Abstract
Background:
Serum surfactant protein (SP) A and SP-D had prognostic value for mortality in patients with idiopathic pulmonary fibrosis (IPF) in prior studies before the reclassification of the idiopathic interstitial pneumonias. We hypothesized that baseline serum SP-A and SP-D concentrations would be independently associated with mortality among patients with biopsy-proven IPF and would improve a prediction model for mortality.
Methods:
We evaluated the association between serum SP-A and SP-D concentrations and mortality in 82 patients with surgical lung biopsy-proven IPF. Regression models with clinical predictors alone and clinical and biomarker predictors were used to predict mortality at 1 year.
Results:
After controlling for known clinical predictors of mortality, we found that each increase of 49 ng/mL (1 SD) in baseline SP-A level was associated with a 3.3-fold increased risk of mortality (adjusted hazard ratio, 3.27; 95% confidence interval, 1.49 to 7.17; adjusted p = 0.003) in the first year after presentation. We did not observe a statistically significant association between serum SP-D and mortality (adjusted hazard ratio, 2.04; p = 0.053). Regression models demonstrated a significant improvement in the 1-year mortality prediction model when serum SP-A and SP-D (area under the receiving operator curve [AROC], 0.89) were added to the clinical predictors alone (AROC, 0.79; p = 0.03).
Conclusions:
Increased serum SP-A level is a strong and independent predictor of early mortality among patients with IPF. A prediction model containing SP-A and SP-D was substantially superior to a model with clinical predictors alone.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrotic interstitial lung disease with a poor prognosis and no proven effective medical treatment.1–3 IPF is characterized histologically by the usual interstitial pneumonia (UIP) pattern. The median survival rate in studies4 performed after the development of the current international consensus classification is between 2 and 4 years after diagnosis, and the 5-year survival rate is between 20% and 40%.5–7 Efforts have been made to identify clinical parameters,3,8,9 histopathologic features,1 and multifaceted prediction models10,11 to estimate risk more accurately for disease progression and death. We showed in a large, well-defined IPF population with long-term follow-up12 that baseline BAL fluid neutrophil percentage was a strong predictor of 1-year mortality.
Surfactant protein (SP) A and SP-D are members of the collectin family. Secreted primarily by alveolar epithelial type II pneumocytes, plasma SP-A and SP-D levels appear to increase early after breakdown in the alveolar epithelium.13,14 SP-A has been shown15,16 to be present in abnormal amounts in the BAL fluid of patients with IPF. These patients demonstrated lower BAL fluid SP-A/surfactant phospholipids ratios compared to control subjects, and those with higher levels had an improved 5-year survival rate.15 That study was performed before the American Thoracic Society and European Respiratory Society4 reclassification of the idiopathic interstitial pneumonias and included patients who now would be classified as having nonspecific interstitial pneumonia (NSIP). Ishii et al17 showed that patients with IPF have a significant increase in serum SP-A level compared to those with NSIP. Takahashi et al18 found that high levels of serum SP-A and SP-D were associated with mortality in a study of 52 patients with IPF but were not associated with the extent of honeycombing seen on high-resolution CT (HRCT) scans. Greene et al19 found that serum SP-A and SP-D levels were predictors of 1-year mortality in a large cohort of patients who had received an IPF diagnosis based on the criteria in use before the current international consensus reclassification.4 In addition, Greene et al19 did not adjust for BAL fluid neutrophil percentage or report detailed comparisons of adjusted multivariate models to reveal the independent relative strength of any given predictor. Thus, although others18,19 have established an association between mortality and serum levels of these proteins, it remains unclear whether such an association provides substantial additive value in the assessment of prognosis related to clinical factors (age and smoking status), functional parameters (FVC, diffusing capacity of the lung for carbon monoxide [Dlco], and alveolar-arterial oxygen gradient), and BAL fluid (neutrophilia) findings.
In the present study, we evaluated the association of serum SP-A and SP-D levels with mortality among a prospective cohort of patients with IPF in whom the presence of the pathologic UIP pattern was confirmed by surgical lung biopsy and BAL fluid cellular analysis. We hypothesized that a higher level of serum SP-A or SP-D at the time of IPF diagnosis would predict mortality in patients with IPF; further, this relationship would persist after adjustment for other established baseline predictors of mortality, including BAL fluid neutrophilia. We also sought to determine whether a prediction model that included SP-A and SP-D improved on models that included clinical predictors and BAL fluid neutrophilia.
Materials and Methods
Study Patients
The study cohort consisted of 82 patients with IPF and a pathologic pattern of UIP seen on a surgical lung biopsy specimen prospectively enrolled into a specialized center of research study at the National Jewish Medical and Research Center (Denver, CO) between 1982 and 1996. The cohort also included a subset of patients (n = 78) previously evaluated by Greene et al19 (n = 111). The remainder of the cohort in the study by Greene et al19 was excluded because these patients did not have a surgical lung biopsy (n = 6), had an eventual alternative diagnosis (connective tissue disease, NSIP, desquamative interstitial pneumonitis, respiratory bronchiolitis, asbestosis, or chronic aspiration; n = 18), or did not have BAL performed (n = 9).
The diagnosis of IPF was made based on established clinical and histologic criteria as described previously.10 Patients were excluded from the study if there was clinical evidence of a connective tissue disease, left ventricular failure, an occupational or environmental exposure that may result in interstitial lung disease, or a history of ingestion of a drug or an agent known to cause pulmonary fibrosis. At the initial visit to the research center, all patients underwent clinical, radiographic, and physiologic assessment before lung biopsy. Patients were designated as current smokers (if they had smoked cigarettes regularly within the previous year), former smokers (if they had not smoked cigarettes in the previous year but had smoked in the past), and never-smokers. All were evaluated as outpatients, and none had clinical evidence of concurrent infection.
Informed consent was obtained from each patient, and the Institutional Human Subject Review Committee approved the protocol. Many of these study participants have been participants in previously reported studies.1,10,15,19
BAL
The BAL procedure was performed in all patients either while awake in an outpatient setting within 3 weeks preceding open lung biopsy or while under anesthesia through an endotracheal tube just prior to the lung biopsy. BAL fluid was analyzed by standard methods, as previously described.15
Evaluation of Serum SPs
Human SP-A and SP-D levels were measured by an enzyme-linked immunosorbent assay kit, as previously described.19,20 All assays were performed on samples from the baseline visit in duplicate, and the data were expressed as the mean.
Physiologic Evaluation
Physiologic assessment included the measurement of thoracic gas volume and total lung capacity, FVC and FEV1, the volume-pressure relationship of the lungs, and the Dlco and arterial blood gases, as previously described.1
Pathologic Assessment
All patients underwent open thoracotomy or video-assisted thoracoscopic lung biopsy. In each patient, tissue was obtained from at least one site from the upper lobe and one site from the lower lobe of the same lung. Only patients with findings consistent with UIP were included in this study.2,21,22
Clinical and Outcome Assessment
A modified American Thoracic Society questionnaire was used to collect demographic and medical information. Vital status was obtained on all patients through July 31, 2006, by linking patient identifiers with the National Death Index. Patients were censored if they were still alive on July 31, 2006, had received a lung transplant, or had died. Survival time was calculated as the time since surgical lung biopsy until censoring.
Treatment Protocol
At study entry, 35 patients (43%) had never received treatment directed at IPF, 38 patients (46%) were not receiving treatment at the time of lung biopsy but had previously received short courses of corticosteroids or other immunosuppressant treatment > 30 days prior to the lung biopsy and 9 patients (11%) had been treated with corticosteroids or immunosuppressant treatment within 30 days of the biopsy. None of the patients had received significant doses of corticosteroid therapy (defined as a total of 2,700 mg of prednisone in any 90-day period) before diagnosis and entry into the study.
Statistical Analysis
Because age-specific, gender-specific, and race-specific clinically established cut points for serum SP concentrations have not been determined among persons with IPF, we categorized study patients into three equal groups for descriptive purposes. Exploratory analysis demonstrated a linear relationship between increasing SP-A level and risk of mortality. Thus, differences in baseline demographic and clinical characteristics were compared across SP-A tertile groups with the use of analysis of variance or the Kruskal-Wallis test for continuous variables and the χ2 test or Fisher exact test for categorical variables, as appropriate.
We used Cox proportional hazards regression model for the primary analysis to evaluate the associations of serum SP-A and SP-D level with mortality. To evaluate the proportional hazards assumptions, we used visual inspection of log-minus-log plots and plots of Schoenfeld residuals vs survival time.23 Visual inspection suggested a violation of the assumption of proportional hazards among the three SP-A tertiles. We took account of this violation by estimating relative hazards by years since study enrollment (censoring study patients with events in earlier years).23 We particularly focused on mortality with the first year, as we believed that this was most clinically relevant, and our prior prognostic study12 of BAL fluid neutrophils had demonstrated that the greatest impact of baseline clinical predictors was within the first year.8
Covariates for adjustment were selected a priori either because they represented important demographic variables (age, gender, and race) or because prior reports3,10 indicated an association with mortality in IPF (age, smoking status, baseline FVC, Dlco, BAL fluid neutrophil percentage, and alveolar-arterial oxygen gradient). Before study inclusion, Pearson product moment correlations between continuous covariates were evaluated to avoid colinearity. None had correlation coefficients > 0.60. We evaluated for multiplicative interactions on the basis of smoking status, which was selected as a candidate effect modifier a priori on the basis of prior research3,8,19 and treatment status. Standard methods do not exist for deriving receiver operating characteristic (ROC) curves for time-to-event data24; thus, we used logistic regression for this part of the analyses. Complementary analyses included a series of logistic regression models to predict 1-year mortality, using odds ratios and 95% confidence intervals (CIs) to estimate relative risk. Our regression models sequentially included the simple clinical, clinical and SP-A and SP-D, and clinical and biomarker (including SP-A, SP-D and BAL fluid neutrophil percentage) models, with evaluation of the discriminatory capability of the models using the C statistic, or the area under the ROC curve (AROC). Two-tailed p values < 0.05 were considered statistically significant. Analyses were performed with a statistical software package (Stata, version 9; StataCorp; College Station, TX).
Results
Among the 82-patient study sample, the mean age was 62 years; 62% were men, and 90% were white. Sixty-two percent of patients were either current or former smokers. The mean serum SP-A level was 106 ng/mL (range, 27 to 276 ng/mL), and the mean serum SP-D level was 641 ng/mL (range, 82 to 2,404 ng/mL). Exploratory analyses revealed that serum SP-A level, but not SP-D level, was associated with early mortality; thus, the baseline characteristics are shown in relation to serum SP-A tertiles (Table 1). No statistically significant associations were found between SP-A tertiles and baseline demographic, clinical, physiologic, or BAL variables, although there was a trend toward higher alveolar-arterial oxygen gradient among patients with higher SP-A levels (p = 0.12).
Table 1.
Baseline Characteristics by SP-A Tertile*
| Characteristics | Overall (n = 82) | Lowest Tertile (n = 28) | Middle Tertile (n = 27) | Highest Tertile (n = 27) | p Value† |
|---|---|---|---|---|---|
| Age at biopsy, yr | 62 (10) | 61 (13) | 62 (9) | 62 (8) | 0.93 |
| Male gender, No. (%) | 51 (62) | 18 (64) | 17 (63) | 16 (59) | 0.92 |
| Race and ethnicity | |||||
| White | 74 | 24 | 25 | 25 | 0.32 |
| Hispanic | 6 | 4 | 1 | 1 | |
| Black | 1 | 0 | 1 | 0 | |
| Asian | 1 | 0 | 0 | 1 | |
| Smoking status | |||||
| Never-smoker | 31 | 8 | 13 | 10 | 0.28 |
| Former smoker | 40 | 17 | 12 | 11 | |
| Current smoker | 11 | 3 | 2 | 6 | |
| Duration of symptoms, mo | 34 | 31 | 36 | 36 | 0.75 |
| Immunosuppressive therapy, No. of patients treated (%) | 47 (57) | 18 (64) | 15 (56) | 14 (52) | 0.63 |
| Baseline pulmonary function tests | |||||
| FVC, % predicted | 64 (18) | 64 (18) | 60 (16) | 68 (18) | 0.25 |
| FEV1, % predicted | 74 (19) | 76 (23) | 70 (18) | 77 (15) | 0.31 |
| Total lung capacity, % predicted | 75 (16) | 75 (15) | 73 (17) | 78 (17) | 0.45 |
| Dlco, % predicted | 54 (16) | 58 (18) | 53 (12) | 50 (17) | 0.15 |
| Baseline oxygen saturation, % | 95 (3) | 95 (3) | 95 (3) | 95 (3) | 0.78 |
| Baseline alveolar-arterial oxygen gradient‡ | 21 (9–36) | 15 (7–27) | 21 (13–36) | 31 (10–42) | 0.12 |
| Baseline BAL fluid neutrophils,‡ % | 6 (3–13) | 6 (4–11) | 3 (2–12) | 11 (2–18) | 0.41 |
*Values are given as the mean (SD), No., or median (interquartile range), unless otherwise indicated. Lowest tertile, ≥ 80.4 ng/mL; middle tertile, 81 to 123 ng/mL; highest tertile, > 123 ng/mL.
†p Values are for comparison among the three subgroups, using χ2 test, Fisher exact test, or analysis of variance, where appropriate.
‡Data were not normally distributed. A Kruskal-Wallis test was performed.
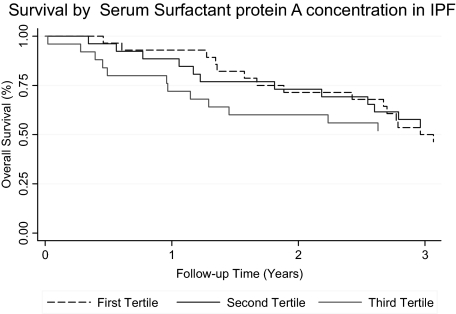
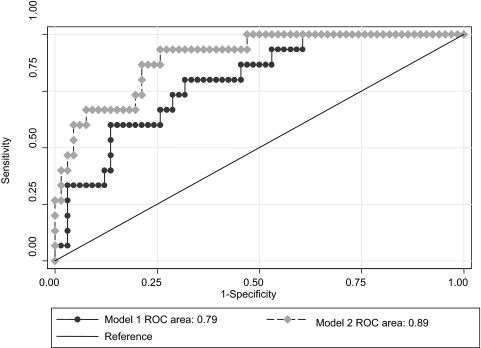
There was a total of 347 person-years of follow-up among the 82 patients. The median follow-up time was 3.0 years (interquartile range, 1.3 to 6.0 years). None of the patients were lost to follow-up. During the first year of follow-up, two patients (7%) in the lowest SP-A tertile, three patients (11%) in the middle tertile, and seven patients (26%) in the highest tertile died or received a lung transplant. Only one of these patients was censored due to lung transplantation, and the remaining patients died. When SP-A was considered as a continuous predictor variable, every increase of 49 ng/mL (1 SD) in concentration was associated with a 3.3 times increased risk for death or lung transplant in adjusted analysis during the first year of follow-up (p = 0.003) [Table 2]. However, this association differed significantly during the follow-up period and was attenuated in subsequent follow-up years. Patient survival divided by tertiles in SP-A levels are shown in Figure 1 (p = 0.009). No evidence was found for effect modification in this relationship based on smoking status (p = 0.72 [for interaction]) or treatment (p = 0.23). In contrast, we did not observe any significant association between SP-D levels and mortality in unadjusted analysis (Table 2); however, there was a trend toward significance in adjusted analysis (adjusted hazard ratio, 2.04; p = 0.053). Regression models demonstrated a significant improvement in the prediction model when serum levels of SP-A and SP-D (AROC = 0.89; p = 0.03) or BAL fluid neutrophilia (AROC = 0.83; p = 0.05) were added to the clinical predictors alone (AROC = 0.79) [Table 3 and Fig 2]. However, the model including serum SP-A and SP-D levels and the clinical parameters was not improved with the addition of BAL fluid neutrophilia.
Table 2.
Comparison Between Predictors for Association With Death or Transplantation During the First Year of Follow-up in Patients With IPF (Using Cox Proportional Hazards Model)
| Per SD Decrease | Hazard Ratio | CI | p Value |
|---|---|---|---|
| FVC % predicted (SD = 18.2) unadjusted | 1.26 | 0.70–2.27 | 0.43 |
| FVC % predicted adjusted* | 1.67 | 0.56–5.00 | 0.36 |
| Dlco % predicted (SD = 16.1) unadjusted | 0.83 | 0.49–1.43 | 0.51 |
| Dlco % predicted adjusted* | 0.50 | 0.21–1.19 | 0.12 |
| Increase per SD (10 yr) | |||
| Age unadjusted | 1.98 | 0.90–4.36 | 0.09 |
| Age adjusted* | 2.97 | 0.85–10.32 | 0.09 |
| Increase per SD (48.7 ng/mL) | |||
| SP-A level unadjusted | 2.09 | 1.21–3.59 | 0.008 |
| SP-A level adjusted* | 3.27 | 1.49–7.17 | 0.003 |
| Increase per SD (394 ng/mL) | |||
| SP-D level unadjusted | 1.22 | 0.95–1.53 | 0.40 |
| SP-D level adjusted* | 2.04 | 0.99–4.22 | 0.053 |
*Adjusted for all others (age, gender, race, smoking status, FVC, Dlco, alveolar-arterial oxygen gradient, and BAL neutrophil percentage).
Figure 1.
The Kaplan-Meier method survival curve of patients with IPF based on serum SP-A level tertiles (≤ 80.4 ng/mL, 81 to 123 ng/mL, and > 123 ng/mL; p = 0.009 [log rank test]; patients at risk: year 1, 68; year 2, 55; year 3, 43).
Table 3.
Comparison of Performance Characteristics Between Multivariate Logistic Regression Prediction Models for 1-Year Mortality in Patients With IPF*
| Models | C Statistic† | 95% CI | p Value† |
|---|---|---|---|
| Clinical alone | 0.79 | 0.67–0.91 | Reference |
| Clinical and SP-A/SP-D levels | 0.89 | 0.81–0.98 | 0.03 |
| Clinical and BAL neutrophil % | 0.83 | 0.73–0.93 | 0.05 |
| Clinical and biomarkers | 0.89 | 0.81–0.97 | 0.04 |
*Clinical model includes age, gender, race, smoking status, FVC, Dlco, and alveolar-arterial oxygen gradient. Biomarkers include SP-A and SP-D levels, and BAL neutrophil percentage.
†Represents the AROC comparison to the clinical-alone model.
Figure 2.
The ROCs for the clinical vs the clinical and serum SP models (p = 0.03) in predicting 1-year mortality in patients with IPF. Model 1 includes clinical parameters (age, gender, race, smoking status, FVC, Dlco, and alveolar-arterial oxygen gradient). Model 2 includes clinical parameters and serum SP-A and SP-D levels.
Discussion
This study examined the association of serum SP-A and SP-D levels with survival in a relatively large and well-characterized cohort of patients with biopsy-proven IPF through long-term and comprehensive follow-up. We demonstrated that serum SP-A levels obtained at the time of initial diagnosis among patients with IPF (confirmed by use of the current consensus pathologic definition) is independently and strongly associated with death or lung transplant within 1 year after presentation. This association was strengthened after statistical adjustment for previously established clinical predictors of mortality. Interestingly, we found that the greatest increase in mortality risk associated with elevated SP-A levels occurred in the first year after measurement, a result similar to our findings12 from an evaluation of BAL fluid neutrophilia and mortality. Furthermore, the present study is the first to evaluate the performance characteristics of multivariate survival prediction models containing SP-A and SP-D levels using ROC curve analysis. These characteristics revealed that models containing SP-A and SP-D in addition to clinical factors demonstrated performance characteristics that were superior to those of a model using clinical characteristics alone. Thus, baseline serum SP-A and SP-D levels provide substantial additive predictive value in prognosis relative to clinical factors (age and smoking status), functional parameters (FVC, Dlco, and alveolar-arterial oxygen gradient), and BAL fluid (neutrophilia) findings in patients with IPF. Interestingly, we found that the greatest increase in mortality associated with SP-A occurred in the first year after measurement, a result similar to that found in our prior study12 evaluating BAL fluid neutrophilia and mortality.
Several potential mechanisms have been postulated19 to increase serum SP-A levels, including increased secretion and loss of cell polarity of SP-A by type II cells, increased secretion because of type II cell hyperplasia, increased leakage from the airspace to the interstitium, and decreased clearance from the vascular compartment. If increased basement membrane permeability is the predominant mechanism by which serum SP-A levels increase, then the relative preservation of the basement membrane in patients with NSIP compared to those with IPF might help to explain the findings of Ishii et al17 of lower SP-A levels in distinct interstitial lung disease. Further, if SP-A is leaking from the alveolar compartment into the systemic circulation, it might explain the previous observation15 that BAL fluid SP-A levels are reduced in patients with IPF.
We believe that the most likely explanation for the association between serum SP-A levels and mortality in patients with IPF is that serum SP-A levels may be a more sensitive indicator of the extent of lung involvement than any of the conventional functional parameters, such as Dlco or FVC. Some advantages of using this measurement as a prognostic indicator include the ease in obtaining blood samples compared to other more invasive measures (BAL) or more expensive measures (HRCT scan), the reproducibility and limited interobserver variability of the test, and the relative strength of its association with mortality compared to other conventional measures.
We did not find a statistically significant association between serum SP-D level and mortality, unlike in the prior study19 from our group. The reasons for this discrepancy are unclear. One potential explanation for the different findings is our strict definition that IPF require a lung biopsy showing the UIP histologic pattern vs the inclusion of patients who would no longer be considered to have IPF but would have other idiopathic interstitial pneumonias, such as NSIP. Therefore, it is possible that the current population is less heterogeneous. Alternatively, our study is smaller than the prior study,19 so it is possible that we would have seen a similar association had we had more patients, given that our point estimate for an association between SP-D and mortality was relatively modest, and the p value approached significance.
The strengths of the current study are its well-characterized patient population, with surgical lung biopsies having been conducted in all subjects and classification accomplished according to the most recent consensus criteria; the length and completeness of the follow-up; the use of multivariate analytic techniques; and a more comprehensive collection of important clinical covariate data. However, several limitations should be considered when interpreting our results. We attempted to mitigate the possibility of confounding variables by collecting and analyzing baseline clinical predictors that have been shown to have an influence on mortality, including the recently demonstrated BAL fluid neutrophil percentage. HRCT scans were not available in the early years of this study; therefore, they were not included in the present analysis. Interestingly, in several more recent studies12,25 evaluating BAL fluid neutrophilia as a predictor of mortality in patients with interstitial lung disease, adjustment for the extent of disease seen on HRCT scan or Dlco did not attenuate this effect. Similarly, in the present study, the impact of serum SP-A level on mortality in patients with IPF was not attenuated by adjustment for Dlco (a reliable surrogate for disease extent seen on HRCT scan in patients with other interstitial lung diseases11,26). These findings suggest the possibility that serum SP-A level may be measuring a separate phenomenon and is not just a surrogate for disease severity or extent as conventionally determined. In addition, the study population was derived from a tertiary referral center. We cannot rule out the possibility that this referral population is somehow different from others that would not have been referred to a tertiary medical center. Finally, we did not have information on the cause of death, so we could not directly address the potential role of acute exacerbations and their relationship to SP-A levels.
In summary, we demonstrate that serum SP-A level at the time of diagnosis of IPF is a strong independent predictor of time to death or lung transplant, particularly during the first year of follow-up. Increased serum SP-A concentrations may identify a subset of patients with more “active” disease that increases the risk of death in the following year and was not predicted by other baseline, noninvasive clinical predictors, such as lung function test results. The results of this and prior studies,18,19 along with the availability of a reference laboratory to regularly perform these assays for clinical purposes, may lead to the increased use of SP-A levels in clinical practice for prognostic purposes and may represent an important surrogate outcome for future therapeutic trials. Future studies will need to clarify whether repeated measures of serum SP-A level provide a more specific marker of mortality risk for long-term follow-up than pulmonary function tests and whether they can be used as a surrogate outcome measure in therapeutic clinical trials.
Abbreviations:
- AROC
area under the receiver operating characteristic curve
- CI
confidence interval
- Dlco
diffusing capacity of the lung for carbon monoxide
- HRCT
high-resolution CT
- IPF
idiopathic pulmonary fibrosis
- NSIP
nonspecific interstitial pneumonia
- ROC
receiver operating characteristic
- SP
surfactant protein
- UIP
usual interstitial pneumonia
Footnotes
This work was supported by a National Institutes of Health T32 training grant and a Clinical Research Loan Repayment Grant (to Dr. Kinder); National Heart, Lung, and Blood Institute Specialized Center of Research (SCOR) grants No. HL-27353 and No. HL-67671; and a Dean's Scholars Clinical Research grant from the University of Cincinnati (to Dr. Kinder).
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians ( www.chestjournal.org/site/misc/reprints.xhtml ).
References
- 1.King TE, Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Brown KK, Bradford WZ, et al. A placebo-controlled trial of interferon γ-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 6.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson AG, Colby TV, Dubois RM, et al. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162:2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, King TE, Jr, Bartelson BB, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TE, Jr, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 11.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 12.Kinder BW, Brown KK, Schwarz MI, et al. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest. 2008;133:226–232. doi: 10.1378/chest.07-1948. [DOI] [PubMed] [Google Scholar]
- 13.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 14.Greene KE, Ye S, Mason RJ, et al. Serum surfactant protein-A levels predict development of ARDS in at-risk patients. Chest. 1999;116:90S–91S. [PubMed] [Google Scholar]
- 15.McCormack FX, King TE, Jr, Bucher BL, et al. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1995;152:751–759. doi: 10.1164/ajrccm.152.2.7633738. [DOI] [PubMed] [Google Scholar]
- 16.Phelps DS, Umstead TM, Mejia M, et al. Increased surfactant protein-A levels in patients with newly diagnosed idiopathic pulmonary fibrosis. Chest. 2004;125:617–625. doi: 10.1378/chest.125.2.617. [DOI] [PubMed] [Google Scholar]
- 17.Ishii H, Mukae H, Kadota J, et al. High serum concentrations of surfactant protein A in usual interstitial pneumonia compared with non-specific interstitial pneumonia. Thorax. 2003;58:52–57. doi: 10.1136/thorax.58.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi H, Fujishima T, Koba H, et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162:1109–1114. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 19.Greene KE, King TE, Jr, Kuroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 20.Cheng IW, Ware LB, Greene KE, et al. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med. 2003;31:20–27. doi: 10.1097/00003246-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Katzenstein ALA, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society, European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 23.Vittinghoff E, Glidden DV, Shiboski SC, et al. New York, NY: Springer Science and Business Media; 2005. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. [Google Scholar]
- 24.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 25.Goh NS, Veeraraghavan S, Desai SR, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 2007;56:2005–2012. doi: 10.1002/art.22696. [DOI] [PubMed] [Google Scholar]
- 26.Wells AU, Hansell DM, Rubens MB, et al. Fibrosing alveolitis in systemic sclerosis: indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum. 1997;40:1229–1236. doi: 10.1002/1529-0131(199707)40:7<1229::AID-ART6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]