Abstract
There is increasing interest in the role of antibodies targeting specific membrane proteins in neurological and other diseases. The target(s) of these pathogenic antibodies is known in a few diseases, usually when candidate cell surface proteins have been tested. Approaches for identifying new antigens have mainly resulted in the identification of antibodies to intracellular proteins, which are often very useful as diagnostic markers for disease but unlikely to be directly involved in disease pathogenesis because they are not accessible to circulating antibodies. To identify cell surface antigens, we developed a “conformational membrane antigen isolation and identification” strategy. First, a cell line is identified that reacts with patient sera but not with control sera. Second, intact cells are exposed to sera to allow the binding of presumptive autoantibodies to their cell surface targets. After washing off non-bound serum components, the cells are lysed, and immune complexes are precipitated. Third, the bound surface antigen is identified by mass spectrometry. As a model system we used a muscle cell line, TE671, that endogenously expresses muscle-specific tyrosine receptor kinase (MuSK) and sera or plasmas from patients with a subtype of the autoimmune disease myasthenia gravis in which patients have autoantibodies against MuSK. MuSK was robustly detected as the only membrane protein in immunoprecipitates from all three patient samples tested and not from the three MuSK antibody-negative control samples processed in parallel. Of note, however, there were many intracellular proteins found in the immunoprecipitates from both patients and controls, suggesting that these were nonspecifically immunoprecipitated from cell extracts. The conformational membrane antigen isolation and identification technique should be of value for the detection of highly relevant antigenic targets in the growing number of suspected antibody-mediated autoimmune disorders. The approach would also be very suitable for the analysis of human or experimental antitumor responses.
Autoimmune diseases are conditions in which aberrant immune responses cause damage to and dysfunction of the body's own tissue. They range from prevalent conditions, such as type 1 diabetes mellitus and rheumatoid arthritis, to various types of autoimmune thyroiditis (1), inflammatory bowel diseases (2), skin conditions such as bullous pemphigoid (3), and rarer neurological disorders such as myasthenia gravis (4).
Understanding of most of these diseases is still highly incomplete. Fundamental knowledge includes the identity of the antigenic target of the immune response and whether the response is predominantly T cell- or antibody-mediated. In some of the above examples, “candidate” antigens have been proposed as a result of study of the pathophysiology of the disease (e.g. see Ref. 5). The detection of a disease-specific autoantibody allows the development of diagnostic tests, and if the target is a cell surface protein it usually implies that the disease will respond clinically to treatments that reduce the levels of the pathogenic antibodies.
In recent years, there has also been increasing interest in natural (or experimental) immune responses to tumor cells that may slow the growth or spread of a tumor. In some cases, however, this immune response may result in pathogenic autoimmunity. For example, antibodies directed to voltage-gated calcium channels expressed on the surface of small cell lung cancer cells can cause neurological dysfunction by binding to similar calcium channels on the motor nerve endings (see Ref. 4). In other cancer-associated (paraneoplastic) disorders, however, there are antibodies to intracellular antigens, which are also shared between the tumor and neuronal tissue, that are highly useful as diagnostic markers for the disorders. In these patients, T cell immunity is thought to be responsible for the neurological disease (see Ref. 6), which generally does not improve with immunosuppressive treatments.
Attempts to identify autoantigens and tumor antigens in many autoimmune and cancer-related syndromes have generally used techniques involving screening of mRNA expression libraries or, more recently, separation of soluble extracts of tissue or cell lines by one- or two-dimensional electrophoresis and blotting of the separated proteins onto membranes where they are probed with patient sera. Typically in any one experiment, a large number of protein bands or spots are bound by serum antibodies, and some of the corresponding bands or spots on the gel are then excised, digested, and analyzed by mass spectrometry (e.g. Refs. 7 and 8). The identified proteins have been claimed as novel antigens associated with the condition with sometimes a whole array of proteins identified from a single experiment and claimed to represent a disease-associated “autoimmune profile.” However, the identified proteins are often common intracellular proteins with the same or closely related proteins repeatedly implicated in seemingly unrelated autoimmune, allergic, and malignant diseases (see “Discussion”). The intracellular location of these proteins where they would be inaccessible to circulating antibodies and their lack of disease specificity cast doubt upon their relevance.
The best understood example of an antibody-mediated disease is myasthenia gravis with acetylcholine receptor antibodies (for a review, see Ref. 9). Another subgroup of myasthenia gravis patients has antibodies to a muscle-specific tyrosine kinase (MuSK).1 These antibodies are known to bind to the cell surface and to inhibit the clustering function of MuSK (10). Although the mechanisms of disease are not fully understood, the patients respond to immunotherapies, and the identification of this antigen by a candidate approach has revolutionized the diagnosis and treatment of this subtype of myasthenia (11). In many other conditions, however, no suitable candidate antigens have yet been proposed, limiting the diagnosis and treatment of the disorders.
To develop a novel proteomics strategy for identifying cell membrane autoantigens, we used a model system involving antibodies from MuSK antibody-positive patients and from MuSK antibody-negative subjects. We first allowed the antibodies to bind to their target(s) on the intact cell surface, rather than after extraction and denaturation in detergents, so that the antibodies could recognize fully conformational epitopes. The cells, with antibodies already bound, were then solubilized, and the ready formed immune complexes were isolated and either visualized by SDS-PAGE and immunoblotting or identified by mass spectrometry. Although we show the current results as a “proof of principle,” the “conformational membrane antigen isolation and identification” (CMAII) technique could easily be adapted for use in studies of other diseases.
EXPERIMENTAL PROCEDURES
Human Samples
Stored sera or plasma samples were used as sources of IgG antibodies. Three myasthenia gravis (MG) samples were MuSK antibody-positive, and one additional MG sample was MuSK antibody-negative. MuSK antibody positivity was determined by radioimmunoprecipitation of 125I-MuSK (RSR Ltd., Cardiff, UK). The antibody titers are typically between 1 and 200 nm (12). Two plasmas and 13 serum samples from healthy individuals were used as controls. Samples were coded and stored at −20 °C until first used after which they were stored at +4 °C for further experiments to avoid repeated freeze/thaw cycles. All serological work was covered by local research ethics as applied at the time of sampling.
Immunocytochemistry with Flow Cytometry
TE671 rhabdomyosarcoma cells were grown as adherent monolayers in Dulbecco's modified Eagle's medium (DMEM; Sigma) with 10% FCS. When approaching confluence, the cells were detached by incubation in 0.025% trypsin, EDTA (Invitrogen) in PBS for 3 min at 37 °C and then washed in fresh PBS. To quantify the binding of the sera to the detached cells, cells were resuspended in DMEM with 10% FCS at a concentration of ∼300,000 cells/ml. 100 μl of patient or control plasma/serum, diluted 1:24 in DMEM with 10% FCS was mixed with 100 μl of cell suspension (30,000 cells) in each well of a round bottomed 96-microwell plate, resulting in a final serum dilution of 1:48. In negative control wells, 100 μl of DMEM with 10% FCS without human serum was added to the cell suspension. After incubation on ice for 1 h, plates were centrifuged at 1200 rpm at 4 °C, the supernatant was discarded, and the cell pellets were washed in ice-cold PBS. The washing step was performed three times. 100 μl of FITC-labeled anti-human IgG (Sigma catalog number F-9512) was added to each cell pellet at 1:32 dilution in PBS. After incubation for 30 min on ice, cells were washed three times as above, and the contents of each well was finally resuspended in 150 μl of 1% formaldehyde in PBS. Cells were then stored in the dark at 4 °C for a maximum of 48 h prior to measurement of the median cell fluorescence of each sample by flow cytometry (BD Biosciences FACSCalibur with CellQuest software). For each sample in a particular experiment a fixed number of “gated events” (usually 10,000) were acquired with the “gate” set to include single cells and exclude cell fragments and clumps of cells.
Biotinylation of the TE671 Cell Membrane
To enable us to demonstrate the presence of membrane proteins after cell solubilization, the cell surface was biotinylated so that the biotinylated membrane proteins could be identified using HRP-conjugated streptavidin. Washed detached cells from one to three 175-cm2 tissue culture flasks were incubated in 1 ml of a 0.5 mg/ml solution of sulfo-NHS-biotin (Pierce) in PBS on a slowly rotating mixer at room temperature for 0.5 h. The biotinylation reaction was quenched by adding 0.05 m Tris base, pH 8 followed by washing in PBS three times. As a standard for the amount of biotinylated protein, we exposed a 10 mg/ml solution of BSA in PBS for 30 min to 1 mg/ml sulfo-NHS-biotin. Unreacted biotin was removed by dialysis against PBS. The final concentration of BSA was measured as 10.3 mg/ml by the bicinchoninic acid (BCA) method (Pierce).
Immunoprecipitation from Extracted Cells
Initially we used a method whereby washed detached biotinylated cells derived from one 175-cm2 flask were first solubilized by incubation on ice in 1 ml of 1% Triton buffer (1% Triton X-100 in 150 mm NaCl, 0.05 m Tris base, pH 8, 0.002 m EDTA, pH 8, 0.001 m PMSF, 10 μl/ml Sigma protease inhibitor mixture, 10 μl/ml Sigma phosphatase inhibitor mixture, 2.5 μl/ml Merck Benzonase) for 0.5 h with intermittent gentle mixing. After centrifuging the samples at 13,000 rpm (10,000 × g) for 15 min to separate out insoluble nuclei and larger intracellular organelles, the solubilized cellular proteins, including cell membrane-derived proteins, were recovered in the supernatant that was then incubated for 1 h with 20 μl of human plasma or serum before incubation with protein G-Sepharose (Sigma), washing, and elution (see below).
Subsequently we changed to a new method whereby washed detached cells, either unbiotinylated or biotinylated, derived from one to three 175-cm2 flasks were incubated in 1 ml of DMEM with 10% FCS containing 50 μl of human plasma or serum (1:20 dilution) on a rotating mixer at room temperature for 1 h. Cells were then washed three times in PBS and solubilized by incubation on ice in 1 ml of 1% Triton buffer for 0.5 h with intermittent gentle mixing. After centrifuging the samples for 15 min, the solubilized cellular proteins, including cell membrane-derived proteins (biotinylated or non-biotinylated), were recovered in the supernatant. Supernatants were left to stand overnight at 4 °C followed by further centrifugation at 13,000 rpm (10,000 × g) to remove poorly soluble proteins that had come out of solution. Final cell extracts were incubated with 50 μl of protein G-Sepharose for 3 h at 4 °C and centrifuged briefly at low speed to collect a pellet of protein G-Sepharose with bound immune complexes. The pellet was washed five times in 1 ml of 1% Triton buffer, and immunoprecipitated proteins were eluted from the protein G beads either by heating in 60 μl of a 1:1 mixture of SDS/2-mercaptoethanol loading buffer (4% SDS, 100 mm Tris base, pH 6.8, 20% glycerol, 0.2% bromophenol blue, 5% 2-mercaptoethanol) and 1% Triton buffer or, if the sample was to be used for mass spectrometry, in 60 μl of 0.1 m glycine-HCl, pH 2.1 followed by neutralization with 6 μl of 1 m Tris base, pH 11.
Visualization of Immunoprecipitated Proteins by SDS-PAGE and Western Blotting
Usually 15 μl (one-quarter) of each eluted immunoprecipitation product dissolved in a 1:1 mixture of SDS/2-mercaptoethanol loading buffer and 1% Triton buffer was loaded onto a 4–12% bis-Tris minigel (Invitrogen). Electrophoresis was performed using an Invitrogen XCell Surelock Mini-Cell and NuPAGE MES SDS running buffer according to the manufacturer's instructions (200 V for 35 min). To visualize protein in the gel, silver staining was performed according to the kit manufacturer's instructions (Bio-Rad). Alternatively to exploit the biotinylation of any immunoprecipitated cell surface proteins, electroblotting of the unstained gel onto nitrocellulose filter paper was carried out using an Invitrogen XCell II Blot Module and NuPAGE transfer buffer (30 V for 1 h). Nitrocellulose filters were blocked in 5% skimmed milk in PBS with 0.1% Tween 20 (PBS Tween) for 20 min at room temperature or overnight at 4 °C and washed in PBS Tween. To immunostain bands of biotinylated proteins, filters were incubated in HRP-conjugated streptavidin (Dako catalog number P0397) diluted 1:10,000 in PBS Tween for 45 min at room temperature, washed in PBS Tween, and stained with 3,3-diaminobenzidine tetrahydrochloride (DAB; Sigma) color reagent (0.5 mg/ml DAB in PBS with 0.03% hydrogen peroxide) for between 5 and 15 min. To immunostain specifically for MuSK, the filter was incubated in goat polyclonal anti-MuSK extracellular domain antibody (R&D Systems catalog number AF562) diluted 1:200 in PBS Tween for 45 min at room temperature, washed in PBS Tween, and incubated in HRP-conjugated anti-goat immunoglobulins secondary antibody (Dako catalog number P0449) diluted 1:1000 in PBS Tween for 30 min at room temperature before DAB color development.
Digestion and Preparation of Immunoprecipitation Products Prior to Liquid Chromatography and Mass Spectrometry
Immunoprecipitation products, eluted in glycine-HCl and neutralized as described above, were lyophilized in a vacuum centrifuge. To increase the quantity of material for analysis, typically two immunoprecipitates (each obtained from three 175-cm2 flasks of cells) were combined for lyophilization in the same vial at this stage so that the final combined immunoprecipitate for each patient or control was derived from six 175-cm2 flasks of cells. Subsequently each sample was redissolved in 40 μl of digestion buffer (6 m urea, 0.05% SDS, 200 mm Tris-HCl, pH 8.1) and incubated in reducing agent tris(2-carboxyethyl)phosphine (Pierce) (final concentration, 5 mm) for 45 min at 37 °C. Each sample was alkylated in 1 mm iodoacetamide for 1 h at room temperature in the dark, and the iodoacetamide was then inactivated by 5 mm dithiothreitol for 5 min. Each sample was diluted 1:5 in water, and having checked that the pH was 7–8, digestion with 2 μg of trypsin (Sigma proteomics grade) was carried out overnight at 37 °C. The following day, to remove excess Tris-HCl, SDS, and urea prior to application of the sample for nanoflow HPLC and to enable the vacuum concentration of the digest, each sample was applied to a strong cation exchange (SCX) column (Applied Biosystems, Warrington, UK), and a volatile eluent system (ammonium acetate/acetic acid) was used to wash and recover the samples (adapted from Ref. 13). In detail, each sample was acidified to pH 4 and diluted by the addition of 600 μl of solvent A (100 mm acetic acid, 2% acetonitrile, 8.54 mm ammonium acetate in water, pH 3–4) and 50 μl of 1 m acetic acid. A 300-μl SCX column was washed in solvent A, and the sample was then loaded onto the column, which was washed again in solvent A. Sequential elution from the column was carried out with 1 ml of solvent B (5.7 mm acetic acid, 2% acetonitrile, 100 mm ammonium acetate in water, pH 7–8) and then with 1 ml of solvent C (5.7 mm acetic acid, 2% acetonitrile, 600 mm ammonium acetate in water, pH 7–8).
Analysis of Immunoprecipitation Products by Liquid Chromatography and Mass Spectrometry
Following digestion and ion exchange purification, immunoprecipitation products eluted in solvent B or solvent C were lyophilized in a vacuum centrifuge. Each sample was redissolved in 0.5 μl of acetonitrile and then in 1 μl of 1% TFA, and then a further 8.5 μl of water were added to give a final solution composition of 5% acetonitrile, 0.1% TFA. The sample was sonicated and drawn up into a reversed phase HPLC machine (Agilent 1100 Nanoflow HPLC system using an 8-μl sample loop). The sample was first loaded onto a trapping column (ZORBAX 300SB-C18, 300 μm × 5 mm, Agilent) via an isocratic pump at 6 μl/min. The trapping column was then connected to the Agilent 1100 nanoflow quaternary pump via a microswitching valve, and the sample was collected from the trapping column at a flow rate of 300 nl/min by an increasing concentration of acetonitrile-containing solvent (90% acetonitrile, 0.085% TFA, water) with solvent concentration increasing by 0.5%/min and separated via a ZORBAX 300SB-C18 75-μm × 150-mm reversed phase column. Fractions were co-spotted with matrix (1 mg/ml α-cyano-4-hydroxycinnamic acid in 76% isopropanol, 17% acetone, 7% water) onto a 386-position AnchorChip target (anchor with 600-μm-diameter positions; Bruker Daltonics) by a PROTEINEER fc spotting device (Bruker Daltonics). Spotting was performed for 30 s per position. A peptide standard mixture was spotted manually on the designated standard positions for external calibration. Liquid chromatography was controlled via the software HyStar (version 3.0, Bruker Daltonics) and the spotter by separate dedicated software (PROTEINEER fc, Bruker Daltonics).
MALDI mass spectrometry was performed using a Bruker Daltonics Ultraflex TOF/TOF machine. Mass spectra acquisition was controlled via the software WARP-LC (version 1.1, Bruker Daltonics) according to the following work flow. First, MS spectra were acquired from all fractions. Subsequently all compounds were subjected to MS/MS using the Potential LIFT technique. 1000 shots were acquired per MS/MS spectrum. Spectra were annotated in an automated procedure using a method defined in the software flexAnalysis using base-line subtraction and smoothing (Savitzky-Golay, width 0.2 m/z, four cycles). MS/MS spectra were matched to human proteins using the International Protein Index (IPI) database (human proteins, IPI human v3.34, 67,764 sequences) in a batch search using the search engine Mascot (version 2.1.0, Matrix Science, London, UK) via an in-house license. Search parameters were as follows: cleavage by trypsin, one missed cleavage site allowed, carbamidomethylcysteine as fixed modification, and oxidized methionine as optional modification. The mass accuracy parameters were routinely set to a mass tolerance of 75 ppm for precursors and 0.7 Da for fragment ions, although, in most cases, the precursor accuracy was better than 50 ppm, and the fragment ion accuracy was better than ±0.2 Da. Default significance criteria (≥95% confidence score as calculated by WARP-LC) were used for accepting hits. The protein result lists were generated based on the WARP-LC evaluation of Mascot hits and displayed with the WARP-LC protein browser tool. All matched MS/MS spectra were also visually inspected to assess their quality. Proteins were only included in the tables of identified proteins if they contained two or more matched peptides.
RESULTS
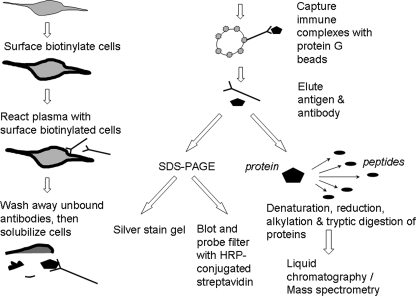
To establish this method, we used a model system involving the TE671 cell line and MuSK antibodies that are known to bind to MuSK on the TE671 cells. Fig. 1 illustrates the immunocapture and proteomics steps used.
Fig. 1.
Depiction of process of immunoprecipitation of cell surface protein and either subsequent visualization of immunoprecipitation products after SDS-PAGE and Western blotting or preparation and analysis of products by mass spectrometry. The first step of the CMAII strategy is to demonstrate by indirect immunofluorescence the presence of plasma antibodies binding to the outside of a cell line; this is measured quantitatively by an analytical flow cytometer. The second step (depicted) is to immunoprecipitate and visualize any cell surface antigen(s) from the cell line (surface-labeled by biotin) using individual plasmas selected from the first step. The third step (depicted) is the preparation and mass spectrometry analysis of patient and control immunoprecipitates to identify the cell surface antigen(s) and to assess other proteins present in the immunoprecipitates.
Step 1: Immunofluorescence with Flow Cytometry to Identify Patients with Antibodies to the Cell Surface
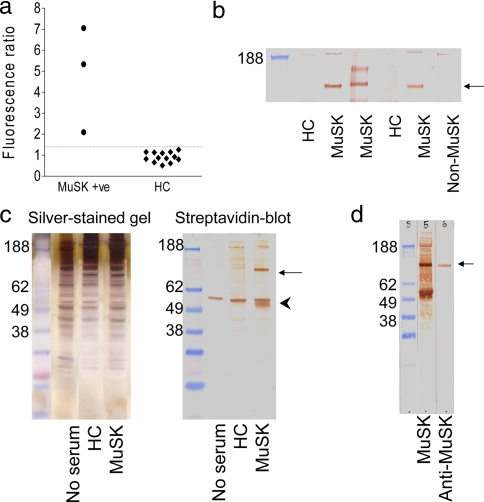
The three test samples from MG patients with MuSK antibodies immunoprecipitated 10–30 nmol of 125I-MuSK/liter of serum (as in Ref. 12). We first confirmed that they also bound selectively to the TE671 muscle cell line. Binding was compared with that of samples that did not contain MuSK antibodies (from healthy controls). Following incubation of the TE671 cells in 1:48 diluted plasma or serum, IgG binding to the cells was measured by flow cytometry after the application of a FITC-labeled anti-human IgG secondary antibody. The three MuSK-MG patients' sera bound to the cells at levels higher than the mean + 2 S.D. of the healthy control sera (Fig. 2a). This preliminary step demonstrated that an IgG antibody in the MuSK-positive plasmas bound to the TE671 cells and that these cells should provide a good starting point for the identification of the antigenic target(s).
Fig. 2.
Evidence for a specific membrane antigen and its immunoprecipitation by MuSK-antibody positive IgG. a, three MuSK-MG plasmas displayed binding to TE671 cells (median fluorescence intensity) greater than the mean + 2 S.D. of the binding of control sera (dotted line). b, incubation of a surface-biotinylated TE671 cell extract with any one of three MuSK-MG plasmas resulted in the immunoprecipitation of a 90-kDa band of biotinylated protein. This band was not present when immunoprecipitation was performed using two healthy control subject sera or using a MuSK-seronegative MG patient plasma. c, the immunoprecipitation technique was refined to reduce nonspecific binding by incubation of intact TE671 cells with plasma followed by washing before cell extraction. The silver-stained gel contained a large number of protein bands matched between the patient, healthy control, and blank immunoprecipitates. On the blot, which was probed with HRP-conjugated streptavidin, there was a strong band at 90 kDa (arrow), which was exclusive to the MuSK-MG immunoprecipitate, and a band around 55 kDa present in all lanes (arrowhead) that must have been nonspecifically captured. d, a goat anti-MuSK antibody bound to a 90-kDa protein band derived from unbiotinylated TE671 cells, which had been immunoprecipitated using a MuSK-MG plasma. This band was of the same molecular mass as the band derived from surface-biotinylated TE671 cells, which had been immunoprecipitated using the same MuSK-MG plasma. HC, healthy control; +ve, positive.
Step 2: Visualization of Antigenic Targets of Patient Antibodies following Immunoprecipitation
We next looked to see whether the test plasmas could immunoprecipitate biotin-labeled MuSK from the cells. We first quantified the amount of MuSK in the TE671 extracts using a competitive radioimmunoprecipitation with 125I-MuSK and precipitation with the MuSK-MG plasma; this gave a value of 14 ng/flask (results not shown). We biotinylated TE671 cells and initially solubilized the cells before exposing them to the patient and control serum samples, immunoprecipitating with protein G-Sepharose beads, and running on one-dimensional SDS-PAGE. To demonstrate polypeptides in the immunoprecipitate, the gels were blotted to nitrocellulose that was incubated in HRP-conjugated streptavidin. The results (Fig. 2b) show that three MuSK-MG samples immunoprecipitated a band of ∼90 kDa that corresponds to MuSK and that the two healthy controls and the sample from an MG patient without MuSK antibodies (non-MuSK) did not precipitate this band. However, these immunoprecipitates contained an excess of IgG that was not bound to MuSK but that bound to the protein G-Sepharose, making it very difficult to identify the antigen in subsequent mass spectrometry experiments. We therefore modified the approach by first incubating the cells with the plasmas and then washing thoroughly to remove unbound antibodies before extracting in detergent and immunoprecipitating with protein G-Sepharose. All proteins in the immunoprecipitates, including both cell surface and intracellularly derived polypeptides, were demonstrated by silver staining of the SDS-PAGE gels. The silver-stained lanes of immunoprecipitates from the MuSK-MG, the healthy control, and the “no serum” control all contained a large number of highly similar protein bands (Fig. 2c) with no apparent bands specific to the MuSK-MG immunoprecipitate. By contrast, when the nitrocellulose blot was probed with HRP-conjugated streptavidin, there were far fewer bands, only some of which were matched between the three immunoprecipitates (Fig. 2c). There was a strong band around 55 kDa present in all lanes that must have been nonspecifically captured by the protein G, and a band of around 90 kDa in the MuSK samples. Finally using another MuSK-MG plasma we repeated the immunoprecipitation using either biotinylated cells or unlabeled cells. After SDS-PAGE and Western blotting, the unlabeled cell immunoprecipitate was probed with a goat antibody to MuSK, which bound to the same 90-kDa band believed to be MuSK (Fig. 2d).
Estimation of Quantity of Immunoprecipitated Antigen
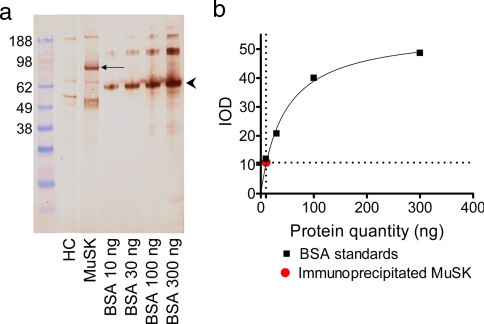
Having found that all three MuSK-MG plasmas bound to and immunoprecipitated a biotinylated 90-kDa protein, we wished to estimate the quantity of immunoprecipitated protein upon which mass spectrometry identification was to be attempted. For this, three 175-cm2 flasks of biotinylated TE671 cells were immunoprecipitated with 50 μl (diluted 20-fold in DMEM) of one MuSK-MG plasma (shown by radioimmunoprecipitation assay to bind to 1.24 ng of MuSK/μl of plasma; data not shown) and protein G beads as above. One-quarter of the immunoprecipitate was separated by SDS-PAGE, blotted, and probed with HRP-conjugated streptavidin along with known quantities of biotinylated BSA (Fig. 3a). Interpolation of the observed optical density of the 90-kDa band from the standard curve of known quantities of BSA, making the assumption that the degree of biotinylation of BSA and MuSK was comparable, gave an approximate estimate for the amount of protein in the 90-kDa band equivalent to 10 ng of biotinylated BSA (Fig. 3b). Because one-quarter of the immunoprecipitate had been loaded for electrophoresis, the entire immunoprecipitate derived from three 175-cm2 flasks of TE671 cells would therefore have contained around 40 ng of the protein. Therefore, in this experiment 50 μl of MuSK-MG plasma (with a maximum MuSK binding capacity of 62 ng) immunoprecipitated ∼40 ng of MuSK from three flasks of cells.
Fig. 3.
Estimation of quantity of immunoprecipitated MuSK. a, the 90-kDa protein immunoprecipitated (arrow) after incubation of TE671 cells with a MuSK-MG plasma was stained with HRP-conjugated streptavidin along with known quantities of biotinylated BSA stained by HRP-conjugated streptavidin that run at 67 kDa (arrowhead). b, the quantity of immunoprecipitated MuSK was estimated by comparison of the integrated optical density of the 90-kDa band with that of the 67-kDa band. HC, healthy control; IOD, integrated optical density.
Step 3: Identification of Immunoprecipitated Antigen by LC-MS
Having shown that we could immunoprecipitate MuSK, for mass spectrometry we used immunoprecipitates from unbiotinylated cells, eluting the bound proteins using glycine-HCl. We found it important to denature eluted proteins with a urea-containing buffer before proteolytic digestion as this seemed to greatly increase the yield of peptides detected by mass spectrometry. Buffer additives unsuitable for direct reversed phase liquid chromatography and mass spectrometry were removed by running the resulting peptide mixture through an SCX column. The peptide mixture was then fractionated by liquid chromatography, spotted onto a MALDI target plate, and subjected to MALDI-TOF/TOF mass spectrometry. For each experiment, we used 100 μl of one MuSK-MG patient plasma or of one control plasma and six 175-cm2 flasks of unbiotinylated TE671 cells. The immunoprecipitates were prepared in parallel and analyzed by mass spectrometry sequentially. Based upon the quantitative data above, the MuSK-MG patient immunoprecipitates initially should each have contained around 80 ng of MuSK.
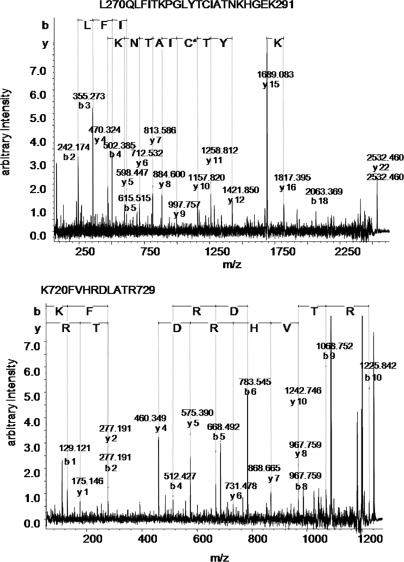
The experiment was performed three times using two different MuSK-MG plasmas each paired with a different healthy control serum and a third MuSK-MG plasma paired with a MuSK-negative MG plasma. On each occasion, MuSK was specifically and robustly detected as the only identifiable plasma membrane protein in each of the three patient samples (see Fig. 4 for examples of two MuSK peptides). Neither MuSK nor any other membrane protein was identified in the control samples. Cumulative Mowse scores derived from the match of observed peptides to MuSK peptides and hence indicating the confidence of identification of the presence of MuSK protein in each experiment ranged from 274 to 655 (Table I ). Thus we found that small amounts of samples (100 μl of plasma), with titers of antibody between 10 and 30 nm, that were shown by flow cytometry to bind to a relevant cell line by 2–7-fold the values of control sera (Fig. 2a), can immunoprecipitate the specific antigen from six 175-cm2 flasks of cells and that the antigen can be confidently detected by mass spectrometry with high Mowse scores.
Fig. 4.
Fragmentation ion spectra of two peptides (parent ion molecular masses, 2532 and 1226 Da) both identified as MuSK peptides
Table I . Mass spectrometry identification of MuSK in the immunoprecipitate using three different MuSK-MG samples.
| Mowse score | Number of peptides identified | Protein sequence coverage | |
|---|---|---|---|
| % | |||
| Experiment 1 | 274 | 9 | 11 |
| Experiment 2 | 655 | 16 | 23 |
| Experiment 3 | 295 | 8 | 11 |
However, disappointingly there were intracellular proteins detected. Table II shows a comparison of selected proteins found in MuSK-MG and control immunoprecipitates. Apart from MuSK itself, many of the proteins, including heat shock proteins, ribonucleoproteins, and tubulin, were present in both patient and control immunoprecipitates. They are intracellular proteins not known to be expressed extracellularly and are likely to have absorbed nonspecifically to the protein G beads. Full details of all proteins identified in each experiment are provided in supplemental Tables S1–S3.
Table II. Representative selection of proteins identified from immunoprecipitates.
Where multiple isoforms or subunits of the same protein were identified, just one isoform or subunit is listed here so that an impression of the spectrum of proteins identified can be obtained. The full comparison of proteins identified is presented in supplementalTable S4.
| Protein name | Accession number | Number of patient precipitates containing protein (total = 3) | Number of control precipitates containing protein (total = 3) | Number of paired patient and control precipitates both containing protein (total = 3) |
|---|---|---|---|---|
| Membrane proteins identified | ||||
| MuSK; muscle, skeletal, receptor tyrosine kinase | IPI00289243 | 3 | 0 | 0 |
| Intracellular proteins found in both patient and control immunoprecipitates | ||||
| ACTB; actin, cytoplasmic 1 | IPI00021439 | 1 | 1 | 0 |
| DDX1; ATP-dependent RNA helicase DDX1 | IPI00293655 | 2 | 1 | 1 |
| FUS; isoform short of RNA-binding protein FUS | IPI00221354 | 2 | 1 | 0 |
| HNRNPA1; isoform A1-B of heterogeneous nuclear ribonucleoprotein A1 | IPI00215965 | 3 | 3 | 3 |
| HNRNPA2B1; isoform B1 of heterogeneous nuclear ribonucleoproteins A2/B1 | IPI00396378 | 3 | 3 | 3 |
| HSPA9; stress-70 protein, mitochondrial precursor | IPI00007765 | 3 | 2 | 2 |
| IGHG4; IGHG4 protein | IPI00550640 | 3 | 1 | 1 |
| ILF2; interleukin enhancer-binding factor 2 | IPI00005198 | 2 | 2 | 1 |
| TUBB; tubulin β chain | IPI00011654 | 1 | 1 | 1 |
| Intracellular proteins found in either patient or control immunoprecipitates | ||||
| ACTB; actin, cytoplasmic 2 | IPI00848058 | 0 | 1 | 0 |
| DDX17; DEAD box polypeptide 17 isoform 1 | IPI00023785 | 0 | 1 | 0 |
| HNRPH1; heterogeneous nuclear ribonucleoprotein H | IPI00013881 | 0 | 1 | 0 |
| IGHG1; IGHG1 protein | IPI00829944 | 0 | 1 | 0 |
| ILF3; isoform 1 of interleukin enhancer-binding factor 3 | IPI00298788 | 0 | 1 | 0 |
| PRDX4; peroxiredoxin-4 | IPI00011937 | 0 | 1 | 0 |
| RPL35; 60 S ribosomal protein L35 | IPI00412607 | 1 | 0 | 0 |
| TUBB2C; tubulin β-2C chain | IPI00007752 | 1 | 0 | 0 |
| VIM; vimentin | IPI00418471 | 0 | 1 | 0 |
For experiments that aim at the identification of unknown antigens, a scoring system is required that highlights the true specific protein identification hit(s). We questioned whether we would have assigned MuSK as our candidate antigen if we had had no prior knowledge of its identity by looking at the frequency of each protein in each of the six immunoprecipitates. Between 11 and 54 proteins were identified in each LC-MALDI-TOF/TOF run. Many of them were identified either equally or almost equally in test and control samples. Very few proteins were identified in two patients or two controls and not in the other group. Only one cell membrane protein, MuSK, was identified, and this was found only in the three patient samples (full comparison data is shown in supplemental Table S4).
DISCUSSION
There is a pressing need to develop reliable techniques for the identification of target antigens for the better diagnosis and treatment of autoimmune diseases. We developed a three step CMAII process and showed how it could have been used for the identification of the antigenic target of a subtype of autoimmune myasthenia gravis. For this model, we demonstrated the presence of the membrane protein MuSK in immunoprecipitates from three patients with myasthenia and in none of the control samples. The identification of MuSK was robust because it was identified on the basis of 8–16 peptides (Table I) sequenced by mass spectrometry. Moreover MuSK was the only surface membrane protein detected in any of the immunoprecipitates and was only found in the MuSK antibody-positive samples; no other membrane protein would have been erroneously assigned as a potential antigen target. The methods that we used, binding and immunoprecipitation by small amounts of patients' serum or plasma, denaturation, digestion, and mass spectrometry, appear to be able to identify disease-specific membrane proteins. Furthermore as the cell line we used had not been genetically manipulated, our approach is applicable to cells or cell lines in which the target membrane protein is present at endogenous expression levels.
We used flow cytometry as the first step to identify the best sera and the most appropriate cell line to use. Like many pathogenic disease-specific antibodies, MuSK antibodies bind with high affinity to their target MuSK (12). It could be that for detection of unknown antigens, more antibody-containing samples would be required. These could be tested against a range of cell lines that express potentially relevant antigens, representative of the in vivo target tissue. For instance, one might choose to screen a large number of multiple sclerosis patient sera for antibodies binding the cell membranes of oligodendroglial cells (as for instance in Ref. 14) or diabetic patient sera for antibodies binding the cell membranes of pancreatic beta islet cells. The aim of this first step would therefore be to select cell-reactive sera and also the most appropriate cell line for the later and more labor-intensive immunoprecipitation steps.
Immunoprecipitation provided the means of physically isolating the antigenic target from the whole cells. Biotinylation of the cell membrane and visualizing the immunoprecipitate on an immunoblot using the highly sensitive HRP-streptavidin method allowed us to demonstrate that an immunoprecipitated cell surface protein was present before proceeding to attempt mass spectrometry identification. Conventional silver staining was not only less sensitive but did not distinguish between cell surface-derived and intracellular proteins (Fig. 2c).
Use of a urea-containing buffer to unfold and denature immunoprecipitated proteins improved the efficiency of proteolytic digestion by trypsin, increasing the yield of peptides detected by mass spectrometry. Buffer additives unsuitable for mass spectrometry were removed with an SCX column. The use of ammonium acetate in this step enabled sufficient elution from the SCX column and allowed us also to resuspend the dried eluate in a small volume (10 μl) as required for its injection in the reversed phase nano-liquid chromatography system. The use of liquid chromatography fractionation prior to mass spectrometry was necessary because the immunoprecipitation process nonspecifically captured many abundant cytoskeletal and other intracellular proteins such as tubulin and various heterogeneous nuclear ribonucleoproteins, and MuSK, although present in reasonable quantities with Mowse scores of between 274 and 655, was still only one of many proteins in the immunoprecipitate. Analysis of an unfractionated peptide mixture would have resulted in the presence of MuSK being obscured by the more abundant nonspecific proteins as we had found in earlier experiments.
A similar approach using immunoprecipitation with MG patient sera and TE671 cell extracts had previously been used to visualize a 110-kDa band subsequently shown to be MuSK by probing the band with specific anti-MuSK antibody (15). Our immunoprecipitation step possessed an important difference with the binding of patient antibody and target taking place on the surface of intact cells. Maintenance of the cells so that they were intact at the time of immune interaction minimized the nonspecific capture of pathologically irrelevant intracellular proteins and favored the capture of antigenic targets exposed on the exterior of the cell and hence with the potential to be bound by antibody under in vivo conditions.
Indeed comparison of the identified proteins in the patient and control immunoprecipitates revealed MuSK as the only protein present in all three patient samples and absent from all three control samples. Even if we had had no prior knowledge of MuSK as the autoantigen in myasthenia gravis, its differential presence and the fact that it was the only cell membrane protein identified would have strongly suggested it as a potential autoantigen. However, mass spectrometry analysis of the entire immunoprecipitate of both patient and control samples also revealed a large number of intracellular proteins that contaminated even the relatively clean immunoprecipitates with many of them being identified in both patient and control samples. It is striking how many of the proteins suggested as autoantigens in studies of various other autoimmune neurological, rheumatological, allergic, infectious, and malignant diseases (Table III) appear on this list, e.g. heterogeneous nuclear ribonucleoproteins in autoimmune hepatitis and HTLV-1-associated myelopathy, heat shock proteins in hepatitis C, and tubulin and actin in acute leukemias. In those studies, soluble extracts of tissue or cell lines were usually submitted to two-dimensional electrophoresis, and separated proteins were blotted onto membranes and probed with patient sera. One suspects that the identification of these proteins may be an artifact arising from their relative abundance in cell extracts, the ability of denatured proteins to bind to antibodies nonspecifically, and possibly the relatively high concentration of protein in the spots that allowed binding of otherwise low affinity antibodies with questionable disease relevance. Hence their identification occurred not because the proteomics techniques were executed poorly but because the immune interaction step was carried out under inappropriate conditions. Indeed using this same technique, intracellular proteins including α-enolase, F-actin capping protein, calreticulin, heterogeneous nuclear ribonucleoproteins L and K, and annexin II were identified as “autoantigens” in a study that examined only serum antibodies of healthy control subjects (16).
Table III . Proteins identified in both patient and control samples that had been previously suggested as autoantigens in other studies.
These proteins were reported in the literature as potential disease-associated antigens. They had previously been identified using approaches in which cell extracts, first separated by two-dimensional electrophoresis and blotted, were probed with patient serum, and immunoreactive spots were excised for mass spectrometry. None of them are membrane proteins. hnRNP, heterogeneous nuclear ribonucleoprotein.
| Possible autoantigen | Number in patient precipitates (total = 3) | Number in control precipitates (total = 3) | Disease | Ref. |
|---|---|---|---|---|
| Ribonucleoprotein hnRNP-A2/B1 | 3 | 3 | Autoimmune hepatitis | Huguet et al. (8) |
| Ribonucleoprotein hnRNP-A1 | 3 | 3 | HTLV-1-associated myelopathy | Levin et al. (17) |
| Heat shock protein HSP70 | 3 | 2 | Hepatitis C | Fukuda et al. (18) |
| Tubulin | 1 | 1 | Relapsing polychondritis | Tanaka et al. (19) |
| Tubulin | 1 | 1 | Allergic rhinitis | Nakamura et al. (20) |
| Tubulin | 1 | 1 | Acute leukemias | Cui et al. (7) |
| Actin | 1 | 1 | Hepatitis C | Fukuda et al. (18) |
| Actin | 1 | 1 | Rheumatoid arthritis | Matsuo et al. (21) |
| Actin | 1 | 1 | Acute leukemias | Cui et al. (7) |
Our CMAII process is capable of identifying pathologically relevant antigens because its immune interaction step occurs under conditions in which the antigens still maintain their native structure and are located on the cell membrane as they would be in vivo. The steps that we illustrate show that one could pursue such an approach with any patient sera that demonstrate significant binding to a relevant cell line by flow cytometry because this would likely indicate the presence of an amount of antigen similar to that found here. There is a growing appreciation of the role of autoantibodies in neurological (9) and other diseases, and this non-candidate approach should provide a powerful and potentially rapid means of identifying previously unknown antigenic targets leading to improved diagnosis of immunotherapy-responsive conditions. It could equally be used to identify the targets of naturally occurring or experimentally induced antitumor immune responses.
Footnotes
* This work was supported by the Oxford Radcliffe Hospitals National Health System Trust (to E. L.), the Wellcome Trust (Oxford Ion Channel Integrative Physiology (OXION) Initiative) (to M. D.), the Myasthenia Gravis Association, and the Muscular Dystrophy Campaign of Great Britain.
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
1 The abbreviations used are:
- MuSK
- muscle-specific tyrosine receptor kinase
- CMAII
- conformational membrane antigen isolation and identification
- MG
- myasthenia gravis
- DMEM
- Dulbecco's modified Eagle's medium
- HRP
- horseradish peroxidase
- NHS
- N-hydroxysuccinimide
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl) propane-1,3-diol
- DAB
- 3,3-diaminobenzidine tetrahydrochloride
- SCX
- strong cation exchange
- IPI
- International Protein Index
- Mowse
- molecular weight search
- TFA
- trifluoroacetic acid
REFERENCES
- 1.Ch'ng C. L., Jones M. K., Kingham J. G. ( 2007) Celiac disease and autoimmune thyroid disease. Clin. Med. Res. 5, 184– 192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Targan S. R., Karp L. C. ( 2007) Inflammatory bowel disease diagnosis, evaluation and classification: state-of-the art approach. Curr. Opin. Gastroenterol. 23, 390– 394 [DOI] [PubMed] [Google Scholar]
- 3.Kasperkiewicz M., Zillikens D. ( 2007) The pathophysiology of bullous pemphigoid. Clin. Rev. Allergy Immunol. 33, 67– 77 [DOI] [PubMed] [Google Scholar]
- 4.Buckley C., Vincent A. ( 2005) Autoimmune channelopathies. Nat. Clin. Pract. Neurol. 1, 22– 33 [DOI] [PubMed] [Google Scholar]
- 5.Vincent A. ( 2002) Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2, 797– 804 [DOI] [PubMed] [Google Scholar]
- 6.Dalmau J., Rosenfeld M. R. ( 2008) Paraneoplastic syndromes of the CNS. Lancet Neurol. 7, 327– 340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J. W., Li W. H., Wang J., Li A. L., Li H. Y., Wang H. X., He K., Li W., Kang L. H., Yu M., Shen B. F., Wang G. J., Zhang X. M. ( 2005) Proteomics-based identification of human acute leukemia antigens that induce humoral immune response. Mol. Cell. Proteomics 4, 1718– 1724 [DOI] [PubMed] [Google Scholar]
- 8.Huguet S., Labas V., Duclos-Vallee J. C., Bruneel A., Vinh J., Samuel D., Johanet C., Ballot E. ( 2004) Heterogeneous nuclear ribonucleoprotein A2/B1 identified as an autoantigen in autoimmune hepatitis by proteome analysis. Proteomics 4, 1341– 1345 [DOI] [PubMed] [Google Scholar]
- 9.Vincent A. ( 2008) Autoimmune channelopathies: John Newsom-Davis's work and legacy. A summary of the Newsom-Davis Memorial Lecture 2008. J. Neuroimmunol. 201–202, 245– 249 [DOI] [PubMed] [Google Scholar]
- 10.Hoch W., McConville J., Helms S., Newsom-Davis J., Melms A., Vincent A. ( 2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 7, 365– 368 [DOI] [PubMed] [Google Scholar]
- 11.Vincent A., Leite M. I. ( 2005) Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr. Opin. Neurol. 18, 519– 525 [DOI] [PubMed] [Google Scholar]
- 12.McConville J., Farrugia M. E., Beeson D., Kishore U., Metcalfe R., Newsom-Davis J., Vincent A. ( 2004) Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann. Neurol. 55, 580– 584 [DOI] [PubMed] [Google Scholar]
- 13.Winnik W. M. ( 2005) Continuous pH/salt gradient and peptide score for strong cation exchange chromatography in 2D-nano-LC/MS/MS peptide identification for proteomics. Anal. Chem. 77, 4991– 4998 [DOI] [PubMed] [Google Scholar]
- 14.Lily O., Palace J., Vincent A. ( 2004) Serum autoantibodies to cell surface determinants in multiple sclerosis. Brain 127, 269– 279 [DOI] [PubMed] [Google Scholar]
- 15.Scuderi F., Marino M., Colonna L., Mannella F., Evoli A., Provenzano C., Bartoccioni E. ( 2002) Anti-p110 autoantibodies identify a subtype of “seronegative” myasthenia gravis with prominent oculobulbar involvement. Lab. Invest. 82, 1139– 1146 [DOI] [PubMed] [Google Scholar]
- 16.Li W. H., Zhao J., Li H. Y., Liu H., Li A. L., Wang H. X., Wang J., He K., Liang B., Yu M., Shen B. F., Zhang X. M. ( 2006) Proteomics-based identification of autoantibodies in the sera of healthy Chinese individuals from Beijing. Proteomics 6, 4781– 4789 [DOI] [PubMed] [Google Scholar]
- 17.Levin M. C., Lee S. M., Kalume F., Morcos Y., Dohan F. C., Jr, Hasty K. A., Callaway J. C., Zunt J., Desiderio D., Stuart J. M. ( 2002) Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat. Med. 8, 509– 513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda Y., Yotsuyanagi H., Ooka S., Sekine T., Koike J., Takano T., Suzuki M., Itoh F., Nishioka K., Kato T. ( 2004) Identification of a new autoantibody in patients with chronic hepatitis. Hum. Immunol. 65, 1530– 1538 [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y., Nakamura M., Matsui T., Iizuka N., Kondo H., Tohma S., Masuko K., Yudoh K., Nakamura H., Nishioka K., Koizuka I., Kato T. ( 2006) Proteomic surveillance of autoantigens in relapsing polychondritis. Microbiol. Immunol. 50, 117– 126 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura M., Tsutsumi K., Ooka S., Sekine T., Koizuka I., Nishioka K., Kato T. ( 2004) Identification of beta-tubulin isoform V as an autoantigen in allergic rhinitis by a proteomic approach. Microbiol. Immunol. 48, 427– 434 [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K., Xiang Y., Nakamura H., Masuko K., Yudoh K., Noyori K., Nishioka K., Saito T., Kato T. ( 2006) Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach. Arthritis Res. Ther. 8, R175. [DOI] [PMC free article] [PubMed] [Google Scholar]