Abstract
Background:
The long allele of a functional promoter polymorphism in the serotonin transporter (SERT) is associated with an increased risk of some forms of pulmonary arterial hypertension. We hypothesized that the long allele or other polymorphisms in SERT would be associated with an increased risk of portopulmonary hypertension (PPHTN) in patients with advanced liver disease.
Methods:
We performed a multicenter case-control study. Subjects undergoing liver transplant evaluation at seven centers were prospectively screened for the presence of PPHTN using transthoracic echocardiography. PPHTN was confirmed by right heart catheterization using standard criteria.
Results:
The study sample included 30 case patients with PPHTN and 109 control subjects with advanced liver disease. There was no significant association between the long allele and case status in an adjusted additive model (odds ratio, 0.63; 95% confidence interval, 0.33 to 1.21; p = 0.17). If anything, LL genotype tended to be associated with a lower risk of PPHTN. There were no associations between other SERT polymorphisms and PPHTN.
Conclusions:
SERT polymorphisms are not associated with the risk of PPHTN in patients with advanced liver disease. Other clinical or genetic risk factors may play a role in this complication of portal hypertension.
Keywords: cirrhosis, gene polymorphism, portal hypertension, pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is present in approximately 6% of individuals evaluated for liver transplantation, termed portopulmonary hypertension (PPHTN).1 The risk of death for individuals with PPHTN is greater than that for those with either PAH or portal hypertension alone.2,3 Furthermore, PPHTN presents a high risk of death during orthotopic liver transplantation, often precluding the only curative option for hepatic failure.4 Early identification of this complication of portal hypertension not only is critical to the management of individual patients, but it also has implications regarding the appropriate allocation of scarce organ resources.
Serotonin plays a key role in the proliferative and vasomotor phenotype of pulmonary vascular endothelial and smooth muscle cells. In addition to serotonin signaling via cell surface receptors, modulation of intracellular serotonin levels by the serotonin transporter (SERT) mediates the effects of this molecule.5 Interest in a potential pathogenetic link between SERT and PAH followed observation of an increased incidence of PAH among users of the anorexigens aminorex and fenfluramine, substrates for SERT.6,7 It has been subsequently shown that transgenic mice overexpressing SERT demonstrate increased susceptibility to pulmonary hypertension.8,9 Similarly, the administration of SERT inhibitors prevents hypoxic-induced pulmonary vascular remodeling in wild-type animals.10
An insertion/deletion polymorphism (in/del) in the gene-encoding SERT modulates both gene expression and protein function, with the long allele associated with significantly higher gene transcription and transporter activity than the short allele.11 In 2001, investigators reported that individuals with idiopathic pulmonary arterial hypertension (IPAH) were much more likely to possess an long allele than were normal control subjects. They went on to demonstrate that the long allele was associated with higher levels of SERT messenger RNA and protein in the lungs of these individuals.12 Subsequent reports linking the SERT polymorphism with pulmonary hypertension in individuals with COPD and left heart failure strengthened the theory that genetic variation in SERT contributed to pulmonary vascular disease.13,14 However, attempts to replicate the initial association between the long allele and PAH were unsuccessful in distinct populations of IPAH and familial PAH.15,16 No data are available regarding patients with the many different forms of PAH associated with other conditions, such as PPHTN.
We therefore aimed to determine whether the long allele of the promoter polymorphism or other single-nucleotide polymorphisms (SNPs) in SERT is associated with the occurrence of PPHTN in patients with portal hypertension.
Materials and Methods
Study Design and Subjects
The Pulmonary Vascular Complications of Liver Disease study enrolled a cohort of 536 patients evaluated for liver transplantation at seven centers in the United States between 2003 and 2006. The only inclusion criterion was the presence of chronic portal hypertension with or without intrinsic liver disease. We excluded patients with evidence of active infection, recent (< 2 weeks) GI bleeding, or who had undergone liver or lung transplantation. The institutional review boards at each of the participating centers approved this study, and informed consent was obtained.
We performed a case-control study within the Pulmonary Complications of Liver Disease study cohort. The study sample included newly referred patients who were evaluated with transthoracic echocardiography during the study period. “Prevalent” patients who met the case definition (see “Case and Control Definitions” section) were also included. Only subjects with analyzable genetic material were included. We excluded patients with pulmonary function test results showing a significant obstructive ventilatory defect, which was defined as an FEV1/FVC ratio < 0.70 with FEV1 of < 80% predicted, and/or a significant restrictive ventilatory defect, which was defined as FVC and (if performed) total lung capacity of < 70% predicted. Patients who otherwise fulfilled the case definition (see “Case and Control Definitions” section) without pulmonary function testing were included if chest radiography did not show hyperinflation or interstitial lung disease; control subjects without pulmonary function testing were excluded. The study sample also excluded patients with HIV infection and the presence of more than moderate aortic or mitral valvular disease or significant left ventricular dysfunction by echocardiography. Severity of liver disease was assessed using the Model for End-stage Liver Disease score, a validated scoring system for chronic liver disease.17,18
Screening with transthoracic echocardiography was standard for liver transplant candidates during the study period. A transthoracic echocardiogram showing right ventricular (RV) systolic pressure > 50 mm Hg with or without abnormal RV morphology was considered an indication for right heart catheterization. RV morphology was graded in a fashion consistent with the guidelines of the American Society of Echocardiography.19
Case and Control Definitions
Case patients with PPHTN met the following criteria: (1) mean pulmonary artery pressure > 25 mm Hg, pulmonary capillary wedge pressure (or left ventricular-end diastolic pressure) ≤ 15 mm Hg, and pulmonary vascular resistance > 240 dyne · s · cm–5; and (2) exclusion of other forms of pulmonary hypertension.20 Prevalent case patients who had previously undergone evaluation and were subsequently being treated were also included; data from the initial evaluation (pretreatment) were used for these case patients. Control subjects met the following echocardiographic criteria: (1) RV systolic pressure < 40 mm Hg (if capable of being estimated); and (2) absence of right atrial or ventricular abnormalities.
Genotyping
Genomic DNA was isolated from peripheral leukocytes using standard procedures (Gentra Puregene; Qiagen, Inc; Valencia, CA). The SERT promoter polymorphism was genotyped using published primers and amplification protocols;21 products were sized using agarose gel and classified using the diallelic L-S system. SNP genotyping was performed using a commercial assay (GoldenGate Assay; Illumina, Inc; San Diego, CA). Seven replicate DNA samples showed 100% reproducibility of genotypes, and all polymorphisms were successfully genotyped in the 139 study subjects.
SNP Selection
Seven SNPs from a 46-kilobase region on chromosome 17 centered on SERT (NM001045) were identified from public (dbSNP and HapMap) and private (Illumina) databases (Table 1). The following criteria were used for SNP selection: (1) minor allele frequency ≥ 0.05; (2) haplotype tagging SNPs (HapMap CEU); and (3) interlocus spacing of approximately 5 kilobases.
Table 1.
Genotyped Serotonin Transporter Polymorphisms
| Locus | Chromosome Position | Location | Alleles |
|---|---|---|---|
| in/del | 25588111 | Promoter | Short/long |
| rs2020933 | 25585881 | Intron 0 | A/T |
| rs2020936 | 25574940 | Intron 0 | C/T |
| rs6354 | 25574024 | Exon 2 | A/C |
| rs2020942 | 25571040 | Intron 3 | G/A |
| rs140701 | 25562658 | Intron 9 | G/A |
| rs1042173 | 25549137 | Exon 15 | T/G |
| rs7224199 | 25547852 | 3' | G/T |
To detect population stratification we genotyped an additional set of 60 SNPs, representing null loci. These were selected from a validated list of Ancestry Informative Markers22 using the following criteria: (1) minor allele frequency ≥ 10%; (2) minimum of 20 Mb between loci; and (3) no linkage (r2 < 0.2) between null and SERT loci. The physical and genetic map positions of the null loci are available from the authors by request.
Statistical Analysis
Unpaired Student t tests, rank sum tests, χ2 tests, and Fisher exact tests were used, as appropriate. Genotype analyses were performed using additive logistic regression models with terms for gender and a diagnosis of autoimmune hepatitis included in the final models. Single-locus association analyses were performed using a statistical software package (SAS/STAT; SAS Institute; Cary, NC). A p value < 0.05 was considered to be significant. Pairwise linkage disequilibrium between loci (D′ and r2) and Hardy Weinberg equilibrium were assessed using appropriate software (Haploview, version 4.0; Broad Institute; Cambridge, MA).23 The potential for population stratification was assessed by comparing allele frequencies of the null loci between case patients and control subjects using the χ2 test with a significance threshold of p < 0.05.24
Our sample size provided > 95% power (α = 0.05) to detect the association between the long allele and PAH shown previously.12 In the event that the association between the long allele and PPHTN was smaller, we still had 80% power to detect an odds ratio (OR) of 2.36. Power analysis was performed using appropriate software (Quanto, version 1.2; University of Southern California; Los Angeles, CA).25
Results
There were 175 patients who met case or control criteria, and 30 case patients with PPHTN and 109 control subjects with liver disease with analyzable genetic material in the study sample. There were no differences in age, gender, race, or Model for End-stage Liver Disease score between the final study sample and excluded patients without genetic material (data not shown).
Age and severity of liver disease were similar between case patients and control subjects (Table 2). Female gender, autoimmune hepatitis, and hepatitis C infection were associated with the risk of PPHTN, as we have previously reported.26
Table 2.
Clinical and Hemodynamic Characteristics*
| Variables | Case Subjects (n = 30) | Control Subjects (n = 109) | p Value |
|---|---|---|---|
| Age, yr | 54 ± 10 | 53 ± 9 | 0.55 |
| Female gender | 20 (67) | 43 (40) | 0.008 |
| Race | 0.24 | ||
| White | 28 (93) | 97 (89) | |
| Black | 0 | 8 (7) | |
| Other | 2 (7) | 4 (4) | |
| Etiology of portal hypertension | |||
| Alcohol | 13 (43) | 48 (44) | 0.95 |
| Hepatitis C infection | 5 (17) | 57 (52) | 0.001 |
| Autoimmune hepatitis | 7 (23) | 4 (4) | 0.002 |
| Nonalcoholic fatty liver disease | 1 (3) | 8 (75) | 0.68 |
| Hepatitis B infection | 1 (3) | 6 (6) | 1.0 |
| Primary sclerosing cholangitis | 1 (3) | 9 (8) | 0.69 |
| Primary biliary cirrhosis | 3 (10) | 3 (3) | 0.12 |
| Cryptogenic | 2 (7) | 8 (7) | 1.0 |
| Model for End-stage Liver Disease score† | 12 ± 3 | 13 ± 5 | 0.86 |
| Echocardiography | |||
| RV dilation | 24 (80) | 0 | 0.001 |
| RV hypertrophy‡ | 11 (39) | 0 | 0.001 |
| RV dysfunction | 17 (59) | 0 | 0.001 |
| RV systolic pressure,§ mm Hg | 77 ± 26 | 31 ± 5 | 0.001 |
| Invasive hemodynamics | |||
| Mean right atrial pressure,‖ mm Hg | 9 ± 6 | ||
| Mean pulmonary artery pressure, mm Hg | 50 ± 9 | ||
| Pulmonary capillary wedge pressure, mm Hg | 10 ± 4 | ||
| Cardiac index, L/min/m2 | 2.9 ± 0.9 | ||
| Pulmonary vascular resistance, dyne · s · cm–5 | 672 ± 376 |
*Variables are given as the mean ± SD or No. (%), unless otherwise indicated.
†Data available for 28 case patients and 108 control subjects.
‡Data available for 28 case patients and 104 control subjects.
§Right ventricular systolic pressure estimable in 27 case patients and 45 control subjects.
‖Mean atrial pressure was measured in 29 case patients.
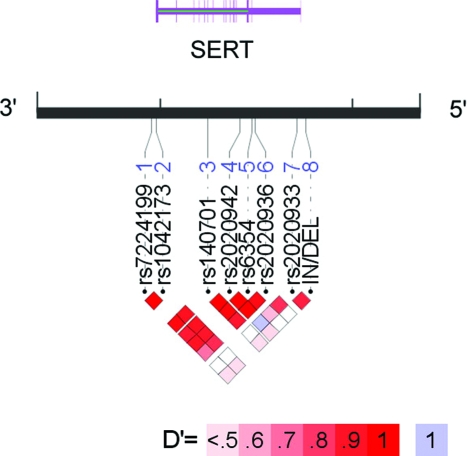
Genotype distribution for all SERT polymorphisms was in Hardy-Weinberg equilibrium (p > 0.05). There was no association between SERT in/del genotype and case status (Table 3). Contrary to our hypothesis, the long allele was (if anything) associated with a lower risk of PPHTN after adjustment for gender and autoimmune liver disease (adjusted OR, 0.63; 95% CI, 0.33 to 1.21; p = 0.17). Similarly, there were no associations between case status and the seven SNPs in and around SERT (Table 4). Pairwise assessment of linkage disequilibrium (a measurement of shared ancestry between neighboring polymorphisms) demonstrated two distinct blocks in the genomic region of SERT. The first block, comprised of six SNPs (pairwise D′ ≥ 0.80), encompassed the coding region of SERT. The second block, containing the tightly linked in/del and rs2020933 (D′ = 0.85), was independent of the first block (D′ ≥ 0.34). Thus, in/del genotype is not an accurate proxy for genetic variation throughout the gene encoding SERT. Figure 1 depicts these relationships between the polymorphisms genotyped in this study.
Table 3.
SERT in/del Genotype in PPHTN Case Subjects and Control Subjects
| Genotype | Case Subjects (n = 30) | Control Subjects (n = 109) | p Value* |
|---|---|---|---|
| SS | 8 (27%) | 26 (24%) | 0.17 |
| LS | 18 (60%) | 54 (50%) | |
| LL | 4 (13%) | 29 (27%) |
*Additive logistic regression model of PPHTN case status with genotype, adjusted for gender and autoimmune liver disease.
Table 4.
Logistic Regression of PPHTN Case Status with Genotype (Additive Models, Adjusted for Gender and Autoimmune Liver Disease)
| Locus | OR | 95% CI | p Value |
|---|---|---|---|
| in/del | 0.63 | 0.43–1.21 | 0.17 |
| rs2020933 | 2.08 | 0.47–9.19 | 0.33 |
| rs2020936 | 0.89 | 0.45–1.78 | 0.75 |
| rs6354 | 0.92 | 0.47–1.81 | 0.81 |
| rs2020942 | 0.83 | 0.44–1.56 | 0.56 |
| rs140701 | 1.16 | 0.64–2.08 | 0.63 |
| rs1042173 | 0.95 | 0.53–1.68 | 0.86 |
| rs7224199 | 0.98 | 0.56–1.73 | 0.95 |
Figure 1.
Linkage disequilibrium (LD) structure of SERT. Pairwise LD between loci (D′ and r2) and haplotype structure were measured using Haploview 4.0. (Broad Institute; Cambridge, MA).23 LD was measured using the in/del and seven SNPs genotyped in the control subjects in this study. The strength of LD is depicted graphically for each pairwise comparison (squares), such that white and blue represent low levels of LD, and red indicates high levels of LD (see color key). The SNPs are identified by their RS numbers and displayed relative to the candidate gene region. The display range of the chromosome (black line) corresponds to the genomic region of SERT. Exon/intron structure of the genes is indicated by thick/thin purple lines according to genome assembly hg17/May 2004. Note that the RefSeq uses the complementary strand of DNA; thus, the promoter region (5′) is on the right. Annotated graphical images were generated using into LocusView 2.0 (Broad Institute; Cambridge, MA).30
There were no significant differences in null loci allele frequencies between case patients and control subjects, and all were in Hardy Weinberg equilibrium (data not shown).
Discussion
This is the first case-control study of SERT-related genetic risk factors for PPHTN. The long allele of the SERT in/del polymorphism was not associated with case status in our population. On the contrary, there was a trend toward a lower risk of PPHTN in patients with this allele. In addition, we did not find associations between other polymorphisms in SERT and the risk of PPHTN.
Our findings add to the knowledge of the controversial relationship between SERT and PAH. Demonstration of an association between possession of the long allele in the SERT in/del and presence of IPAH12 generated interest in the serotonergic contribution to PAH, and offered the first common genetic risk factor for the disease. Subsequent attempts to replicate these findings in larger, geographically distinct cohorts of PAH patients were unsuccessful.15,16 Similarly, although plasma serotonin levels were increased in PAH patients in older studies,27,28 they were not increased in a more recent study that included patients with PPHTN.29 Possible explanations for these discrepant findings include population differences between studies, the existence of additional causal genetic variants in SERT, pathogenetic contributions from redundant mechanisms with serotonin signaling pathways, or type I error. Thus, the true nature of the role of serotonin signaling in PAH pathogenesis remains cryptic.
There are several potential explanations for the negative findings in our study. First, inadequate power is always a concern (type II error). Our study had more than sufficient power to detect the strong association previously reported in IPAH.12 Coupled with the trend toward the LL genotype being protective, this explanation is unlikely. Second, confounding by other genetic differences between case patients and control subjects could have masked an association. The absence of population stratification demonstrated by the null loci results makes this less likely. Third, it is possible that the in/del polymorphism serves as a marker of the true causal genetic variant in SERT in some populations, but not in ours, because our analysis found that the in/del polymorphism was not linked to genetic variation in the coding region. Fourth, SERT in/del genotype may contribute to an intermediate phenotype in patients with advanced liver disease, not captured by our study. Last, PPHTN and idiopathic forms of the disease could result from different pathogenetic mechanisms. We believe that these data demonstrate that PPHTN is independent of genetic variation in SERT.
Limitations of this multicenter study include the use of transthoracic echocardiography as a screening modality. This technique is subject to interobserver variability in performance and interpretation as well as technical limitations specific to individual subjects. In addition, missing data may lead to misclassification of case patients and control subjects. This could have resulted in bias to the null, although we used strict accepted criteria for both case patients and control subjects to minimize this possibility.
This is the first reported candidate genetic association study in a well-phenotyped and prospectively recruited cohort of subjects with portal hypertension and PAH. We have shown that common genetic variation in SERT does not account for the risk of PPHTN in patients with advanced liver disease. Future studies should focus on other mechanistic components of this disease.
Abbreviations:
- IPAH
idiopathic pulmonary arterial hypertension
- OR
odds ratio
- PAH
pulmonary arterial hypertension
- PPHTN
portopulmonary hypertension
- RV
right ventricular
- SERT
serotonin transporter
- SNP
single-nucleotide polymorphism
Appendix
Centers and Members of the Pulmonary Vascular Complications of Liver Disease Study Group
Columbia University:
Jenna Reinen, BA; Jeffrey Okun, BA; Daniel Rabinowitz, PhD; Debbie Rybak, BA.
The Mayo Clinic:
Linda Stadheim, RN; Vijay Shah, MD; and Russell Wiesner, MD.
The University of Alabama:
Dottie Faulk, BA; J. Stevenson Bynon, MD; Devin Eckhoff, MD; Harpreet Singh, MPH; and Rajasekhar Tanikella, MPH.
The University of Colorado:
Ted Perry, MS; and Lisa Forman, MD.
The University of North Carolina at Chapel Hill:
Carrie Nielsen, RN; and Roshan Shrestha, MD.
The University of Pennsylvania:
Vivek Ahya, MD; Michael Harhay, BS; Harold Palevsky, MD; and Rajender Reddy, MD.
The University of Southern California:
Neil Kaplowitz, MD.
Footnotes
This work was supported by National Institutes of Health grants DK064103, DK065958, RR00645, RR00585, RR00046, RR00032, HL67771, and HL089812.
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians ( www.chestjournal.org/site/misc/reprints.xhtml ).
References
- 1.Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401–409. doi: 10.1053/jhep.2003.50060. [DOI] [PubMed] [Google Scholar]
- 2.Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107–1111. doi: 10.1002/lt.20459. [DOI] [PubMed] [Google Scholar]
- 3.Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492–498. doi: 10.1016/s0735-1097(10)80121-4. [DOI] [PubMed] [Google Scholar]
- 4.Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: implications for liver transplantation. Clin Chest Med. 2005;26:587–597. vi. doi: 10.1016/j.ccm.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Lee SL, Wang WW, Moore BJ, et al. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res. 1991;68:1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- 6.Gurtner HP. Aminorex and pulmonary hypertension: a review. Cor Vasa. 1985;27:160–171. [PubMed] [Google Scholar]
- 7.Abenhaim L, Moride Y, Brenot F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension: International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 8.MacLean MR, Deuchar GA, Hicks MN, et al. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–2155. doi: 10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- 9.Guignabert C, Izikki M, Tu LI, et al. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res. 2006;98:1323–1330. doi: 10.1161/01.RES.0000222546.45372.a0. [DOI] [PubMed] [Google Scholar]
- 10.Marcos E, Adnot S, Pham MH, et al. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2003;168:487–493. doi: 10.1164/rccm.200210-1212OC. [DOI] [PubMed] [Google Scholar]
- 11.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 12.Eddahibi S, Humbert M, Fadel E, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddahibi S, Chaouat A, Morrell N, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108:1839–1844. doi: 10.1161/01.CIR.0000091409.53101.E8. [DOI] [PubMed] [Google Scholar]
- 14.Olson TP, Snyder EM, Frantz RP, et al. Repeat length polymorphism of the serotonin transporter gene influences pulmonary artery pressure in heart failure. Am Heart J. 2007;153:426–432. doi: 10.1016/j.ahj.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Willers ED, Newman JH, Loyd JE, et al. Serotonin transporter polymorphisms in familial and idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;173:798–802. doi: 10.1164/rccm.200509-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado RD, Koehler R, Glissmeyer E, et al. Genetic association of the serotonin transporter in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;173:793–797. doi: 10.1164/rccm.200509-1365OC. [DOI] [PubMed] [Google Scholar]
- 17.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 18.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 22.Seldin MF, Shigeta R, Villoslada P, et al. European population substructure: clustering of northern and southern populations. PLoS Genet. 2006;2:e143. doi: 10.1371/journal.pgen.0020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauderman W, Morrison J. QUANTO 1.1: a computer program for power and sample size calculations for genetic-epidemiology studies. [Accessed March 7, 2008]; Available at: http://hydra.usc.edu/gxe .
- 26.Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196–203. doi: 10.1002/hep.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kereveur A, Callebert J, Humbert M, et al. High plasma serotonin levels in primary pulmonary hypertension: effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler Thromb Vasc Biol. 2000;20:2233–2239. doi: 10.1161/01.atv.20.10.2233. [DOI] [PubMed] [Google Scholar]
- 28.Herve P, Launay JM, Scrobohaci ML, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 29.Lederer DJ, Horn EM, Rosenzweig EB, et al. Plasma serotonin levels are normal in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2008;21:112–114. doi: 10.1016/j.pupt.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petryshen T, Kirby A, Ainscow M. LocusView 2.0. Cambridge, MA: Broad Institute; 2003. [Google Scholar]