Abstract
Background:
The obesity epidemic has prompted remarkable changes in the proportion of obese children who are referred for habitual snoring. However, the contribution of obesity to adenotonsillar hypertrophy remains undefined.
Methods:
In our study, 206 nonobese habitually snoring children with polysomnographically diagnosed obstructive sleep apnea (OSA) were matched for age, gender, ethnicity, and obstructive apnea-hypopnea index (OAHI) to 206 obese children. Size estimates of tonsils and adenoids, and Mallampati class scores were obtained, and allowed for the assessment of potential relationships between anatomic factors and obesity in pediatric OSA.
Results:
The mean OAHI for the two groups was approximately 10.0 episodes/h total sleep time. There was a modest association between adenotonsillar size and OAHI in nonobese children (r = 0.22; p < 0.001) but not in obese children. The mean (± SEM) adenotonsillar size was larger in nonobese children (3.85 ± 0.16 vs 3.01 ± 0.14, respectively; p < 0.0001), and conversely Mallampati class scores were significantly higher in obese children (p < 0.0001).
Conclusion:
The magnitude of adenotonsillar hypertrophy required for any given magnitude of OAHI is more likely to be smaller in obese children compared to nonobese children. Increased Mallampati scores in obese children suggest that soft-tissue changes and potentially fat deposition in the upper airway may play a significant role in the global differences in tonsillar and adenoidal size among obese and nonobese children with OSA.
Since its initial description, obstructive sleep apnea (OSA) has emerged as a highly prevalent condition in the pediatric age range, affecting 2 to 3% of school-aged children and associated with an extensive array of emerging morbidities, particularly affecting cognition and behavior as well as cardiovascular and metabolic systems.1–6 Although it is accepted overall that the primary pathophysiologic mechanism involved in pediatric OSA consists of hypertrophy of adenoid and tonsillar tissues in the upper airway,7–11 several studies12–16 have thus far failed to demonstrate the anticipated corollary to such findings, namely, a very strong association correlation between upper airway adenotonsillar size and OSA severity. These findings suggest that OSA represents the end point of the interactions between multiple factors contributing to upper airway collapsibility during sleep, which also include neuromotor responses as well as other important anatomic factors such as retrognathia and upper airway length.17,18
The prevalence and severity of overweight and obesity in children and adolescents has witnessed dramatic increases in the last few decades worldwide.19,20 For example, the prevalence of childhood overweight doubled among children 6 to 11 years of age and tripled among children 12 to 17 years of age in the United States between 1980 and 2000.21,22 It has become apparent that obese children may be at increased risk for OSA.23–29 Indeed, the proportion of respiratory disturbances during sleep was found markedly increased among obese children.25,26 In a case-control study design, Redline and colleagues23 examined the risk factors for sleep-disordered breathing (SDB) in children age 2 to 18 years, and they found that the risk among obese children was increased fourfold to fivefold. In fact, for every increment in body mass index (BMI) of 1 kg/m2 beyond the mean BMI for age and gender, the risk of OSA increased by 12%. Similar trends demonstrating an increased risk of OSA among obese and overweight children have been reported from all over the world. In this context, we have reported30,31 that the presence of obesity appears to modify the end-organ susceptibility to OSA and prescribes some of the differences in phenotypic manifestations and clinical presentations. Thus, similar to adults, obese children appear to be at increased risk for the development of SDB, and the severity of OSA seems to be proportional to the degree of obesity.23,27,29 Conversely, hypertrophic adenotonsillar tissues may not always be the main contributing factor to the development of OSA in obese children, and even among nonobese children, tonsil size correlated with the severity of OSA only among younger children (ie, < 7 years of age).13,32,33 However, the respective contributions of adenotonsillar size and BMI on pediatric OSA have not been critically examined. Therefore, we conducted the present study to test several related hypotheses, as follows: (1) that obese children with OSA will have less adenotonsillar hypertrophy compared to nonobese children with OSA of matched severity; (2) that this effect will not differ among children below or above the age of 7 years after controlling for gender and ethnicity; and (3) obese children with OSA will have a higher Mallampati classification score34 when compared to nonobese children with OSA of matched severity. To examine these issues, we conducted a retrospective examination of polysomnographic findings in a large cohort of age-, gender- and ethnicity-matched obese and nonobese children with OSA of similar severity who received a diagnosis in our pediatric sleep center.
Materials and Methods
This retrospective study was approved by the University of Louisville Human Research Committee, and it included as the first phase the database review of all habitually snoring children between 1 and 16 years of age who were evaluated with an overnight polysomnography (PSG) evaluation from October 2003 until September 2007 for suspected SDB. All patients fulfilling obesity criteria were initially identified. Of these, the charts of those children with PSG evidence of OSA were reviewed for retrieval of all the necessary and pertinent data. If complete information was not available, or if any of the children had chronic diseases, including asthma (declared by parents and requiring treatment with regular daily bronchodilators and/or inhaled corticosteroids), or from craniofacial and genetic syndromic conditions, they were excluded. On completion of this process, all nonobese children referred for evaluation of habitual snoring during the same period were identified, and all necessary and pertinent data for nonobese children fulfilling the OSA criteria were retrieved. Careful evaluation of the charts enabled matching of a nonobese eligible child with OSA and with complete information in their clinical charts to one of the previously identified obese children with OSA. Age (within 6 months), gender, ethnicity, and the obstructive apnea-hypopnea index (OAHI) [within one episode per hour of total sleep time (TST)] served as the required matching criteria.
The clinical characteristics of the two cohorts of OSA patients were documented from a review of their clinical charts. The data extracted from the sleep studies and clinical charts included age, gender, height, weight, ethnicity, and estimates of adenoidal size (likert scale range, 0 to 4), Mallampati score (likert scale range, 1 to 4),34 and tonsillar size (likert scale range, 0 to 4). The Mallampati score assigns a score of 1 when the protruded tongue does not obfuscate the visual sighting of the soft palate, fauces, uvula, and tonsillar pillars; it assigns a score of 4 only when the hard palate can be visualized.34 Adenoid size was determined from a blind review of lateral neck radiographs, which were obtained using standard techniques in the radiology department at Kosair Children's Hospital. The neck was extended and the patient was instructed to breathe through the nose. The adenoidal/nasopharyngeal ratio was then measured according to the method of Fujioka and colleagues35 by two of the investigators who were blinded to the group assignment and PSG findings of the subjects. A score of 0 corresponded to the absence of adenoid tissues; a score of +1 indicated an adenoid size of 0 to 25% of the retropalatal airway; a score +2 indicated an adenoid size of 25 to 50% of the retropalatal airway; a score of +3 indicated an adenoid size of 50 to 75% of the retropalatal airway; and a score of +4 indicated an adenoid size of > 75% of the retropalatal airway lumen.
Individual Mallampati scores and tonsillar size scores were extracted from the chart and related to the initial assessment by the evaluating physician during visual inspection. They were scored using pictorial guides in the chart. As with adenoid size scores, tonsil size was assigned a score of 0 (no tonsils present) to 4 (kissing tonsils).36 The sum of the adenoid and tonsil scores was used as the global estimate of adenotonsillar size. The diagnostic criteria for OSA used in this study included an OAHI > 2/h TST, with a nadir oxygen saturation value of at least < 92%.37
PSG Evaluation
A description of the procedures followed for an overnight sleep study has been described in great detail elsewhere.37 In brief, subjects accompanied by a caretaker reported to the sleep laboratory around 7:30 pm, and the sleep studies began between 9:00 and 9:30 pm. The following parameters were measured: chest and abdominal wall movement by respiratory impedance or inductance plethysmography; heart rate by ECG; and air flow with a sidestream end-tidal capnograph (BCI SC-300; BCI; Menomonee Falls, WI), which also provided breath-by-breath assessment of end-tidal carbon dioxide levels, nasal pressure, and an oral-nasal airflow thermistor. Pulse oximetric saturation (Spo2) was assessed using a pulse oximeter (Nellcor N 100; Nellcor Inc; Hayward, CA) with a simultaneously recorded pulse waveform. Bilateral electrooculogram, eight channels of EEG, chin and anterior tibial electromyograms, and analog output from a body position sensor (Braebon Medical Corp; Ogdensburg, NY) were also monitored. All measures were digitized using a commercially available computerized PSG system (Rembrandt; MedCare Diagnostics; Buffalo, NY). Tracheal sound was monitored with a microphone sensor (Sleepmate; Midlothian, VA), and digital time-synchronized video images were collected. The sleep technician followed patient behavior and confirmed sleep position by the infrared camera inside the room. All the studies were initially scored by a certified technician and then reviewed by physicians experienced in pediatric PSG. The scorer had no prior knowledge of the physical examination findings in the upper airways of the children. Sleep architecture was assessed by standard techniques.38 The proportion of time spent in each sleep stage was expressed as the percentage of TST. Central, obstructive, and mixed apneic events were counted. OSA was defined as the absence of airflow with continued chest wall and abdominal movement for duration of at least two breaths.37 Hypopneas were defined as a decrease in oronasal flow of ≥ 50% with a corresponding decrease in Spo2 of ≥ 4% and/or arousal.37 The OAHI was defined as the number of apneas and hypopneas per hour of TST. The mean oxygen saturation, as measured by Spo2 in the presence of a pulse waveform signal void of motion artifact, and the nadir Spo2 were recorded.
BMI:
The BMI was calculated based on standardized percentile curves and the Centers for Disease Control and Prevention reference values ( http://www.cdc.gov/epiinfo/ ).39,40 Obesity was defined as a BMI in the > 95th percentile for gender and age, corresponding to a BMI z score of 1.67; therefore, nonobese children were included if their BMI z score was < 1.67.
Statistical Analysis
Data are presented as the mean ± SEM, unless otherwise indicated. All analyses were conducted using a statistical software package (SPSS, version 16.0; SPPS; Chicago, IL). Comparisons of demographics according to group assignment were made with independent t tests or analysis of variance followed by post hoc comparisons, with p values adjusted for unequal variances when appropriate (Levene test for equality of variances), or χ2 analyses with the Fisher exact test (dichotomous outcomes). Nonparametric tests (Mann-Whitney) and logistic multivariate analyses were used to assess the independent contributions of adenotonsillar size, Mallampati scores, age, gender, ethnicity, and BMI z score to OAHI. All p values reported are two tailed with statistical significance set at < 0.05.
Results
Cohort Selection
Initially, we identified all children who were obese and who fulfilled the diagnostic criteria for OSA (see following). This process allowed for the identification of 1,236 obese children with OSA of 2,217 obese habitually snoring children (55.7%). After the exclusion of incomplete records or of patients not fulfilling entry criteria, 206 obese children with OSA were retained for analyses. The database was then evaluated, and it identified 3,656 nonobese children who were referred during the same period for suspected SDB. Among these nonobese children, OSA was diagnosed in 1,428 children (39%). Thus, the relative risk for OSA was significantly greater among habitually snoring obese children (1.43; 95% confidence interval, 1.35 to 1.51; p < 0.0000001). A total of 206 age-, gender-, ethnicity-, and OAHI-matched nonobese children were then identified for subsequent analyses.
Demographic and PSG Characteristics
A total of 412 children with OSA (40.3% female; 56% white; and 37% African American), with a mean age of 6.5 ± 0.2 years (age range, 1 to 14 years), were included (206 obese habitually snoring otherwise healthy children with PSG-diagnosed OSA; and 206 nonobese matching children) [Table 1]. An additional stratification of these two cohorts was done based on age above and below 7 years old. Table 2 shows the PSG characteristics of these four groups.
Table 1.
Demographic Characteristics and BMI z Score in 206 Obese and 206 Matched Nonobese Children With OSA
| Nonobese OSA |
Obese OSA |
||||
|---|---|---|---|---|---|
| Variables | Young | Old | Young | Old | p Value |
| Age, yr | 4.43 ± 0.13 | 11.06 ± 0.45 | 4.45 ± 0.16 | 11.08 ± 0.39 | NS |
| Gender, % female | 38 | 33 | 38 | 33 | NS |
| African American, % | 40 | 35 | 41 | 36 | NS |
| BMI z score | 1.04 ± 0.36 | 0.94 ± 0.32 | 2.08 ± 0.37 | 2.25 ± 0.39 | < 0.0001 |
| OAHI, episodes/h TST | 10.8 ± 2.4 | 9.2 ± 2.6 | 11.3 ± 2.4 | 9.4 ± 2.7 | NS |
NS = not significant. Obese category defined as BMI z score > 1.67.
Table 2.
PSG Findings in the Four Groups of Children With OSA
| Variables | YNOB (n = 101) | ONOB (n = 105) | YOB (n = 101) | OOB(n = 105) | p Value |
|---|---|---|---|---|---|
| TST, min | 458.31 ± 5.24 | 445.71 ± 7.06 | 437.86 ± 5.43 | 481.03 ± 46.78 | NS |
| Sleep efficiency, % | 89.41 ± 0.79 | 88.20 ± 1.04 | 87.52 ± 0.91 | 98.36 ± 10.62 | NS |
| Sleep stage, % | |||||
| 1 | 9.6 ± 0.56 | 8.38 ± 0.78 | 8.13 ± 0.55 | 8.51 ± 0.76 | NS |
| 2 | 41.78 ± 1.65 | 42.45 ± 1.45 | 43.98 ± 0.95 | 46.25 ± 1.99 | NS |
| SWS | 24.31 ± 0.61 | 24.07 ± 1.16 | 23.32 ± 0.79 | 23.98 ± 1.27 | NS |
| REM sleep | 18.25 ± 0.48 | 16.39 ± 0.88 | 15.91 ± 0.55 | 16.18 ± 1.15 | NS |
| OAHI, episodes/h TST | 10.8 ± 1.0 | 9.2 ± 1.7 | 11.3 ± 1.0 | 9.1 ± 1.1 | NS |
| Obstructive apnea index, episodes/h TST | 3.96 ± 0.56 | 3.29 ± 1.11 | 3.51 ± 0.45 | 3.79 ± 0.72 | NS |
| Spo2 | |||||
| Nadir % | 81.56 ± 0.97 | 82.65 ± 12.46 | 82.42 ± 8.08 | 82.15 ± 9.30 | NS |
| Mean | 93.24 ± 0.20 | 92.81 ± 13.53 | 92.64 ± 8.95 | 92.95 ± 10.52 | NS |
| Mean ETCO2, mm Hg | 37.10 ± 4.25 | 37.67 ± 5.55 | 37.43 ± 3.72 | 37.04 ± 4.36 | NS |
| Total arousal index episodes/h TST | 14.20 ± 0.79 | 12.99 ± 0.95 | 14.81 ± 1.21 | 15.89 ± 2.78 | NS |
| Respiratory arousal index, episodes/h TST | 6.45 ± 0.55 | 5.63 ± 0.75 | 7.48 ± 0.93 | 6.89 ± 0.91 | NS |
Values are given as the mean ± SEM, unless otherwise indicated. ETCO2 = end-tidal carbon dioxide; NOB = nonobese; ONOB = old nonobese; OOB = old obese; REM = rapid eye movement; SWS = slow-wave sleep; YNOB = young nonobese. See Table 1 for abbreviation not used in the text.
Effect of BMI on Respiratory Disturbance During Sleep in Children With OSA
As evidence of the careful matching process described earlier, there were no significant differences between mean OAHI of nonobese OSA and obese OSA (Table 2). In addition, further allocation of each of the two cohorts into those younger or older than 7 years of age showed that no differences occurred for the OAHI, apnea index, or for any of the other PSG measures (Table 2). There was no linear association between BMI z score and OAHI (p value, not significant). Similarly, there were no global differences in the effect of obesity on OAHI among girls or boys, although after age 13 years, there was a small, albeit significant, trend toward an increased impact of BMI in boys compared to girls (p < 0.04).
Adenotonsillar Size and Respiratory Disturbance During Sleep
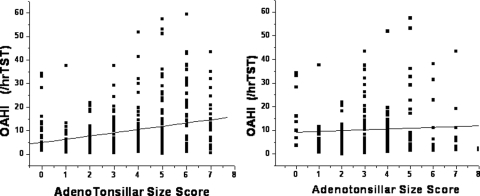
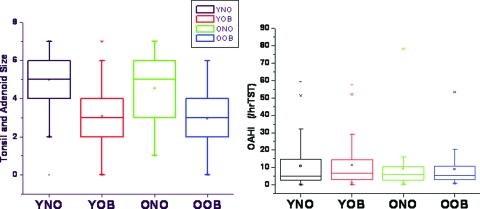
A positive, albeit modest, linear relationship between the sum of adenoid and tonsillar size scores and the OAHI emerged among the nonobese children, but it was not present among the obese children (Fig 1). For the whole cohort, no differences emerged in adenotonsillar size scores among the two age groups; however, there were significant differences in adenotonsillar scores in the context of BMI, such that adenotonsillar size was markedly larger in nonobese children for the whole cohort, and also after subdivision of the BMI-based cohorts according to age groups (p < 0.0001) [Fig 2].
Figure 1.
Scatterplot between the OAHI as a measure of OSA severity and adenotonsillar size sum score in 206 nonobese children (left) [r = 0.22; p < 0.001] and 206 OAHI-matched obese habitually snoring children with OSA (right) [difference not significant].
Figure 2.
Box plots of adenotonsillar size scores among young obese (YOB) and old obese (OOB) children and nonobese children with OSA (left) and OAHIs (right). No significant differences are present among the OAHI groups, but adenotonsillar size scores are significantly lower in both YOB and OOB children compared to their nonobese counterparts (young nonobese [YNO] and old nonobese [ONO]) [p < 0.0001].
Mallampati Scores in Obese and Nonobese Children With OSA
Categorical assignment of the OSA cohort into four groups based on age and BMI Z score revealed that Mallampati class scores were significantly higher among obese children (p < 0.0001) in both age groups (Fig 3). Furthermore, a significant association between BMI Z score and Mallampati scores emerged for the whole cohort (r = 0.26; p < 0.00001).
Figure 3.
Mallampati class scores among YOB, OOB, and nonobese children with OSA. Mallampati class scores were significantly higher in both YOB and OOB children compared to YNOB and ONOB children (p < 0.0001). See the legend of Figure 2 for abbreviations not used in the text.
Discussion
In this study, we retrospectively identified two large cohorts of closely OAHI-matched pediatric patients with OSA who were also matched for age, gender, and ethnicity, and who differed only in their BMI. This approach, which aimed for further understanding of the relative contribution of obesity to OSA, revealed that adenotonsillar size is correlated with the severity of OSA in nonobese children but not among obese children. More importantly, however, our study shows that the magnitude of adenotonsillar hypertrophy among obese children with OSA is markedly smaller at any given level of OAHI than among nonobese children, and that conversely, Mallampati scores, which provide a simple estimate of how crowded the upper airway is, are markedly increased among obese children compared to nonobese OSA patients when all other confounders such as age, gender, ethnicity, and OAHI are kept constant. These findings suggest that obesity is an important contributor to the pathophysiology of OSA in children irrespective of age.
Our study, of course, suffers from several methodological issues that need to be discussed before we proceed with the interpretation of our findings. First, this was a retrospective study, whereby for every eligible obese child in whom the diagnosis of OSA was determined by PSG, a nonobese, closely matched child was then identified in the database. Using such an approach, we were able to include only < 10% of all children evaluated for SDB during that period. Comparisons between the retained cohorts of OSA and those excluded from analyses did not reveal significant differences in age distribution, gender, ethnicity, or OSA severity, both among the obese and nonobese children (data not shown), such that current findings should retain their validity and applicability. Second, the evaluation of adenoid and tonsillar size and of Mallampati scores was conducted by multiple clinicians rather than by a designated investigator. Although such an approach is undoubtedly fraught with large interindividual variability, we submit that it further strengthens the validity of the findings because it would not be affected by any potential individual investigator bias in the categorical assignment of such scores. Furthermore, we recognize as a limitation that 1+ of tonsillar size may not be equivalent to 1+ of adenoidal size. Therefore, a score of 1+ tonsils plus 2+ adenoids may not cause the same increase in upper airway resistance compared to a score of 2+ tonsils plus 1+ adenoids. Third, there was a relative overrepresentation of African-American children when compared to the ethnic demographic characteristics of the general population in Louisville, KY. This was not surprising, considering the higher rates of both OSA and obesity reported for African Americans.21,41–43
Of note, there was no evidence for a significant correlation between the degree of obesity and the severity of OSA. This overall finding, which was generally anticipated, considering that the presence of obesity alone does not mandate the existence of respiratory abnormalities during sleep, does not detract, however, from potentially substantial contributions of adiposity to the occurrence and severity of OSA. Indeed, several investigators12,24–29,43,44 have previously noted the higher prevalence and severity of OSA among obese children in the absence of a strong relationship between the degree of obesity and the severity of respiratory disturbance during sleep. Verhulst and colleagues45 reported the presence of OSA in 19% of obese patients and 41% of overweight children who were referred for initial evaluation and management in an obesity clinic but did not find any correlation between BMI z score and OAHI. Indeed, the validity of a “straightforward” association between overweight/obesity and increased prevalence of SDB has been recently questioned.46 The reason for such discrepant findings may reside in the limitations imposed by the reporting of obesity in terms of BMI. Clearly, BMI does not reflect body habitus and does not measure adiposity directly. Therefore, the potential mass effect of adipose tissue on the upper airway may not be reflected by traditional BMI measures, which will also fail to point toward interactions of fat tissues with a developing upper airway system or to the biologic effects of fat itself on upper airway function, through either local and systemic inflammation and/or changes in respiratory control.
The most important finding of the present study resides in the reciprocal interactions found between adenotonsillar size and Mallampati scores. In other words, at any level of OSA severity, the more crowded the airway, the lesser the size of adenotonsillar tissues required to elicit OSA of any particular magnitude. This relationship was present independent of age, gender, and ethnicity, and therefore would strongly support a major role for obesity in the pathophysiology of OSA. However, and as mentioned earlier, although the assessment of BMI fails to reveal the mechanisms by which excess fat contributes to SDB, the increased Mallampati scores found among obese children would suggest that fat deposition in the soft tissues of the upper airway are more likely to occur in the context of obesity and will lead to reductions in upper airway diameter, thereby facilitating the occurrence of OSA when lymphadenoid hypertrophy occurs, even if the latter is mild. This assumption appears not only theoretically plausible but has in fact been substantiated by the high rates of residual OSA after adenotonsillectomy among obese children.47–49 Conversely, interventions aiming to reduce the degree of adiposity have also been associated with improvements in OSA.50,51
In summary, the present study conclusively demonstrates that for any level of OSA severity the habitually snoring obese child requires less adenotonsillar hypertrophy when compared to nonobese children of the same age, gender, and ethnicity. These differences appear to be mediated, at least in part, by the presence of a more crowded airway in obese children, as a corollary to increased fat deposits in the airway. Prospective studies should evaluate whether the efficacy of adenotonsillectomy in obese children with OSA can be predicted from equation models incorporating both estimates of adenoid and tonsillar size and Mallampati scores.
Abbreviations:
- BMI
body mass index
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SDB
sleep-disordered breathing
- Spo2
pulse oximetric saturation
- TST
total sleep time
Footnotes
This research was supported by National Institutes of Health grants HL-065270, HL-086662, and HL-083075; the Commonwealth of Kentucky Research Challenge for Excellence Trust Fund; and the Children's Foundation Endowment for Sleep Research (to Dr. Gozal).
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians ( www.chestjournal.org/site/misc/reprints.xhtml ).
References
- 1.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4–5 year olds. Arch Dis Child. 1993;68:360–363. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old: an epidemiologic study of lower limit of prevalence. Chest. 1995;107:963–966. doi: 10.1378/chest.107.4.963. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish L. Disorders of breathing during sleep. In: Chernick V, Boat T, Wilmott R, et al., editors. Kendig's disorders of the respiratory tract in children. Philadelphia, PA: Elsevier; 2006. pp. 1046–1070. [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 5.Sans Capdevila O, Kheirandish-Gozal L, Dayyat E, et al. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozal D, Sans Capdevila O, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among non-obese and obese pre-pubertal children. Am J Respir Crit Care Med. 2008;177:1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss SG, Lynn AM, Bratton SL, et al. Ventilatory response to CO2 in children with obstructive sleep apnea from adenotonsillar hypertrophy. Anesth Analg. 1999;89:328–332. doi: 10.1097/00000539-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- 9.Greenfeld M, Tauman R, DeRowe A, et al. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol. 2003;67:1055–1060. doi: 10.1016/s0165-5876(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 10.Shintani T, Asakura K, Kataura A. Adenotonsillar hypertrophy and skeletal morphology of children with obstructive sleep apnea syndrome. Acta Otolaryngol Suppl. 1996;523:222–224. [PubMed] [Google Scholar]
- 11.Shintani T, Asakura K, Kataura A. Evaluation of the role of adenotonsillar hypertrophy and facial morphology in children with obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 1997;59:286–291. doi: 10.1159/000276955. [DOI] [PubMed] [Google Scholar]
- 12.Lam YY, Chan EY, Ng DK, et al. The correlation among obesity, apnea-hypopnea index, and tonsil size in children. Chest. 2006;130:1751–1756. doi: 10.1378/chest.130.6.1751. [DOI] [PubMed] [Google Scholar]
- 13.Fregosi RF, Quan SF, Kaemingk KL, et al. Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children. J Appl Physiol. 2003;95:2030–2038. doi: 10.1152/japplphysiol.00293.2003. [DOI] [PubMed] [Google Scholar]
- 14.Li AM, Wong E, Kew J, et al. Use of tonsil size in the evaluation of obstructive sleep apnoea. Arch Dis Child. 2002;87:156–159. doi: 10.1136/adc.87.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdamar B, Suoglu Y, Cuhadaroglu C, et al. Evaluation of clinical parameters in patients with obstructive sleep apnea and possible correlation with the severity of the disease. Eur Arch Otorhinolaryngol. 2001;258:492–495. doi: 10.1007/s004050100367. [DOI] [PubMed] [Google Scholar]
- 16.Brooks LJ, Stephens BM, Bacevice AM. Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr. 1998;132:682–686. doi: 10.1016/s0022-3476(98)70360-9. [DOI] [PubMed] [Google Scholar]
- 17.Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:253–262. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magarey AM, Daniels LA, Boulton TJ. Prevalence of overweight and obesity in Australian children and adolescents: reassessment of 1985 and 1995 data against new standard international definitions. Med J Aust. 2001;174:561–564. doi: 10.5694/j.1326-5377.2001.tb143435.x. [DOI] [PubMed] [Google Scholar]
- 20.Lobstein T, Baur L, Uauy R, et al. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5(suppl):S4–S104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 23.Redline S, Tishler PV, Schluchter M, et al. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Curtis S, Koerner CB, et al. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–183. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Mallory GB, Jr, Fiser DH, Jackson R. Sleep-associated breathing disorders in morbidly obese children and adolescents. J Pediatr. 1989;115:892–897. doi: 10.1016/s0022-3476(89)80738-3. [DOI] [PubMed] [Google Scholar]
- 26.Silvestri JM, Weese-Mayer DE, Bass MT, et al. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993;16:124–129. doi: 10.1002/ppul.1950160208. [DOI] [PubMed] [Google Scholar]
- 27.Sogut A, Altin R, Uzun L, et al. Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3-11-year-old Turkish children. Pediatr Pulmonol. 2005;39:251–256. doi: 10.1002/ppul.20179. [DOI] [PubMed] [Google Scholar]
- 28.Sulit LG, Storfer-Isser A, Rosen CL, et al. Associations of obesity, sleep-disordered breathing, and wheezing in children. Am J Respir Crit Care Med. 2005;171:659–664. doi: 10.1164/rccm.200403-398OC. [DOI] [PubMed] [Google Scholar]
- 29.Chay OM, Goh A, Abisheganaden J, et al. Obstructive sleep apnea syndrome in obese Singapore children. Pediatr Pulmonol. 2000;29:284–290. doi: 10.1002/(sici)1099-0496(200004)29:4<284::aid-ppul8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood OSA: one or two distinct disease entities? Clin Sleep Med. 2007;2:433–444. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sans Capdevila O, Kheirandish-Gozal L, Dayyat E, et al. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath. 2008;12:25–31. doi: 10.1007/s11325-007-0132-z. [DOI] [PubMed] [Google Scholar]
- 33.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 34.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–434. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 35.Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. 1979;133:401–404. doi: 10.2214/ajr.133.3.401. [DOI] [PubMed] [Google Scholar]
- 36.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–1569. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery-Downs HE, O'Brien LM, Gulliver TE, et al. Polysomnographic characteristics in normal preschool and early school-age children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. Washington, DC: National Institutes of Health; 1968. publication No. 204. [Google Scholar]
- 39.Hammer LD, Kraemer HC, Wilson DM, et al. Standardized percentile curves of body mass index for children and adolescents. Am J Dis Child. 1991;145:259–263. doi: 10.1001/archpedi.1991.02160030027015. [DOI] [PubMed] [Google Scholar]
- 40.Cole TJ, Faith MS, Pietrobelli A, et al. What is the best measure of adiposity change in growing children: BMI, BMI%, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 41.Redline S, Tishler PV, Hans MG, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 42.Schechter MS. American Academy of Pediatrics technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:1–20. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 43.Rudnick EF, Walsh JS, Hampton MC, et al. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg. 2007;137:878–882. doi: 10.1016/j.otohns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Wing YK, Hui SH, Pak WM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child. 2003;88:1043–1047. doi: 10.1136/adc.88.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–208. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohler MJ, van den Heuvel CJ. Is there a clear link between overweight/obesity and sleep disordered breathing in children? Sleep Med Rev. 2008;12:347–361. doi: 10.1016/j.smrv.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137:43–48. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Apostolidou MT, Alexopoulos EI, Chaidas K, et al. Obesity and persisting sleep apnea after adenotonsillectomy in Greek children. Chest. 2008;134:1149–1155. doi: 10.1378/chest.08-1056. [DOI] [PubMed] [Google Scholar]
- 50.Kalra M, Mannaa M, Fitz K, et al. Effect of surgical weight loss on sleep architecture in adolescents with severe obesity. Obes Surg. 2008;18:675–679. doi: 10.1007/s11695-008-9472-4. [DOI] [PubMed] [Google Scholar]
- 51.Ng DK, Lam YY, Chan CH. Dietary intervention combined with exercise improves vascular dysfunction but also obstructive sleep apnea in obese children. Circulation. 2004;110:e314. doi: 10.1161/01.CIR.0000142199.13107.F2. [DOI] [PubMed] [Google Scholar]