.001 |
NOTE. ART, antiretroviral therapy; BMI, body mass index (calculated as the weight in kilograms divided by the square of the height in meters); BP, blood pressure; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Prevalence of Significant Coronary Stenosis
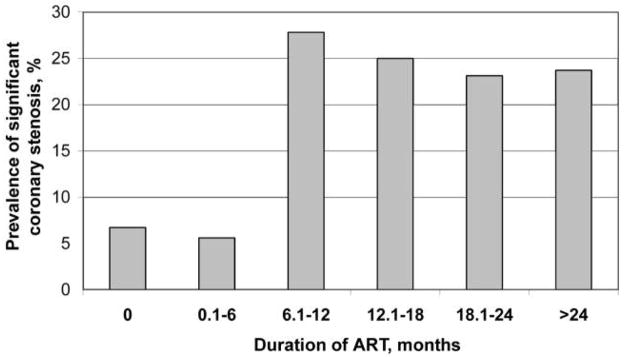
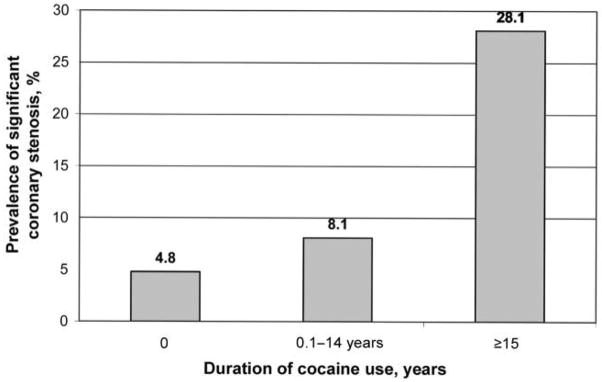
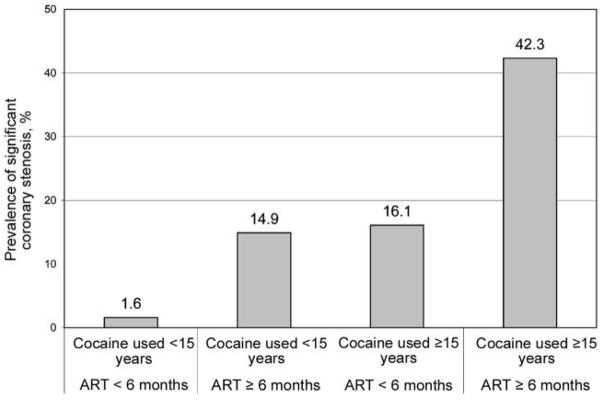
The overall prevalence of the presence of significant coronary stenosis was 15% (95% CI, 9.5%–20.9%). The prevalence by duration of ART is presented in figure 1. There was no statistically significant difference in the prevalence of significant stenosis between those who were ART naive and those who received ART for ⩾6 months (P = 1.00). Nevertheless, compared with the prevalence among those who were ART naive (6.8%), the prevalence among those who received ART for >6 months (24.7%) was significantly higher (P = .003). The prevalence by duration of cocaine use is shown in figure 2, and the prevalence by durations of cocaine use and ART exposure is shown in figure 3.
Figure 1.
Prevalence of significant coronary stenosis, by duration of antiretroviral therapy (ART). The prevalences were 6.8% (5 of 74 patients), 5.6% (1 of 18), 27.8% (5 of 18), 25.0% (1 of 4), 23.1% (3 of 13), and 23.7% (9 of 38) among those who had never received ART, those who had received ART for ⩾6 months, those who had received ART for 6–12 months, those who had received ART for 12–18 months, those who had received ART for 18–24 months, and those who had received ART for >24 months, respectively.
Figure 2.
Prevalence of significant coronary stenosis, by cocaine use status. The prevalences were 4.8% (1 of 21 patients), 8.1% (7 of 87), and 28.1% (16 of 57) among those who had never used cocaine, those who had used cocaine for ⩾15 years, and who had used cocaine for <15 years, respectively. ART, antiretroviral therapy.
Figure 3.
Prevalence of significant coronary stenosis, by durations of cocaine use and antiretroviral (ART) exposure. The prevalences were 1.6% (1 of 61 patients), 14.9% (7 of 47), 16.1% (5 of 31), and 42.3% (11 of 26) among those who had never used cocaine or used it for ⩾15 years and never received ART or received ART for <6 months, those who had never used cocaine or used it for <15 years but received ART for ⩾6 months, those who used cocaine for ⩾15 years and never received ART or received ART for <6 months, and those who had used cocaine for ⩾15 years and received ART fo ⩾6 months, respectively.
Factors Associated with the Presence of Significant Coronary Stenosis
By univariate logistic regression analyses, conventional risk factors associated with the presence of significant coronary stenosis included age ⩾50 years, male sex, diastolic blood pressure ⩾80 mm Hg, total serum cholesterol level ⩾160 mg/dL, serum low-density lipoprotein cholesterol level ⩾100 mg/dL, triglyceride level ⩾130 mg/dL, and Framingham risk score of ⩾3. Non-conventional risk factors associated with the presence of significant coronary stenosis included cocaine use for ⩾15 years, use of nonnucleoside reverse-transcriptase inhibitors for ⩾6 months, and exposure to any ART for ⩾6 months. Heroin use was not associated with significant coronary stenosis.
The final model indicated that a significant risk of the presence of significant stenosis was associated with previously described conventional risk factors, including male sex (adjusted OR, 5.04; 95% CI, 1.19–31.5), diastolic blood pressure ⩾80 mm Hg (adjusted OR, 5.94; 95% CI, 1.58–25.5), and low-density lipoprotein cholesterol level ⩾100 mg/dL (adjusted OR, 6.10; 95% CI, 1.69–25.2). The analysis also revealed that both cocaine use for ⩾15 years (adjusted OR, 7.75; 95% CI, 2.26–31.2) and receipt of ART for ⩾6 months (adjusted OR, 4.35; 95% CI, 1.30–16.4) were independently associated with the presence of significant coronary stenosis (table 2). Interaction between use of cocaine for ⩾15 years and receipt of ART for ⩾6 months was not statistically significant in the multiple logistic regression model.
Table 2.
Demographic, laboratory, and clinical factors in relation to the risk of significant coronary stenosis: exact logistic regressions analysis.
| Significant (⩾ 50%) coronary stenosis
|
|||
|---|---|---|---|
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) | P |
| Age | |||
| <50 years | 1.00 | … | |
| ⩾50 years | 3.89 (1.05–13.3) | … | |
| Sex | |||
| Female | 1.00 | 1.00 | |
| Male | 4.58 (1.28–25.1) | 5.04 (1.19–3) | .023 |
| Family history of CAD | |||
| No | 1.00 | … | |
| Yes | 1. 77 (0.60–4.89) | … | |
| CD4 cell count | |||
| <350 cells/mm3 | 1.00 | … | |
| ⩾350 cells/mm3 | 0.80 (0.23– 2.77) | … | |
| HIV RNA level | |||
| <400 copies/mL | 1.00 | … | |
| ⩾400 copies/mL | 1.40 (0.38–5.35) | … | |
| Cocaine use | |||
| Never | 1.00 | … | |
| Ever | 2.73 (0.38–120.2) | … | |
| Duration of cocaine use | |||
| <15 years | 1.00 | 1.00 | |
| ⩾15 years | 4.83 (1.79–14.1) | 7.75 (2.26–31.2) | <.001 |
| Cigarette smoking | |||
| Never | 1.00 | … | |
| Ever | 1.92 (0.42–18.1) | … | |
| Alcohol use | |||
| No | 1.00 | … | |
| Yes | 0.65 (0.16–3.91) | … | |
| CRP level | |||
| <2 mg/dL | 1.00 | … | |
| ⩾2 mg/dL | 0.65 (0.23–1.74) | … | |
| Systolic BP | |||
| <120 mm Hg | 1.00 | … | |
| ⩾120 mm Hg | 1.99 (0.67–5.55) | … | |
| Diastolic BP | |||
| ⩾80 mm Hg | 1.00 | 1.00 | |
| >80 mm Hg | 3.09 (1.15–8.33) | 5.94 (1.58–25.5) | .006 |
| Fasting glucose level | |||
| <85 mg/dL | 1.00 | … | |
| ⩾85 mg/dL | 1.10 (0.41–2.86) | … | |
| BMI | |||
| <24 | 1.00 | … | |
| ⩾24 | 0.74 (0.27–1.92) | … | |
| Total cholesterol level | |||
| <160 mg/dL | 1.00 | … | |
| ⩾160 mg/dL | 4.60 (1.55–16.7) | … | |
| LDL cholesterol level | |||
| <100 mg/dL | 1.00 | 1.00 | |
| ⩾100 mg/dL | 3.43 (1.30–9.43) | 6.10 (1.69–25.2) | .004 |
| HDL cholesterol level | |||
| <50 mg/dL | 1.00 | … | |
| ⩾50 mg/dL | 0.68 (0.25–1.76) | … | |
| Triglyceride level | |||
| <130 mg/dL | 1.00 | … | |
| ⩾130 mg/dL | 2.65 (1.01–7.11) | … | |
| Framingham risk score | |||
| <3% | 1.00 | … | |
| ⩾3% | 13.2 (2.02–559.6) | … | |
| Duration of NRTI use | |||
| <6 months | 1.00 | … | |
| ⩾6 months | 2.20 (0.82–5.85) | … | |
| Duration of NNRTI use | |||
| <6 months | 1.00 | … | |
| ⩾6 months | 3.42 (1.03–10.6) | … | |
| Duration of PI use | |||
| <6 months | 1.00 | … | |
| ⩾6 months | 2.51 (0.95–6.69) | … | |
| ART exposurea | |||
| ART naïve | 1.00 | … | |
| ART for <6 months | 0.99 (0.19–9.86) | … | |
| ART for ⩾6 months | 4.24 (1.41–15.5) | … | |
| ART exposurea | |||
| ART for <6 months | 1.00 | 1.00 | |
| ART for ⩾6 months | 4.65 (1.64–15.2) | 4.35 (1.30–16.4) | .014 |
NOTE. ART, antiretroviral therapy; BMI, body mass index (calculated as the weight in kilograms divided by the square of the height in meters); BP, blood pressure; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
In the first “ART exposure,” ART <6 months and ART ⩾6 months was compared with ART naive. Because ART <6 months was not different from ART naive, ART <6 months and ART naive were combined for the second “ART exposure.”
Associations between ART and the Presence of Significant Coronary Stenosis
Receipt of ART
After controlling for cocaine use for ⩾15 years and the above-mentioned conventional risk factors, use of nucleoside reverse-transcriptase inhibitors was marginally associated with significant stenosis (adjusted OR, 3.86; 95% CI, 0.87–21.8; P = .08) (table 3).
Table 3.
Associations between antiretroviral (ARV) therapy and the risk of significant coronary stenosis: exact logistic regression analysis.
| Significant (⩾ 50%) coronary stenosis
|
||||
|---|---|---|---|---|
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) | P | |
| ARV therapy status | ||||
| NRTIs | ||||
| Naïve | 1.00 | 1.00 | ||
| Ever | 3.75 (1.03–17.2) | 3.86 (0.87–21.8) | .08 | |
| NNRTIs | ||||
| Naïve | 1.00 | 1.00 | ||
| Ever | 5.06 (1.15–26.0) | 3.54 (0.54–26.8) | .23 | |
| Pis | ||||
| Naïve | 1.00 | 1.00 | ||
| Ever | 4.02 (1.22–17.3) | 2.28 (0.57–11.1) | .31 | |
| Duration of ARV therapy | ||||
| NRTIs | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 4.32 (1.17–19.9) | 5.13 (1.10–31.4) | .035 | |
| NNRTIs | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 7.02 (1.55–37.2) | 4.50 (0.67–36.4) | .14 | |
| Pis | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 4.59 (1.28–20.9) | 2.75 (0.54–14.5) | .28 | |
| Use of an individual ARV drug | ||||
| Zidovudine | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 2.18 (0.04–26.8) | 4.26 (0.04–414.0) | .78 | |
| Didanosine | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 5.01 (0.63–35.4) | 5.25 (0.34–87.6) | .30 | |
| Stavudine | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 8.44 (1.28–57.3) | 14.5 (0.59–∞) | .12 | |
| Lamivudine | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 5.79 (1.09–33.5) | 11.0 (0.78–679.1) | .085 | |
| Combivir | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 3.96 (0.92–20.0) | 4.27 (0.81–27.9) | .096 | |
| Efavirenz | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 4.79 (1.06–25.6) | 2.82 (0.36–24.5) | .43 | |
| Nevirapine | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 7.34 (0.52–75.1) | 15.9 (0.66–∞) | .10 | |
| Nelfinavir | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 3.04 (0.25–24.7) | 5.66 (0.13–542.2) | .59 | |
| Indinavir | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 9.64 (0.64–115.3) | 1.78 (0.01–292.9) | 1.00 | |
| Kaletra | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 4.83 (1.10–24.8) | 3.08 (0.67–39.9) | .45 | |
| Atazanavir | ||||
| Naïve | 1.00 | 1.00 | ||
| Used | 10.5 (1.81–67.3) | 4.63 (0.39–∞) | .23 | |
| Duration of an individual ARV drug | ||||
| Zidovudine | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 2.18 (0.04–26.2) | 4.26 (0.04–414.0) | .78 | |
| Didanosine | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 7.39 (0.88–56.8) | 9.87 (0.48–651.7) | .17 | |
| Stavudine | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 8.44 (1.28–57.3) | 18.1 (1.51–∞) | .02 | |
| Lamivudine | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 6.80 (1.25–40.2) | 10.7 (0.80–636.0) | .08 | |
| Combivir | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 4.44 (1.02–22.6) | 5.94 (1.05–44.5) | .043 | |
| Efavirenz | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 6.45 (1.33–35.5) | 3.32 (0.41–29.9) | .34 | |
| Nevirapine | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 9.65 (0.64–115.3) | 19.9 (0.82–∞) | .07 | |
| Nelfinavir | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 3.78 (0.30–31.8) | 5.66 (0.13–542.2) | .59 | |
| Indivavir | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 7.28 (0.10–171.2) | 4.15 (0.05–372.3) | .76 | |
| Kaletra | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 5.58 (1.26–28.9) | 3.61 (0.34–50.6) | .38 | |
| Atazanavir | ||||
| Naïve | 1.00 | 1.00 | ||
| ⩾6 months | 9.78 (1.45–68.9) | 4.30 (0.34–∞) | .26 | |
NOTE. ORs could not be estimated for zalcitabine, delavirdine, abacavir, amprenavir, ritonavir, and saquinavir because of the small number of observations. Adjusted ORs were obtained after controlling for sex, duration of cocaine use (<15 years vs. ⩾15 years); diastolic BP (⩾80 mm Hg vs. >80 mm Hg); and low-density lipoprotein cholesterol level (<100 mg/dL vs. ⩾100 mg/dL). NNRTI, nonnucleosidereverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Duration of exposure to ART
After controlling for cocaine use for ⩾15 years and the above-mentioned conventional risk factors, exposure to nucleoside reverse-transcriptase inhibitors for ⩾6 months was significantly associated with significant coronary stenosis (adjusted OR, 5.13; 95% CI, 1.10–31.4; P = .035) (table 3).
Receipt of a regimen containing an individual antiretroviral Drug
After controlling for cocaine use for ⩾15 years and the above-mentioned conventional risk factors, both receipt of a regimen containing lamivudine and receipt of a regimen containing combivir were marginally associated with significant stenosis (table 3).
Duration of receipt of a regimen containing an individual antiretroviral drug
After controlling for cocaine use for ⩾15 years and the above-mentioned conventional risk factors, both stavudine use for ⩾6 months (adjusted OR, 18.1; 95% CI, 1.51–∞, P = .02) and combivir use for ⩾6 months (adjusted OR, 5.94; 95% CI, 1.05–44.5; P = .043) were significantly associated with significant stenosis. Atazanavir use for ⩾6 months was univariately significantly associated with significant stenosis; nevertheless, the association became insignificant after controlling for the potential confounding factors (table 3).
Proportion of variation of the presence of significant stenosis explained by ART
According to the proportion of variation explained analysis, female sex, cocaine use for ⩾15 years, diastolic blood pressure ⩾80 mm Hg, low-density lipoprotein cholesterol level ⩾100 mg/dL, and receipt of ART for ⩾6 months combined explained 35.9% of variation. Although female sex, cocaine use for ⩾15 years, diastolic blood pressure ⩾80 mm Hg, and low-density lipoprotein cholesterol level ⩾100 mg/dL explained 6.9%, 7.7%, 6.9%, and 8.7% of the variation, respectively, the adjusted (for the other predictors) proportion of variation explained for receipt of ART for ⩾6 months was 1.6%, implying that only 1.6% of cases of significant stenosis could be explained by receipt of ART for ⩾6 months.
DISCUSSION
To our knowledge, this is the first study to examine whether exposure to ART is associated with silent CAD in African American adults with HIV infection who do not have cardiovascular symptoms. Overall, although African Americans account for only ~13% of the US population, one-half of the estimated new HIV and AIDS diagnoses in the United States in 2004 were among African Americans [18]. According to the 2 US National Health and Nutrition Examination Surveys, among non-His-panic African Americans, a history of use of cocaine or other illicit drugs had the strongest effect on risk of HIV infection, and >40% of HIV-infected individuals used cocaine or other illicit drugs [19]. Thus, up to 20% of HIV-infected persons in this country may abuse cocaine.
This study suggests that short-term receipt of ART was not associated with an elevated risk of significant stenosis. The crude OR for receipt of ART for <6 months was 0.99; however, after controlling for potential confounding factors, the adjusted OR for receipt of ART for <6 months decreased to 0.63, although the change was not statistically significant.
The findings from this study suggest that long-term ART (duration of ART, ⩾6 months) is significantly associated with an increased risk of significant stenosis. The finding is consistent with those derived from the Data Collection on Adverse Events of Anti-HIV Drugs study [20].
Long-term cocaine use has been implicated in premature atherosclerotic CAD in postmortem studies. Quantitative studies of the aorta and coronary arteries in humans at autopsy have noted moderate to severe coronary atherosclerosis in young persons who used cocaine and were otherwise at low risk for coronary atherosclerosis [21–26]. Cocaine use has also been linked to the presence of coronary calcification [27, 28]. According to this study, the magnitude of increased risk of significant stenosis associated with long-term cocaine use could be 5 times higher than that associated with long-term ART use. Therefore, this study highlights the importance of implementing effective screening and intervention programs for cocaine abuse in health care settings.
This study suggests that the prolonged use of certain nucleoside reverse-transcriptase inhibitors (specifically stavudine or lamivudine plus zidovudine), not protease inhibitors, may lead to occult CAD, independent of conventional risk factors and other related factors. This study also suggests that longer duration of receipt of these drugs may be associated with significant stenosis. In fact, it has been previously reported that regimens containing stavudine increase the risk of myocardial infarction [29] and may increase triglyceride and total cholesterol levels and induce insulin resistance [30]. Great caution should be used when interpreting the results of our study. First, most patients were receiving thymidine analogues. Most of the antiretroviral drugs assessed had adjusted ORs ranging from 3.3 to 19.9, including nevirapine, efavirenz, nelfinavir, didanosine, zidovudine, indinavir, and kaletra; the identification of stavudine and lamivudine plus zidovudine may be related to selection bias in how drugs were prescribed. Second, because most participants had been exposed to multiple drugs simultaneously or sequentially, these analyses could not identify the net effect of each drug. Actually, it is unlikely that such a limitation could be overcome, because current treatment regimens involve multiple drugs.
This study also suggests that the magnitude of risk associated with ART is quite low after adjustment for traditional risk factors and cocaine use in this population; only 1.6% of the cases of significant stenosis could be explained by receipt of long-term ART. In addition, probably because of the small sample size, this study did not identify any dose-response relationship between the duration of ART and the presence of significant stenosis. Nevertheless, the effect of ART on silent CAD was significant, even on the basis of a very conservative exact logistic regression model, and actions should be taken to lower the risk.
This study has several limitations. First, because the study population consisted of African Americans and was not a random sample of persons with HIV infection, the results should be interpreted with caution. Second, because the original design was to evaluate the effects of cocaine use and protease inhibitor–based HAART on subclinical atherosclerosis, those with hypertension and other existing cardiovascular conditions were excluded. Thus, the study population was healthier than the target population. Third, because the majority of participants were smokers, the effects of cigarette smoking on significant stenosis could not be evaluated, both individually and combined. Fourth, the study is cross-sectional in nature. Fifth, because of the nature of cross-sectional design, there was no adjustment for some hidden confounding factors, such as socioeconomic status. Furthermore, because this study was performed among African Americans living in innercity Baltimore, where cocaine use is often intertwined with other drug addictions, the effects of these drugs (or multiple-drug interactions) on CAD could not completely be controlled for by statistical analyses. Sixth, although noninvasive coronary CT angiography provides an excellent diagnostic tool, revealing the atherosclerotic burden, 10%–14% of coronary arterial segments visualized by coronary CT angiography are not assessable [31]. Nevertheless, recent studies have reported excellent diagnostic accuracy for 64-slice MDCT in the detection of significant stenosis in smaller coronary artery segments and side branches (sensitivity, 86%–94%; specificity, 93%–97%) [32–35]. Seventh, although a synergistic effect of long-term cocaine use and ART use was suggested (figure 1), the synergistic effects of these 2 factors were not significant because of the sample size. A larger study with at least 800 participants is needed to identify the synergistic effects, according to a post hoc estimation.
Despite its limitations, this study’s findings of an unexpectedly high rate of silent CAD in HIV-infected African American men and women who did not have cardiovascular symptoms have disturbing but important implications for early prevention of CAD and the management of clinically silent CAD in this population. The study strongly suggests that, for HIV-infected persons who are receiving ART, an aggressive reduction of traditional risk factors for CAD, including lowering low-density lipoprotein cholesterol levels and maintaining optimal blood pressure, is ultimately vital to lower the risk of CAD. Because long-term cocaine use imposes an alarming risk of CAD, treatment for substance abuse is critical. This study could not examine the net effect of a particular antiretroviral agent on subclinical CAD, although duration of HAART itself was a risk. Primary prevention strategies, including regular exercise and smoking cessation, should also be recommended for all patients with HIV infection. However, the most important intervention for HIV-infected persons receiving ART is lifestyle modification, including cocaine use cessation.
Acknowledgments
We thank the study participants for their contributions.
Financial support. National Institute on Drug Abuse, National Institutes of Health (R01-DA 12777 and DA15020).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–30. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 2.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1–infected patients. AIDS. 1998;12:F167–73. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Vergis EN, Paterson DL, Wagener MM, Swindells S, Singh N. Dysli-pidaemia in HIV-infected patients: association with adherence to potent antiretroviral therapy. Int J STD AIDS. 2001;12:463–8. doi: 10.1258/0956462011923507. [DOI] [PubMed] [Google Scholar]
- 4.Galli M, Ridolfo AL, Adorni F, et al. Body habitus changes and metabolic alterations in protease inhibitor-naive HIV-1–infected patients treated with two nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2002;29:21–31. doi: 10.1097/00126334-200201010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular risk factors in HIV patients—association with antiretroviral therapy: results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor–associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 7.The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2004. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 8.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immuno-deficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 9.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV infection. J Acquir Immune Defic Syndr. 2001;27:251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Haubrich RH, Little S, Currier JS, Forthal DN, Kemper C. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS. 1999;13:1099–107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 11.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23:386–95. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artert disease: report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council of Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 14.Clopper C, Pearson S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 15.Hirji KF, Mehta CR, Patel NR. Computing distributions for exact logistic regression. J Am Stat Assoc. 1987;82:1110–7. [Google Scholar]
- 16.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Mittlbock M, Schemper M. Explained variation for logistic regression. Stat Med. 1996;15:1987–97. doi: 10.1002/(SICI)1097-0258(19961015)15:19<1987::AID-SIM318>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Cases of HIV infection and AIDS in the United States. [Accessed 15 March 2006];2004 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2004report/default.htm.
- 19.McQuillan GM, Kruszon-Moran D, Kottiri BJ, et al. Prevalence of HIV in the US household population: the National Health and Nutrition Examination Surveys, 1988 to 2002. J Acquir Immune Defic Syndr. 2006;41:651–6. doi: 10.1097/01.qai.0000194235.31078.f6. [DOI] [PubMed] [Google Scholar]
- 20.The DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 21.Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–8. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- 22.Kolodgie FD, Virmani R, Cornhill JF, Herderick EE, Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated coronary vasospasm and thrombosis. J Am Coll Cardiol. 1991;17:1553–60. doi: 10.1016/0735-1097(91)90646-q. [DOI] [PubMed] [Google Scholar]
- 23.Dresser FA, Malekzadeh S, Roberts WC. Quantitative analysis of amounts of coronary arterial narrowing in cocaine addicts. Am J Cardiol. 1990;65:303–8. doi: 10.1016/0002-9149(90)90292-9. [DOI] [PubMed] [Google Scholar]
- 24.Kolodgie FD, Farb A, Virmani R. Pathobiological determinants of cocaine-associated cardiovascular syndromes. Hum Pathol. 1995;26:583–6. doi: 10.1016/0046-8177(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 25.Virmani R. Cocaine-associated cardiovascular disease: clinical and pathological aspects. NIDA Res Monogr. 1991;108:220–9. [PubMed] [Google Scholar]
- 26.Kolodgie FD, Virmani R, Cornhill JF, et al. Cocaine: an independent risk factor for aortic sudanophilia: a preliminary report. Atherosclerosis. 1992;97:53–62. doi: 10.1016/0021-9150(92)90050-q. [DOI] [PubMed] [Google Scholar]
- 27.Lai S, Lai H, Meng Q, et al. Effect of cocaine use on coronary calcium among black adults in Baltimore, Maryland. Am J Cardiol. 2002;90:326–8. doi: 10.1016/s0002-9149(02)02475-x. [DOI] [PubMed] [Google Scholar]
- 28.Lai S, Lima JAC, Lai H, et al. HIV-1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165:690–5. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 29.Moore RD, Keruly JC, Lucas GM. Increasing incidence of cardiovascular disease in HIV-infected persons in care [abstract 130]. Program and abstracts of the 10th Conference on Retroviruses and Opportunistic Infections; Boston. 2003. [Google Scholar]
- 30.Saves M, Raffi F, Capeau J, et al. Antiproteases Cohorte (APROCO) Study Group. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34:1396–405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 31.Pannu HK, Jacobs JE, Lai S, Fishman EK. Coronary CT angiography with 64-MDCT: assessment of vessel visibility. AJR Am J Roentgenol. 2006;187:119–26. doi: 10.2214/AJR.05.0908. [DOI] [PubMed] [Google Scholar]
- 32.Raff GL, Gallagher MJ, O’Neill WW, et al. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 33.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 34.Leschka S, Alkadhi H, Plass A, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26:1482–7. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 35.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]