Abstract
TGF-β has been implicated in the proliferation and differentiation of chondrocytes and osteoblasts. However, the in vivo function of TGF-β in skeletal development is unclear. In this study, we investigated the role of TGF-β signaling in growth plate development by creating mice with a conditional knockout of the TGF-β type I receptor ALK5 (ALK5CKOCKO) in skeletal progenitor cells using Dermo1-Cre mice. ALK5CKO mice had short and wide long bones, reduced bone collars, and trabecular bones. In ALK5CKO growth plates, chondrocytes proliferated and differentiated, but ectopic cartilaginous tissues protruded into the perichondrium. In normal growth plates, ALK5 protein was strongly expressed in perichondrial progenitor cells for osteoblasts, and in a thin chondrocyte layer located adjacent to the perichondrium in the peripheral cartilage. ALK5CKO growth plates had an abnormally thin perichondrial cell layer and reduced proliferation and differentiation of osteoblasts. These defects in the perichondrium likely caused the short bones and ectopic cartilaginous protrusions. Using tamoxifen-inducible Cre-ER™-mediated ALK5-deficient primary calvarial cell cultures, we found that TGF-β signaling promoted osteoprogenitor proliferation, early differentiation, and commitment to the osteoblastic lineage through the selective MAPKs and Smad2/3 pathways. These results demonstrate the important roles of TGF-β signaling in perichondrium formation and differentiation, as well as in growth plate integrity during skeletal development.
Keywords: ALK5 (TGF-β type I receptor), skeletal progenitor cells, osteoblasts, chondrocytes, growth plate, perichondrium, conditional knockout mice
Introduction
Endochondral and intramembranous ossifications are two major processes that control skeletogenesis. In endochondral ossification, precursor mesenchymal cells condense in the areas destined to become bone and differentiate into chondrocytes. Differentiated chondrocytes proliferate and undergo further differentiation processes to mature hypertrophic chondrocytes that subsequently are replaced by bone cells (Kronenberg, 2003). Mesenchymal cells at the periphery of the condensation give rise to the perichondrium, which differentiates into osteoblasts and forms a bone collar. The perichondrium consists of the outer fibrous layer and inner osteoprogenitor cell layer (Kronenberg, 2003). In intramembranous ossification, condensed mesenchymal cells directly differentiate into osteoblasts and form bone (Nakashima and de Crombrugghe, 2003) .
Transforming growth factor-β (TGF-β) and its related factors, including bone morphogenetic proteins (BMPs) and activins, regulate diverse cellular processes, such as proliferation, differentiation, apoptosis, and extracellular matrix formation during embryogenesis. TGF-β signaling is mediated by two types of transmembrane serine/threonine kinase receptors, type I (ALK5) and type II receptors, which form a heteromeric complex. In this signaling complex, following TGF-β binding to the type II receptor (TGFβR2), the type II receptor phosphorylates and activates ALK5. Activated ALK5 then induces signaling cascades through Smad-dependent and Smad-independent pathways. In the Smad-dependent pathway, the TGF-β-receptor complex activates Smad2/3, whereas the BMP-receptor complex activates Smad1/5/8 (Feng and Derynck, 2005).
TGF-β signaling has been implicated in cartilage and bone formation in a number of studies. However, this conclusion is controversial, in part because of multiple signaling cascades and redundant expression of three TGF-β isoforms (TGF-β1, -β2 and -β3). Genetic manipulations of TGF-β signaling molecules in mice have clarified some of their roles in skeletogenesis. However, since gene targeting of TGF-β signaling molecules has resulted in variable phenotypes, ranging from early embryonic lethality to normal phenotype at birth, the precise role of TGF-β signaling in skeletal development is not yet fully understood. For example, targeted germline deletions of Tgfbr2 and Alk5 result in early embryonic lethality because of defects in hematopoiesis and vasculogenesis before skeletal elements are formed (Larsson et al., 2001; Oshima et al., 1996). In contrast, Col2a1-Cre-mediated conditional inactivation of Tgfbr2 in chondrocytes does not show obvious defects in long bone formation (Baffi et al., 2004), while Prx1-Cre-mediated Tgfbr2 deletion in the limb mesenchyme results in short limbs and fusion of the joints of the phalanges (Seo and Serra, 2007; Spagnoli et al., 2007). A genetic deletion of Smad3, a known substrate for ALK5 and an important mediator of the canonical Smad-dependent pathway, displays normal phenotype at birth (Borton et al., 2001), suggesting that the TGF-β-Smad2/3 signaling may not be required for limb development. On the other hand, mice deficient in TGF-β2 suffer perinatal lethality with abnormal skeletal formation, such as reduced cranial ossification, bifurcation of the sternum, irregular and fused ribs, and shortened limbs (Sanford et al., 1997), suggesting that TGF-β signaling is indispensable for skeletogenesis.
ALK5 is one of the most prominent receptors for TGF-β superfamily members in skeletal tissues. Recent studies suggest that ALK5 may also serve as a receptor for some other TGF-β superfamily proteins, such as myostatin (GDF8) and GDF11 (Andersson et al., 2006; Rebbapragada et al., 2003; Tsuchida et al., 2008; Wu et al., 2003). Deficiency of ALK5 should eliminate Smad-dependent and Smad-independent signaling for all TGF-β isoforms and other potential TGF-β superfamily proteins. In the present study, conditional knockout mice have been created in which ALK5 was inactivated in skeletal progenitor cells by Dermo1-Cre expression in mice and tamoxifen-inducible Cre-ER™ expression in vitro. This allowed us to circumvent the early embryonic lethality observed in a germline of ALK5-null mice (Larsson et al., 2001) in order to investigate the role of ALK5 in skeletogenesis. We demonstrated that ALK5 is expressed in the skeletal primordium and that Dermo1-Cre-mediated ALK5 conditional knockout (ALK5CKO) results in bone growth retardation, defects in perichondrium, and abnormal cartilaginous protrusions. Our studies indicate that ALK5 regulates the commitment of progenitor cells to the osteoblastic lineage, followed by osteoblast proliferation and differentiation through selective downstream pathways.
Materials and methods
Mouse lines
ALK5-floxed (Alk5flox/flox) mice and Dermo1-Cre knock-in mice were kindly provided by Dr. Stefan Karlsson (Department of Molecular Medicine and Gene Therapy. Institute of Laboratory Medicine, Lund University Hospital, Lund, Sweden) (Larsson et al., 2003) and Dr. David M. Ornitz (Department of Molecular Biology and Pharmacology, Washington University Medical School, St. Louis, MI) (Yu et al., 2003), respectively. Skeletal progenitor-specific ALK5 conditional knockout ALK5CKO (Alk5flox/flox; Dermo1Cre/wt) mice were created by crossing Alk5flox/flox homozygous females with Alk5flox/wt; Dermo1Cre/wt double heterozygous males. ROSA26 Cre-reporter mice were created by Dr. Philippe Soriano’s laboratory (Fred Hutchinson Cancer Research Center, Seattle, Washington) (Soriano, 1999) and obtained from Jackson Labs (Bar Harbor, ME). The ROSA26 promoter confers ubiquitous expression of LacZ. Rosa26 mice were crossed with Dermo1-Cre mice to create Dermo1Cre/WT;Rosa26 mice to trace Dermo1 expression. A Cre-ER™ mouse line (CAGG-Cre-ER™) created by Drs. Hayashi and McMahon (Harvard University, Cambridge, MA) (Hayashi and McMahon, 2002) was obtained from Jackson Labs. In Cre-ER™ mice, Cre recombinase is fused to the modified mouse estrogen receptor ER™ under the control of the chicken β-actin promoter and cytomegalovirus (CMV) enhancer, and Cre activity can be induced by tamoxifen. These two lines were crossed and double homozygous (Rosa26; Cre-ER™) mice generated. The double homozygous mice were crossed with ALK5-floxed mice to generate tamoxifen-inducible ALK5-deficient mice (ALK5-Cre-ER, Alk5flox/flox; Rosa26; Cre-ER™). CreER-negative Alk5flox/flox and wild type mice were used to prepare control calvarial cells. The animal protocol approved by the NIDCR ACU Committee was used for maintaining and handling mice, and all animals were housed in an American Association for the Accreditation of Laboratory Animal Care-accredited mouse facility.
Reagents and chemicals
TGF-β2 and BMP-2 were obtained from R&D systems (Minneapolis, MN). SB203580, U0126 and SP600125 were purchased from Tocris Bioscience (Ellisville, MO). SIS3 was purchased from EMD Bioscience (La Jolla, CA). The enhanced chemiluminescent (ECL) blotting detection reagents were purchased from Amersham Biosciences Corp. (Piscataway, NJ). Tamoxifen and Oil Red O were purchased from Sigma, and Nile Red from Invitrogen.
Skeletal preparation
Embryos were dissected, fixed in 100% ethanol overnight, and then stained with Alcian blue, followed by Alizarin Red S, according to standard protocols (McLeod, 1980).
Metatarsal explant culture
Metatarsal rudiments were cultured as previously described (Haaijman et al., 1999). Metatarsal rudiments were dissected from embryos at E15.5 and cultured in α-minimum essential medium without nucleosides (Invitrogen) supplemented with 0.05 mg/mL ascorbic acid (Sigma), 0.05 mg/mL gentamycin (Invitrogen), 1 mM β-glycerophosphate (Sigma), and 0.2% FBS in a humidified atmosphere of 5% CO2 in air at 37 °C. One day after starting the culture, the rudiments were incubated in 400 μL of the same medium containing 10 ng/mL of TGF-β2 (R&D), or without TGF-β2, for an additional 4 days. The explants were cultured with BrdU (bromodeoxyuridine; 10 μM) for 2.5 h at the fourth day of the culture. Stereomicroscopic photographs using Zeiss Stemi and NIH Image J software were used to measure the length of cultured explants that had been processed for histological examinations.
BrdU staining
Pregnant mice bearing E18.5 embryos were intraperitoneally injected with BrdU labeling reagent (10 μL/g body weight; Zymed Laboratories). The mice were euthanized for BrdU staining 2 h later. Metatarsal explants were cultured with BrdU (10 μM) for 2 h at day 5. The explants were fixed in paraformaldehyde at 4 °C overnight, embedded in paraffin, and cut into 5-μm sections. Incorporated BrdU was detected using a BrdU staining kit (Invitrogen).
Isolation of calvarial cells (Osteoblast-enriched cells)
Calvaria of newborn ALK5-CreER mice (Alk5flox/flox; Rosa26; Cre-ER™) and CreER-negative Alk5flox/flox and wild type mice were incubated six times for 10 min (for a total of 1 h) with 0.1% collagenase, type 1 (Worthington, Lakewood, NJ) and 0.2% dispase II (Roche Applied Science, Indianapolis, IN) in phosphate-buffered saline solution (PBS). The last two fractions were centrifuged at 1500 rpm for 5 min, resuspended in culture medium consisting of α-minimum essential medium (Invitrogen, Rockville, MD) with 10% fetal bovine serum (HyClone, Logan, UT), 100 U/mL of penicillin and 100 μg/mL of streptomycin. For cell proliferation assays, cells were seeded at a low density (2,500 cells/cm2). The next day, cells were treated with 1μM tamoxifen, and allowed to proliferate for an additional 3 days in the presence of TGF-β2 or inhibitors. For differentiation assays, cells were seeded at a higher density (25,000/cm2). Similarly to the proliferation assay, on the following day cells were treated with tamoxifen and differentiation was initiated by the addition of 50 μg/mL ascorbic acid and 5 mM β-glycerophosphate. To test the reactivity of the calvarial cells to TGF-β2 during differentiation, cells were briefly exposed to TGF-β2 (1 ng/mL) for 1 h just before cells were collected; the lysate was subjected to Western blot analysis using phospho-Smad2 specific antibody. Alkaline phosphatase staining was carried out using Fast Red Violet LB salt as the diazonium salt.
Alizarin Red S staining
Primary calvarial cells were fixed with 60% isopropanol and stained with 1% (w/v) Alizarin Red S (Sigma). After washing 5 times with PBS, cultures were photographed and calcium deposition was quantified by extracting the Alizarin Red S stain with 10% cetylpyridinium chloride (Sigma) and measuring the OD at 550 nm.
Nile Red and Oil Red O staining
Primary calvarial cells were fixed for 10 min with 4% paraformaldehyde in PBS. After washing with PBS, they were incubated for 5 min with either 0.1 μg/mL Nile Red in PBS or 0.3% Oil Red O. For quantifications of Nile Red positive areas, after 14 d culture, seven different fields (10x) per well were randomly chosen and photographed. Images were captured using Adobe Photoshop CS (Adobe Systems, San Jose, CA), a threshold was set to automatically compute the area positive for the staining, and the data were imported as a TIFF file into NIH Image J software (National Institutes of Health, Bethesda, MD). All experiments were repeated in triplicate.
Western blot analysis
Western blot analysis and cell extract preparation were carried out as previously described (Matsunobu et al., 2006). The antibodies used were as follows: mouse polyclonal anti-TGF-βRI (Abnova, Taiwan), rabbit polyclonal anti-MAPK, anti-phospho-MAPK (Thr202/Tyr204), anti-p38, anti-phospho-p38 MAP Kinase (Thr180/Tyr182), anti-SAPK/JNK, anti-phospho-SAPK/JNK (Thr183/Tyr185), anti-Smad2, rabbit monoclonal anti-phospho-Smad2 (Ser465/467)(Cell Signaling, Danvers, MA), and mouse anti-α-tubulin (Sigma, St. Louis, MO). Horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit IgG were obtained from US Biological (Swampscott, MA). α-tubulin was used as a loading control.
Immunohistochemistry and immunofluorescence
Tissue slides were deparaffinized using xylene and rehydrated through an alcohol gradient series to water. Antigens were retrieved by microwave treatment for 20 min in citrate buffer (pH 6.0). Endogenous peroxidase enzyme activity was blocked using 3% hydrogen peroxidase in methanol for 10 min at room temperature. The slides were washed in distilled water and in PBS, and then incubated overnight at 4°C with the primary antibodies: rabbit polyclonal anti-TGF-βRI 1:50 (Abcam, Cambridge, MA), rabbit polyclonal anti-Sp7/osterix 1:100 (Abcam), goat polyclonal anti-osteocalcin 1:100 (Biomedical Technologies, Stoughton, MA), rabbit polyclonal anti-osteopontin 1:50 (Ogbureke and Fisher, 2004), and mouse monoclonal anti-aggrecan (University of Iowa Hybridoma Bank). The incubation of the slides with primary antibodies was followed by chromogenic visualization with the SuperPicTure polymer detection kit (Invitrogen) or fluorescence visualization with the appropriate secondary antibodies.
Real-time quantitative RT-PCR
DNase-free RNA was prepared using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. After DNase treatment, RNA was reverse-transcribed to cDNA using the SuperscriptIII kit (Invitrogen) according to the manufacturer’s instructions. The cDNA was subjected to real-time PCR using SYBR Green PCR Master Mix (Bio-Rad) with DNA Engine Chromo 4 Real-time System (MJ Research, Waltham, MA). Primer sequences and PCR conditions are described in supplemental Table I. Gene expression was normalized to Ppia. Reactions were run in triplicate.
Results
ALK5 is expressed in developing limbs and calvaria
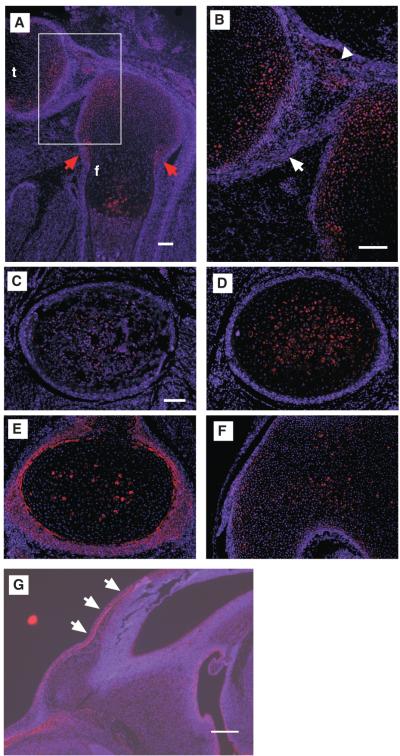
Immunostaining of ALK5 was carried out to examine the expression of ALK5 protein in normal developing limbs (Fig. 1). In E18.5 femurs, ALK5 expression was observed in resting and hypertrophic chondrocytes. ALK5 was also strongly expressed in a thin chondrocyte layer at the periphery of cartilage located adjacent to the perichondrium (Fig. 3F’, arrows, and Supplemental Fig. S3). Expression was also noted within the perichondrium with the highest level seen at the ossification groove of Ranvier (Fig. 1A, E, red arrows). The ALK5 expression in the perichondrium diminished toward the center of diaphyses where osteoblasts were differentiated (Figs. 1A, C). ALK5 expression was also observed in synovial tissues and ligaments (Figs. 1A, B, arrowhead and white arrow, respectively), and in E14.5 calvarial primordium (Fig. 1G.).
Fig. 1.
Expression patterns of ALK5 protein in normal E18.5 knee joint. (A) Sagittal section of E18.5 knee joint. The boxed area is enlarged in (B). (C-F) Transverse sections of E18.5 femur. Panels C, D, E, and F correspond to bone marrows, hypertrophic chondrocytes, proliferating chondrocytes, and resting chondrocytes (epichondyle), respectively. ALK5 protein was expressed in the perichondrium, synovium, ligaments, and resting and hypertrophic chondrocytes. f, femur; t, tibia. Red arrows, Ranvier’s ossification groove; arrow, posterior cruciate ligament; arrowhead, synovium. Bars, 100 μm. (G) Expression of ALK5 in normal E14.5 calvarial primordium. Arrows, frontal bone. Bar, 200 μm.
Fig. 3.
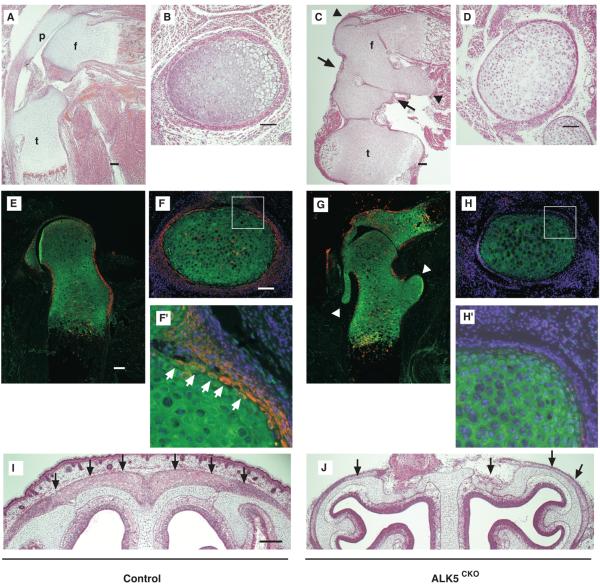
Thin perichondrium layer and ectopic cartilaginous protrusion in ALK5CKO growth plates. (A-D) Histological sagittal (A and C) and transverse sections (B and D) of E18.5 hindlimbs of control (A and B) and ALK5CKO embryos (C and D) stained with H-E. The transverse sections are at the level of the boundary between proliferative chondrocytes and hypertrophic chondrocytes in the distal femur. ALK5CKO mice exhibited partial knee joint fusion, ectopic cartilaginous protrusions and eccentric hypertrophic maturation of chondrocytes in tibiae with thin perichondrium. Arrows, knee joint; arrowheads, ectopic cartilaginous protrusions. f, femur; t, tibia; p, patella. (E-H) Double-immunostaining for ALK5 (red) and aggrecan (green) of E17.5 control (E) and ALK5CKO hip joints (G). Arrowheads, ectopic cartilaginous protrusions. (F, H) Double-immunostaining for ALK5 (red) and aggrecan (green) with DAPI nuclear staining of E18.5 control (F) and ALK5CKO femur (H). The boxed areas in (F) and (H) are enlarged in (F’) and (H’), respectively. ALK5 protein expression was diminished, especially in the perichondrium, and ectopic cartilaginous protrusions were formed. White arrows indicate ALK5-expressing chondrocytes located at the periphery of cartilage. Bar, 100 μm. (I, J) Coronal sections of E18.5 control (I) and ALK5CKO heads (J) stained with H-E. Calvaria of ALK5CKO mice were very thin. Arrows, calvaria. Bar, 200 μm.
ALK5 is required for skeletogenesis and body wall formation
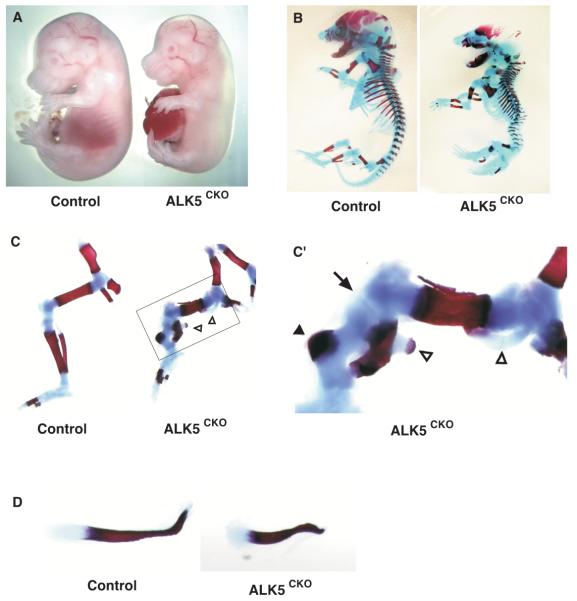
To determine the role of TGF-β signaling during skeletogenesis, Alk5flox/flox homozygous females were crossed with Alk5flox/wt; Dermo1Cre/wt double heterozygous males, creating skeletal progenitor-specific ALK5 conditional knockout ALK5CKO (Alk5flox/flox; Dermo1Cre/wt) mice. Dermo1-Cre expression starts as early as embryonic day (E) 9.5, and mesenchyme-specific expression was observed in the limb buds, craniofacial mesenchyme and body wall of E10.5 Dermo1Cre/wt;Rosa26 mice (Supplemental Fig. S1) (Soriano, 1999; Yu et al., 2003). Analysis of E17.5 hindlimbs of Dermo1Cre/wt;Rosa26 reporter mice revealed positive X-gal staining in the chondrocytes in growth plate cartilage, perichondrial cells, ligament cells, and synovial cells (Supplemental Fig. S2). Double-heterozygous mice, Alk5flox/wt;Dermo1Cre/wt, used as littermate controls in this study, showed no apparent defects or embryonic lethality, and these mice were viable and fertile. ALK5CKO embryos had hydramnion and most of them died shortly after birth, possibly because of respiratory distress due to severe midline fusion defects. ALK5CKO embryos developed dwarfism, characterized by shorter limbs, and most of the visceral organs such as the heart, liver, and intestine were herniated through a body wall defect and covered with a thin and transparent membrane (Fig. 2A). The defect in the body wall formation in ALK5CKO embryos became obvious from E12.5 and was conspicuously earlier than the limb abnormality during embryogenesis (data not shown). E15.5 ALK5CKO embryos also exhibited hypoplastic skull bases (Fig. 2A). Calvarial bone formation was severely defective in ALK5CKO mice, and the facial bones of mutant embryos were smaller than those of control embryos (Fig. 2A).
Fig. 2.
Skeletal abnormalities of ALK5CKO (Alk5flox/flox; Dermo1Cre/wt) mice. (A) Macroscopic lateral views of control (left) and ALK5CKO (right) embryos at E15.5. ALK5CKO embryos developed short-limbed dwarfism with midline-fusion defect. (B-C) Skeletal preparations of whole embryos (B) and hindlimbs (C) from control (left) and ALK5CKO (right) embryos at E18.5. (C’) Higher magnification of the boxed area shown in (C). Cartilage was stained by Alcian blue and bone was stained by Alizarin Red S. Arrow, knee joint; arrowhead, eccentric mineralization of tibia; open arrowheads, ectopic cartilaginous protrusion. (D) Skeletal preparation of clavicles from littermate control (top) and ALK5CKO (bottom) embryos at E16.5.
Skeletal preparations of E18.5 ALK5CKO mice stained with Alcian blue and Alizarin Red S revealed that the cranial vault developed poor ossification and that the clavicle was shorter (Fig. 2B, D). ALK5 was therefore important for intramembranous ossification during embryogenesis. All bones formed through endochondral ossification were also short and malformed (Fig. 2B, C). In the axial skeletal system, mutant mice had a shortened body axis and developed severe scoliosis and kyphosis with myelomeningocele (Fig. 2B; data not shown). Mutant rib cages were abnormally straight rather than curved. In ALK5CKO mice, the sternum was formed, but failed to fuse. The appendicular skeletal system in ALK5CKO embryos was also severely impaired (Fig. 2C). The E16.5 ALK5CKO mice had short femurs and two distinct, but incomplete, elements of zeugopods, in which fibulae were mineralized but short and curved, whereas tibiae were not mineralized (data not shown). At E18.5, instead of forming a central bone shaft, tibiae of mutant embryos had eccentric hypertrophic chondrocytes with an ossified bone collar (Fig. 2C’, arrowhead), while fibulae bent sharply, and a distinct knee joint space was not clear (Fig. 2C’, arrow). Ectopic cartilaginous protrusions in pelvis, femurs and zeugopods were noticeable and some protrusions in proximal metaphyses of femurs extended to the mineralized diaphyses (Fig. 2C’, open arrowheads).
ALK5 is required for joint development and perichondrium formation
To characterize the skeletal abnormalities in more detail, histological analysis was performed (Fig. 3). E18.5 ALK5CKO embryos developed partial knee joint fusion at the peripheral region (Fig. 3C, arrows). Although long bones were shorter in length and wider, compared to those of control mice, the three principal layers of chondrocytes, consisting of resting, proliferative, and hypertrophic zones, were formed in ALK5CKO femurs (Fig. 3C). The E18.5 ALK5CKO femurs also developed ectopic cartilaginous protrusions (Fig. 3C, arrowhead) and thin perichondrium, in which fibrous and osteoprogenitor layers were thinner and contained fewer and smaller cells (Fig. 3C, D and Supplemental Fig. S5).
The abolishment of ALK5 expression in ALK5CKO mice was confirmed by immunostaining for ALK5 (red). Strong expression of ALK5 was observed in the perichondrium in control mice (Fig. 3E, F), as shown in Fig. 1. Double immunostaining with aggrecan (green), a marker of chondrocytes, distinguished the perichondrium and cartilage. We found the presence of a thin chondrocyte layer, which was positive for both ALK5 and aggrecan, in the peripheral cartilage adjacent to the perichondrial layer (Fig. 3F’, arrows). This ALK5-expressing chondrocyte layer also stained positively for Sox9, another chondrocyte marker (Supplemental Fig. S3). The expression of ALK5 protein was substantially diminished in ALK5CKO femurs (Fig. 3G, H, H’) and in calvaria (data not shown). Some signals observed in ALK5CKO skeletons (Fig. 3G) may have been produced by cells escaping from Dermo1-Cre-mediated recombination, since some X-gal-negative chondrocytes were found in the femurs in Dermo1Cre/wt;Rosa26 reporter mice (Supplemental Fig. S2). Therefore, some cells that escaped from the recombination probably also expressed ALK5 protein in the ALK5CKO mice.
Pathological changes were observed in the skeletal tissues of ALK5CKO mice in which endogenous expression of ALK5 had been abolished. In ALK5CKO hindlimbs, strong ALK5 expression in the perichondrium was eradicated and formation of the perichondrium was impaired (Fig. 3, Supplemental Figs. S4, S5). It is interesting to note that ectopic cartilaginous protrusions were most typically formed from the cartilage at the level of the ossification groove of Ranvier (Shapiro et al., 1977), where ALK5 protein expression was strong in control mice but abolished in ALK5CKO mice (Fig. 3C, G, arrowheads). The perichondrium surrounding the ectopic protrusion was very thin (Supplemental Fig. S4). Moreover, serial transverse sections of mutant femurs revealed the formation of multiple protrusions, as well as solitary ectopic cartilage (Supplemental Fig. S4, asterisks and arrowhead). Calvaria of E18.5 ALK5CKO heads were thinner than those of control mice, similar to the defect observed in the perichondrium (Fig. 3I, J).
ALK5 is required for perichondrial cell proliferation and differentiation
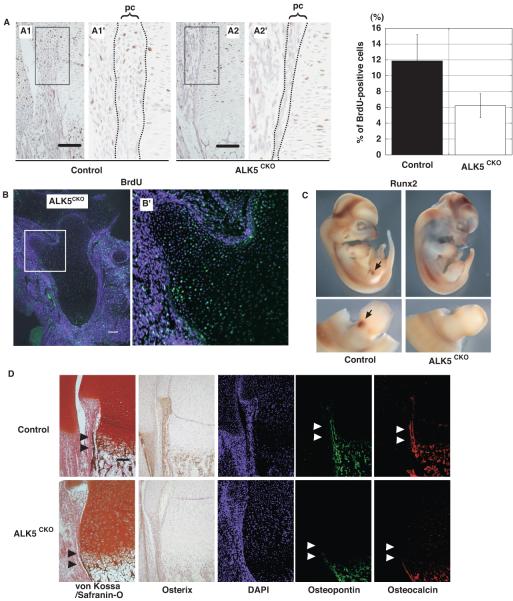
To characterize the perichondrium of ALK5CKO growth plates, the proliferation activity of perichondrial cells was examined by BrdU incorporation. Proliferation of perichondrial cells was markedly reduced in E18.5 ALK5CKO femurs (Fig. 4A). In contrast to these observations for perichondrial cells, the proliferation activity of chondrocytes at the base of the protrusion increased (Fig. 4B). These data suggest that the abnormally thin perichondrium and enhanced proliferation activity of chondrocytes in the periphery of cartilage may account, at least in part, for the formation of the ectopic cartilaginous protrusions.
Fig. 4.
Reduced proliferation and differentiation of the perichondrium and osteoprogenitor cells in ALK5CKO mice. (A) (Left) BrdU staining of the distal end of femurs at E18.5. Higher magnifications of the boxed areas in panels A1 and A2 are shown in A1’ and A2’, respectively. (Right) The number of BrdU-labeled cells in perichondrium was quantified. Proliferation activity of perichondrial cells was markedly reduced in ALK5CKO femurs. pc, perichondrium. Bar, 100μm. (B) BrdU staining (green) of the proximal end of E17.5 ALK5CKO femur. Higher magnification of the boxed area is shown in B’. Note that contrary to perichondrial cells, proliferation activity of chondrocytes at the base of the protrusion was increased. Bar, 100μm. (C) Whole mount in situ hybridization of runx2 at E11.5 in control and ALK5CKO embryos. Lower panels show magnified lateral view of hindlimbs in C. Runx2 expression was decreased in limb buds of ALK5CKO embryos. (D) von Kossa and safranin-O staining, and immunostaining with antibodies to osterix, osteopontin, and osteocalcin. Mineralization and expression of osteoblast markers for early and late differentiation were reduced in ALK5CKO femurs. DAPI, 4,6-diamidino-2-phenylindole. Arrowheads, bone collar. Bar, 100μm.
Cartilage- and bone-mineralization were next examined in hindlimbs by expression analysis of bone-specific proteins as differentiation markers. In E11.5 control limb buds, Runx2, a master regulator of osteoblast differentiation, was expressed in mesenchymal condensation, while the expression in ALK5CKO limb buds was significantly decreased (Fig. 4C). Staining with von Kossa/safranin-O showed less calcified cartilage and bone matrix in E18.5 ALK5CKO femurs. Mineralization, especially in the bone collar, was also significantly reduced (Fig. 4D).
Osterix is an osteoblast-specific transcription factor and a marker for early osteoblast differentiation (Nakashima et al., 2002). In control femurs, osterix-positive cells lined the perichondrium, as well as the surface of the trabecular bone in the bone marrow (Fig. 4D). On the other hand, these areas of ALK5CKO femurs had less osterix-positive cells. Osteopontin and osteocalcin, which are bone matrix proteins and late differentiation markers, respectively, were expressed in control femurs, whereas their expression levels were substantially lower in ALK5CKO femurs (Fig. 4D). Their expression levels were also markedly reduced in the bone collar. These data suggest that an ALK5 deficiency in skeletal progenitor cells at the early stage of skeletogenesis caused the inhibition of osteoblast proliferation and maturation observed in limbs.
ALK5 regulates calvarial cell proliferation and differentiation
Primary neonatal calvarial cells were used to obtain a better understanding of the mechanism of ALK5 functions in osteoblast proliferation and differentiation. Since bone formation of ALK5CKO was poor, sufficient cell numbers could not be obtained from ALK5CKO calvaria. Therefore, a tamoxifen-inducible Cre mouse line (Cre-ER™) was employed in place of the Dermo1-Cre mouse line to allow preparation of sufficient number of primary cells. Cre-ER™ mice were crossed with Rosa26 reporter mice (Soriano, 1999) to enable the tracking and visualization of Cre-mediated inactivation of ALK5. By cross-mating Alk5flox/flox mice with those mice, tamoxifen-inducible ALK5 deficient mice (ALK5/Cre-ER, Alk5flox/flox; Rosa26; Cre-ER™) were created that were able to provide sufficient calvarial cells from neonates. We also prepared CreER-negative control calvarial cells from Alk5flox/flox and wild type mice.
First, the proliferative activity of calvarial cells was examined, as shown in the scheme presented in Fig. 5D. After a 3-day treatment with tamoxifen, the cell number was reduced by 40% (Fig. 5A). Addition of TGF-β2 increased the cell number in the absence of tamoxifen, but not in the presence of tamoxifen (Fig. 5A). This indicated that TGF-β signaling promoted proliferation of control calvarial cells through ALK5, but not in tamoxifen-treated cells. For analysis of cell differentiation, calvarial cells were cultured at a higher density in the osteogenic medium to induce osteoblast differentiation. Alkaline phosphatase (ALP) activity, an early osteoblast differentiation marker, was then analyzed as shown in Fig. 5D. ALP was not expressed at day 0 of the induction (data not shown) but had been induced by 3 days after osteoinduction in control cells. In tamoxifen-treated cells, ALP activity was reduced (Fig. 5B, right panel). Tamoxifen-induced ALK5 inactivation was also visualized by X-gal staining of calvarial cells from ALK5/Cre-ER/ROSA26 mice (Fig. 5B, left panel) and confirmed by deleted allele-specific genomic PCR (data not shown). The double staining of X-gal and ALP revealed the striking finding that X-gal-positive cells showed little ALP activity (Fig. 5B). These data suggest that TGF-β signaling is important in the early differentiation of osteoblasts.
Fig. 5.
Tamoxifen-induced ALK5 inactivation results in the inhibition of proliferation and differentiation of primary calvarial cells. Calvarial cells were prepared from newborn ALK5/Cre-ER mice. (A) The number of cells was counted 3 days after treatment with ethanol (control) or tamoxifen. Inactivation of ALK5 resulted in growth inhibition of calvarial cells. (B) Representative microscopic pictures of X-gal/ALP-staining of primary calvarial cell cultures in the absence or presence of tamoxifen at day 3 after osteoinduction. Overall views of ALP-stained wells are shown in the insets. X-gal-positive cells showed little ALP activity. (C) Representative pictures of Alizarin Red S staining of primary calvarial cells on day 17 after osteoinduction (left). Quantification of the staining is shown (right). Inactivation of ALK5 resulted in reduced mineralization of calvarial cells. Note that TGF-β2 (1 ng/mL) inhibited mineralization of normal calvarial cells, but not of ALK5-deficient cells. Data represent means ± SD. (D) Schematic outlines of the experimental design.
In the mineralization assay, using Alizarin Red S staining for terminal differentiation of osteoblasts, calvarial cells were treated in the same manner as for the ALP assay, except that cells were analyzed at 17 days after osteoinduction (Fig. 5C, left pictures), and the staining for Alizarin Red S was quantified (Fig. 5C, right graph). The cells cultured with tamoxifen were less mineralized than the control cells cultured without tamoxifen. When TGF-β2 was included in the culture at osteoinduction initiation, complete inhibition of mineralization was observed. However, TGF-β2 failed to inhibit mineralization in ALK5 deficient cells induced by tamoxifen (Fig. 5C). The maturation defect observed when ALK5 inactivation was induced at the early osteoinduction condition (Fig. 5D) was caused by the early differentiation defect (Fig. 5B). When cells were treated with tamoxifen at 4 days or later after osteoinduction, no mineralization defect was observed (data not shown). In control experiments, we tested the effect of tamoxifen on proliferation, ALP activity, and mineralization of calvarial cells from wild type and Alk5flox/flox mice. Tamoxifen did not affect these activities in wild type calvarial cells or in Cre-negative Alk5flox/flox cells. There were no substantial differences in proliferation and differentiation between wild-type and Alk5flox/flox cells in the absence or presence of tamoxifen (Supplemental Fig. S8). These results indicate that tamoxifen affects cellular activities specific to Alk5flox/flox;CreERTM/+ cells, but not to CreER-negative Alk5flox/flox and wild type cells. Thus, TGF-β signaling is apparently required for early, but not late, osteoblast differentiation. These combined results suggest that ALK5 positively regulates proliferation and early differentiation of osteoblasts in calvarial cell culture.
ALK5 is required for the commitment of progenitor cell differentiation to the osteoblast lineage
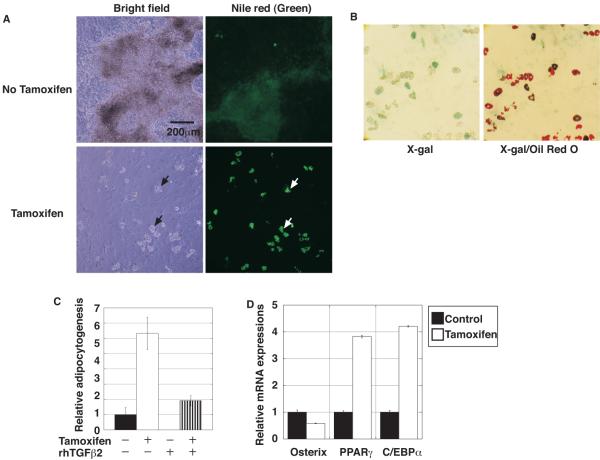
While tamoxifen inhibited osteogenic differentiation of ALK5/Cre-ER calvarial cells, we noticed that droplet-containing cells became visible at about 1 week after tamoxifen treatment and became prominent throughout the culture. The droplets were stained with the lipophilic and fluorogenic dye, Nile Red, indicating that the calvarial culture contained adipocytes (Fig. 6A). To investigate the relationship between adipocytogenesis and ALK5 inactivation, double-staining with X-gal was performed, followed by staining with another lipophilic dye, Oil Red O. Many Oil Red O-positive cells were also positive for X-gal staining (Fig. 6B). These data indicated that inactivation of ALK5 caused differentiation of calvarial cells to adipocytes, instead of osteoblasts. Using Nile Red, a method was established for quantifying Nile Red-positive areas under fluorescence microscopy, as described in Materials and Methods. Even in the absence of tamoxifen, small areas were positive for Nile Red. In the presence of tamoxifen, the positive areas dramatically increased (Fig. 6C). On the other hand, addition of TGF-β2 completely blocked adipocytogenesis in the absence of tamoxifen (Fig. 6C). The inhibition of adipocytogenesis by TGF-β2 was also observed even in the presence of tamoxifen (Fig. 6C). This inhibitory effect likely occurred because the level of tamoxifen-mediated Alk5 deletion was not 100 %. These data were consistent with the results obtained by FACS analysis using Nile Red (data not shown).
Fig. 6.
Tamoxifen-induced ALK5 inactivation increased adipocytogenesis in primary calvarial cell cultures. (A) Representative light and fluorescent microscopic images of Nile Red (green signal) staining of primary calvarial cell cultures in the absence or presence of tamoxifen at day 18 after osteoinduction. Droplets observed in light microscopy (left, black arrows) were positive for Nile Red lipophilic fluorescent dye (right, white arrows). (B) Microscopic pictures of X-gal/Oil Red O-staining of primary calvarial cell cultures in the absence or presence of tamoxifen at day 20 after osteoinduction. Note that many Oil Red O-positive cells were also positive for X-gal staining. (C) Adipocytogenesis was quantified by the Nile Red positive area as described in Materials and Methods. Data represent means ± SD. Inactivation of ALK5 resulted in enhanced adipocytogenesis of calvarial cells. (D) Real-time quantitative RT-PCR for the osteoblast-specific osterix and adipocyte-specific differentiation markers PPARγ and C/EBPα. cDNA, prepared using mRNA from calvarial cells on the 6th day of the osteogenic culture, was subjected to real-time quantitative PCR. Osteogenic marker expression was downregulated while adipocyte marker expression was upregulated in the presence of tamoxifen.
Expression of osterix mRNA as an osteoblast marker and of PPARγ and C/EBPα mRNAs as adipocyte markers in calvarial cells were examined on the 6th day under the osteogenic condition by real-time quantitative RT-PCR. Consistent with the immunohistochemistry results for osterix observed in vivo (Fig. 4C), the expression of osterix mRNA was significantly downregulated in the tamoxifen-treated calvarial cells (Fig. 6D). On the other hand, expression of PPARγ and C/EBPα mRNA was markedly upregulated in the tamoxifen-treated cells (Fig. 6D). Tamoxifen treatment did not affect adipocytogenesis of Cre-negative Alk5flox/flox control calvarial cells (Supplemental Fig. S9). Taken together, these results suggest that TGF-β signaling promotes the commitment of progenitor cells to the osteoblast lineage and inhibits adipocytogenesis through ALK5.
ALK5 regulates osteoblast lineage, proliferation, and differentiation through selective Smad2/3 and MAPKs pathways
To examine the regulatory mechanism of calvarial cell proliferation and differentiation, the MAPK and Smad2/3 pathways, two major downstream pathways of TGF-β signaling, were analyzed. First, to examine the activation status of TGF-β signaling pathways during osteoblastic differentiation of calvarial cells, the expression level of ALK5 protein and the phosphorylation level of Smad2, as a representative direct substrate for ALK5, were analyzed by Western blotting. Osteogenic induction increased ALK5 protein expression at 3 days after induction, but ALK5 expression was gradually decreased thereafter (Fig. 7A). Since anti-phospho-ALK5 antibody is not available and Smad2 is a substrate of phosphorylation by ALK5, we examined the phosphorylation level of Smad2 with anti-phospho-Smad2. The phosphorylation level of Smad2 was transiently induced and then decreased as differentiation proceeded (Fig. 7B), in a similar pattern to that seen for ALK5 expression. These results suggest that ALK5 expression and activity were diminished, as well as Smad2/3, as direct downstream molecules of ALK5 became inactive with osteoblast maturation.
Fig. 7.
MAPK and Smad signaling during osteogenic differentiation of primary calvarial cells. (A) Expression level of ALK5 protein during normal primary calvarial cell differentiation. Cultures of primary calvarial cells from control mice were incubated in osteoinduction medium and cell lysates were prepared at the indicated times. α-Tubulin was used as a loading control in this experiment. ALK5 protein expression diminished with osteoblast maturation. (B) Reactivity of primary calvarial cells to TGF-β2 during differentiation. The primary calvarial cells cultured in osteoinduction medium were incubated with TGF-β2 for 1 h at various time points as indicated. Lysates were analyzed for phospho-Smad2 by Western blot analysis. Activation of Smad2 by TGF-β2 decreased during maturation of calvarial cells. (C) Effects of ALK5 inactivation on activation of JNK, Erk1/2, and p38. Primary calvarial cells were grown for 3 days in osteoinduction medium, in the absence or presence of tamoxifen, and lysates were prepared and subjected to Western blot analysis. Inactivation of ALK5 resulted in decreased activity of MAPKs. Ctrl, control; Tam, tamoxifen.
Next, the effect of ALK5 inactivation on phosphorylation of JNK, Erk1/2, and p38 was examined. At the 3rd day after osteoinduction, the phosphorylation levels of JNK, Erk1/2, and p38 were decreased in the presence of tamoxifen (Fig. 7C). Tamoxifen did not affect the activity of these signaling molecules in Cre-negative Alk5flox/flox control cells (Supplemental Fig. S10). These results suggest that, at the early stage of osteoblast differentiation, MAPK pathways are activated through ALK5.
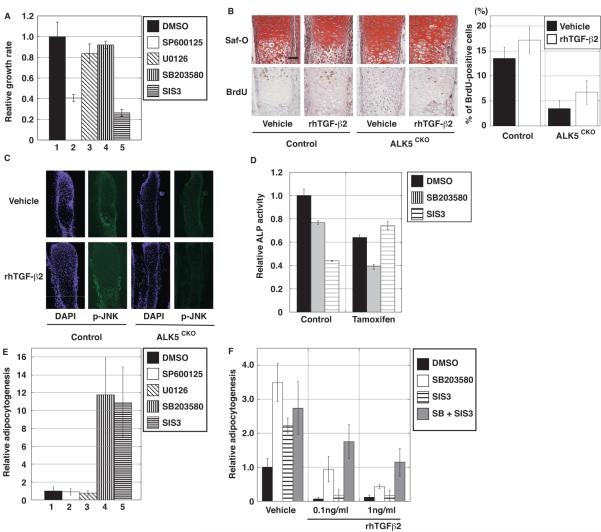
Several inhibitors of MAPK and Smad pathways were tested to examine the involvement of these signaling pathways in control calvarial cells. SP600125 and SIS3, which are JNK and Smad3 inhibitors (Jinnin et al., 2006), respectively, significantly inhibited proliferation of normal calvarial cells (Fig. 8A). In contrast, U0126 and SB203580, which are Erk1/2 and p38 inhibitors, respectively, had little effect on calvarial cell proliferation (Fig. 8A). In order to examine the involvement of the JNK pathway in proliferation of perichondrial cells, primordial metatarsal cartilage was isolated from E15.5 ALK5CKO and control mice, and explant organ culture experiments were carried out. Consistent with the result of BrdU incorporation (Fig. 4A), there were fewer BrdU-positive cells in the perichondrium of ALK5CKO metatarsals than in the control explants (Fig. 8B). Addition of TGF-β2 increased BrdU-positive cells in control metatarsals, whereas TGF-β2 had little effect on BrdU incorporation into the perichondrium of ALK5CKO explants (Fig. 8B). The involvement of the JNK pathway in metatarsal explant cultures was also examined. At the 4th day of control metatarsal culture, phosphorylation of JNK was substantially induced in the perichondrium in the presence of TGF-β2, compared with that in the absence of TGF-β2. On the other hand, in ALK5CKO metatarsal culture, phosphorylation of JNK was low regardless of the absence or presence of TGF-β2 (Fig. 8C). TGF-β signaling therefore positively regulated progenitor cell proliferation, in part through the JNK and Smad2/3 pathways.
Fig. 8.
TGF-β signaling pathways in proliferation and differentiation of primary calvarial cells and metatarsal explants. (A) Effects of MAPKs and Smad3 inhibitors on normal primary calvarial cell proliferation. Inhibitors for JNK and Smad3, but not for Erk1/2 and p38, reduced proliferation. (B) Proliferation of control and ALK5CKO metatarsal organ culture with and without TGF-β2. Safranin-O staining (top) and BrdU staining (bottom) of metatarsal rudiments at the 4th day after treatment. Safranin-O staining shows a thin perichondrial layer compared with the control layer. (Right) The number of BrdU-labeled cells in the perichondrium was quantified. BrdU-positive cells in the ALK5CKO perichondrium were reduced. TGF-β2 increased BrdU-positive cell numbers in the control perichondrium, but did not affect the ALK5CKO perichondrium. Black bars, without TGF-β2. (C) Immunostaining for phospho-JNK in metatarsal rudiments using the same samples used for panel B at day 4 after TGF-β2 treatment. The expression of phospho-JNK was detected in the control perichondrium and its expression level was increased in the presence of TGF-β2. There was little phospho-JNK expression in the ALK5CKO perichondrium with or without TGF-β2. (D) Effect of p38 and Sma d3 inhibitors on ALP activity of primary calvarial cells at day 3 after osteoinduction. Tamoxifen was added one day before osteoinduction, as illustrated in Fig. 5D. In tamoxifen-induced ALK5-deficient calvarial cells, ALP activity was reduced. Treatment with the Smad3 inhibitor, SIS3, did not change ALP activity, but the p38 inhibitor, SB203580, further reduced the activity. (E) Effects of MAPKs and Smad3 inhibitors on adipocytogenesis of normal primary calvarial cells. Adipocytogenesis was quantified by Nile Red-positive area. SIS3 and SB203580, but not the JNK inhibitor SP600125 or Erk1/2 inhibitor U0126 promoted calvarial cell differentiation to adipocytes. (F) Additive effects of p38 and Smad3 inhibitors on adipocytogenesis. Normal primary calvarial cells were cultured in osteoinduction medium containing either SB203580 or SIS3, or both in the presence of TGF-β2. DMSO, control; SP600125, JNK inhibitor; U0126, Erk1/2 inhibitor; SB203580, p38 inhibitor; SIS3, Samd3 inhibitor.
The effect of MAPKs and Smad3 inhibitors on ALP activity, an early osteoblast differentiation marker, in calvarial cells was also examined. In the absence of tamoxifen, treatment with SB203580 and SIS3 inhibited ALP activity (Fig. 8D), but inhibitors for Erk1/2 and JNK did not (data not shown). Tamoxifen did not affect the inhibitory activities of SB203580 and SIS3 in ALP activity of Cre-negative Alk5flox/flox control calvarial cells (Supplemental Fig. S11). Consistent with the ALP staining results (Fig. 5B), in the presence of tamoxifen, ALP activity was inhibited. Treatment with SB203580 further reduced ALP activity, while SIS3 did not have any additive inhibitory effects (Fig. 8D). Thus, in the early differentiation phase, TGF-β signaling appears to control osteoblast differentiation, in part through the p38 and Smad2/3 pathways.
The role of the MAPKs and Smad3 pathways in adipocytogenesis of normal calvarial cells was also examined. In contrast to the ALP inhibition results, SB203580 and SIS3 substantially promoted adipocytogenesis, but SP600125 and U0126 had negligible effect on adipocytogenesis (Fig. 8E). As shown in Fig. 6C, even low concentrations of TGF-β2 (0.1 ng/mL) almost completely blocked adipocytogenesis of calvarial cells (Fig. 8F). However, simultaneous treatment with SB203580 and SIS3 partially blocked the inhibitory effect of TGF-β2 (Fig. 8F). These results suggest that TGF-β signaling suppresses adipocytogenesis, in part through the p38 and Smad2/3 pathways. Taken together with the ALP inhibition data, the data presented here suggest that TGF-β signaling positively a nd negatively regulates osteoblastogenesis and adipocytogenesis, respectively, in part through the p38 and Smad2/3 pathways.
Discussion
Despite many investigations, the role of TGF-β signaling in endochondral bone formation remains controversial, as often opposing results have been reported (Dunker and Krieglstein, 2000). Although many studies suggest the importance of TGF-β signaling in skeletogenesis, its in vivo role is not yet fully understood. ALK5 is the target receptor for a broad range of TGF-β signaling pathways. In this report, we created ALK5CKO mice, in which ALK5 was conditionally inactivated in skeletal progenitors at the early stage of bone development, in order to address the physiological role of TGF-β signaling in skeletogenesis. TGF-β signaling appears to be critical for perichondrium formation, progenitor cell commitment, osteoblast proliferation, and early differentiation, through selective Smad2/3 and MAPKs signaling pathways.
Skeletal defects in ALK5CKO mice
Conditional ALK5 inactivation by Dermo1-Cre overcame early embryonic lethality and allowed for survival until birth. In ALK5CKO mice, both intramembranous and endochondral ossification were impaired. In this study, severe bone formation defects in ALK5CKO mice during embryogenesis were identified, including short-limbed dwarfism, less mineralization, and defects in bone integrity and isotropy. These abnormalities detected in the ALK5CKO mice correlate mostly with the expression pattern of ALK5 in developing limbs and calvaria. In mouse embryos, both ALK5 and Dermo1-Cre were expressed in developing articular cartilage, ligaments, and synovium (Supplemental Fig. S2). One of the striking pathological changes observed in ALK5CKO mice was the abnormal formation of the perichondrium. Immunofluorescent staining in developing limbs showed that ALK5 protein was strongly expressed in the perichondrium and in a unique, thin chondrocyte layer located peripherally in the cartilage adjacent to the ALK5-expressing perichondrial layer (Fig. 3F, F’ and Supplemental Fig. S3). ALK5 expression in chondrocytes of the growth plate has been reported (Horner et al., 1998; Iseki et al., 1995; Sakou et al., 1999). We also observed that ALK5 was expressed in resting and hypertrophic chondrocytes. The expression of ALK5 in these chondrocytes was weaker than that in the perichondrium and the unique chondrocyte layer.
The perichondrium is implicated in regulating the growth of the long bone (Alvarez et al., 2001; Alvarez et al., 2002; Di Nino et al., 2001), and when the perichondrial layer is enzymatically removed, TGF-β loses its power to regulate metatarsal growth (Alvarez et al., 2001). These results suggest the importance of TGF-β signaling in the perichondrium in long bone development. Newborn mice with a chondrocyte-specific conditional deletion of Tgfbr2 using Col2a1-Cre have normal bone length and mineralization in limbs and joints (Baffi et al., 2004). In contrast to Dermo1-Cre expression, Col2a1-Cre is expressed strongly in differentiating chondrocytes and its expression in the perichondrium is low (Ovchinnikov et al., 2000). Differences in Cre expression stages and levels may account for the phenotypic differences in these two mouse models.
In the ALK5CKO mice, the proliferation activity and differentiation of perichondrial cells was reduced and a thin perichondrial layer was observed to form (Figs. 3, 4A). Consistent with the in vivo data, both the perichondrium in metatarsal rudiment explants and the primary calvarial cells showed reduced proliferation activity when ALK5 was inactivated (Figs. 5A, 8B). The perichondrial cells in the ossification groove of Ranvier have the highest proliferation activity (Shapiro et al., 1977) and in our study expressed ALK5 strongly (Fig. 1). Thus, TGF-β signaling is likely responsible for the high proliferation activity. Furthermore, TGF-β signaling is required for the formation of the perichondrium. The abnormal perichondrium of ALK5CKO mice may cause ectopic cartilaginous protrusions. In addition, the unique ALK5-expressing chondrocyte layer located in the peripheral cartilage may contribute to preventing ectopic protrusion in wild-type limbs. These cells express aggrecan and Sox9, chondrocyte markers (Fig. 3 and Supplemental Fig. S3), similar to chondrocytes in other parts of cartilage. However, these cells’ strong ALK5 expression is unique and they are a previously unidentified chondrocyte population. TGF-β signaling in these cells may negatively regulate proliferation and have specific cellular activity that limits the lateral expansion of the cartilage. Without TGF-β signaling, the cells lose these activities and allow abnormal lateral expansion through the ossification groove of Ranvier. The results of the present study suggest that TGF-β signaling regulates perichondrium formation and is essential for maintaining the proper integrity, size, and shape of cartilage during the development of the growth plate.
It has been demonstrated that TGFβRII is expressed in the articular cartilage of the interphalangeal joints (Spagnoli et al., 2007) and in the perichondrium (Horner et al., 1998). Germline null mutations of TGFβRII and ALK5 in mice result in early embryonic lethality around E10.5 because of defects in hematopoiesis and vasculogenesis before skeletal elements are formed (Larsson et al., 2001; Oshima et al., 1996). This suggests that TGFβRII and ALK5 form a heteromeric complex and share their roles in hematopoiesis and vasculogenesis. There are similarities between the abnormal phenotypes in ALK5CKO mice and Prx1-Cre-mediated Tgfbr2 conditional knockout mice. Both Dermo1-Cre and Prx1-Cre are expressed in mesenchymal progenitors, and those mice exhibit short-limbed dwarfism, abnormal sternums, and defects in joints (Seo and Serra, 2007; Spagnoli et al., 2007), suggesting that TGFβRII and ALK5 also form a complex in mesenchymal progenitor cells. However, there are differences between Prx1-Cre Tgfbr2 and ALK5CKO mice. Prx1-Cre Tgfbr2 mice develop fusion of the joints of the phalanges, while ALK5CKO mice had normal phalange joints but developed partial fusion of the knee joints. In addition, there are some differences with regard to the causes of the short long bones in these mutant mice. In Prx1-Cre Tgfbr2 mice, the short length of the long bones is primarily due to a decrease in chondrocyte proliferation and a delay in late hypertrophic differentiation. In contrast, there were no substantial decrease in chondrocyte proliferation and differentiation in ALK5CKO growth plates. The alteration of the long bone length in ALK5CKO mice is mainly due to a decrease in the proliferation and differentiation of the perichondrium. During development, Prx1-Cre is expressed earlier than Dermo1-Cre in mesenchymal progenitors (Stephen J. Rodda 2006). It is also possible that the expression levels of Dermo1-Cre and Prx1-Cre may differ in mesenchyme progenitors, perichondrial cells and chondrocytes. These differences may contribute to the phenotypic difference in these mouse models. It has been reported that ALK5 can form a complex with other type II receptors such as ACTRII (Andersson et al., 2006; Rebbapragada et al., 2003; Tsuchida et al., 2008; Wu et al., 2003). Therefore, it is possible that ALK5 may exert its activity independent of TGFβRII in certain tissues and developmental stages. Indeed, this may also explain the differences in these mice.
For ligand specificity, any TGF-β (i.e., TGF-β1, -β2, and -β3) can bind to the TGFβRII/ALK5 receptor complex. Although TGF-β1 is the most abundant among the three TGF-βs in the bone matrix, all TGF-βs are expressed in the perichondrium (Millan et al., 1991; Pelton et al., 1991). TGF-β1 knockout mice are born normally but develop a multifocal inflammatory disease that leads to death by weaning age (Kulkarni et al., 1993). In contrast, TGF-β2 and -β3 double knockout mice develop herniation of visceral organs (Dünker and Krieglstein, 2002), which is similar to our ALK5CKO mice. Therefore, it can be asserted that TGF-β2 and -β3 may be major ligands for ALK5.
TGF-β signaling promotes osteoblast proliferation through Smad-dependent and Smad - independent pathways
Smad2/3 are known as direct substrates for ALK5. Consistent with our results from ALK5CKO growth plates (Fig. 4A) and explants (Fig. 8B), the Smad3-specific inhibitor SIS3 (Jinnin et al., 2006) was found to inhibit proliferation of normal primary calvarial cells (Fig. 8A). In ALK5-deficient calvarial cells, phosphorylation of Smad2 was not induced, even in the presence of TGF-β (data not shown). In MAPK pathways, mediators between the TGF-β receptor complex and MAPK kinase kinases (MAPKKKs) have not yet been established. However, considerable evidence suggests that TGF-β signaling is able to initiate MAPK pathways. The results from our metatarsal explants suggest that TGF-β signaling regulates osteoblast proliferation, at least in part, through JNK. JNK was originally identified as a stress-activated protein kinase, but it is now known to regulate diverse cellular processes, including osteoblast proliferation (Hipskind and Bilbe, 1998). In addition, JNK activation by TGF-β phosphorylates Smad3 and facilitates Smad-dependent transcriptional activity (Engel et al., 1999). Therefore, it is likely that the JNK and Smad2/3 pathways cooperatively regulate osteoblast proliferation.
TGF-β signaling is required for early, but not late, osteoblast differentiation
It was unclear whether TGF-β signaling promotes or inhibits osteoblast differentiation in vivo. Similar to the different effects of TGF-β on osteoblast proliferation in culture (Antosz et al., 1989), the effects of TGF-β on osteoblast differentiation in vitro are often controversial, primarily because they are affected by culture conditions. We found that the expression of the early osteoblast differentiation markers, runx2, osterix, and ALP, was reduced when ALK5 was inactivated at the early stage by Dermo1-Cre in vivo (Figs. 4C, D) and tamoxifen-inducible Cre in vitro (Figs. 5B, D; 8D). Therefore, we conclude that TGF-β signaling regulates early differentiation of osteoblasts through ALK5.
Smad2 and Smad 3 are activated by TGF-β and Activin, whereas Smad1, Smad5, and Smad8 are activated by BMP signaling. BMP signaling induces osteoblast differentiation, and shares a common partner, Smad4, with TGF-β signaling. Pharmacological inhibition of TGF-β signaling may cause an increase in the pool of Smad4 available for BMP signaling (Inman et al., 2002). Therefore, we performed immunostaining of phospho-Smad1/5/8 in order to examine whether ALK5 inactivation causes an increase in BMP signaling. We found that the expression level of phospho-Smad1/5/8 in the perichondrium of ALK5CKO embryos was similar to that in control embryos (Supplemental Fig. S7). In normal growth plates, ALK5 expression in the perichondrium decreased when cells differentiated into mature osteoblasts (Figs. 1; 3). During differentiation of calvarial cells in culture, ALK5 expression and activation of Smad2 were initially induced, but later gradually decreased (Fig. 7A, B). These expression patterns suggest that ALK5 is not necessary for the late differentiation of osteoblasts. Indeed, no defects were observed in endochondral ossification in Col1a1-Cre-mediated ALK5 knockout mice, in which Col1a1-Cre was expressed in differentiated osteoblasts (data not shown). In addition, no mineralization defects were found in ALK5/Cre-ER calvarial cells when ALK5 inactivation was induced by the addition of tamoxifen, on or after the 4th day of osteoinduction (data not shown). TGF-β2 did not inhibit mineralization of ALK5-deficient calvarial cells (Fig. 5C), suggesting that differentiating osteoblasts become 16 mature independent of TGF-β signaling. These data indicate that TGF-β signaling is not required for the maturation of osteoblasts. It has been reported that the expression level of TGF-β receptors decreases during retinoic acid-induced osteoblastic differentiation of multipotent mesenchymal cells (Gazit et al., 1993) and that TGF-β binding to the receptors is reduced in differentiated osteogenic cells (Takeuchi et al., 1996). Together with the results of previous reports, our results suggest that the TGF-β signaling is attenuated in the maturation stage of osteoblast differentiation, and that sustained activation of TGF-β signaling may perturb osteoblast maturation.
TGF-β signaling regulates the fate of osteoprogenitors
We found that some ALK5-deficient calvarial cells differentiated into adipocytes under the osteogenic induction condition (Fig. 6). Addition of TGF-β completely abolished adipocytogenesis of control calvarial cells. These results suggest that TGF-β signaling promotes the commitment of progenitor cells to the osteoblast lineage. It has been reported that some calvarial cells differentiate into adipocytes (Bellows and Heersche, 2001; Sabatakos et al., 2000). About 95% of fetal rat calvarial cells are committed to the osteoblastic cell lineage and the rest (∼5%) are common osteoblast/adipocyte progenitors (Bellows and Heersche, 2001). By FACS analysis, this study found that Nile Red-positive cells constituted, at most, 4% of the primary calvarial cells (data not shown), similar to a previous report (Bellows and Heersche, 2001). A mouse study for the comparison of adipogenic and osteogenic abilities of bone marrow stem cells isolated from aged and young mice showed that the aged cells expressed fewer TGF-β signal molecules than did the young cells, and these differentiated into adipocytes rather than to osteoblasts (Moerman et al., 2004). Considering these results, we conclude that TGF-β signaling regulates commitment of progenitor cells to the osteoblast lineage through ALK5.
Signaling pathways in osteoblast differentiation and cell fate commitment
During calvarial cell differentiation, JNK, ErK1/2, and p38 were activated (Supplemental Fig. S6). The activation of these signaling molecules was reduced in tamoxifen-induced ALK5-deficient calvarial cells (Fig. 7C). Inhibitors for Smad3 and p38 inhibited early osteoblast differentiation (Fig. 8D). JNK inhibitor did not affect differentiation (data not shown). This may be because JNK is involved in other cellular events.
Smad3 inhibitor also inhibited early differentiation (Fig. 8D). Smad2 was activated during early osteoinduction (Fig. 7B). These results suggest that Smad2/3 and p38, but not JNK, regulate osteoblast differentiation. Phenotypes of Smad3 mutant mice vary depending upon which exon of the Smad3 gene is targeted. Mice with Smad3 mutations in exon 1 or 2 are viable while a Smad3 mutation in exon 8 is lethal between 1 and 8 months of age (Borton et al., 2001; Datto et al., 1999; Yang et al., 1999; Zhu et al., 1998). Smad3 is known to physically interact with many signaling molecules such as runx2, β-catenin, and vitamin D receptor (Alliston et al., 2001; Li et al., 2006; Yanagi et al., 1999). Further investigation into the regulatory mechanism of osteoblast differentiation through Smad3 is required.
In contrast to osteoblast differentiation, inhibitors of Smad3 and p38 promoted differentiation of calvarial progenitor cells into adipocytes (Fig. 8E). JNK and Erk1/2 inhibitors did not affect adipocytogenesis. It has been reported that TGF-β signaling inhibits adipocytogenesis by inhibition of C/EBP via physical interaction with Smad3. Marrow stem cells isolated from Smad3-null mice were resistant to hypoxia-mediated inhibition of adipocyte differentiation. The inhibitory role of p38 in adipocytogenesis was also found using dietary and genetically obese mice and p38-null ES cells (Aouadi et al., 2006). These previous results are consistent with our findings in calvarial cells. Taken together, Smad3 and p38 pathways suppress adipocyte differentiation of calvarial progenitor cells in an opposite manner to that seen for osteoblast differentiation. Our data suggest that TGF-β signaling regulates the commitment of calvarial cell progenitors to the osteoblast lineage.
Although TGF-β signaling is attenuated and is likely dispensable for osteoblast maturation, the JNK, Erk1/2, and p38 pathways remained active throughout osteoblast differentiation (Supplemental Fig. S6). p38 has been implicated in BMP-induced osteoblast differentiation (Guicheux et al., 2003), while FGF and BMP signaling are known to regulate osteoblast differentiation via MAPK pathways (Xiao et al., 2002a; Xiao et al., 2002b). This suggests that the sustained activity of these signaling molecules is regulated by other extracellular signaling molecules, such as FGF and BMP, during the late differentiation stage, when TGF-β signaling is switched off.
In conclusion, the present study reveals the critical roles of TGF-β signaling in joint and perichondrium formation, in progenitor commitment, and in osteoblast proliferation and early differentiation, through the operation of selective Smad-dependent and -independent pathways, during fetal bone development.
Supplementary Material
Acknowledgments
We thank Drs. Stefan Karlsson, David M. Ornitz, and Andrew McMahon for ALK5-floxed mice, Dermo1-Cre mice, and CAGG-Cre-ER™ mice, respectively. This work was supported by the Intramural Program of the NIDCR, National Institutes of Health. Tomoya Matsunobu was supported in part by a Fellowship from the Japan Society for the Promotion of Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–72. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Horton J, Sohn P, Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev Dyn. 2001;221:311–21. doi: 10.1002/dvdy.1141. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Sohn P, Zeng X, Doetschman T, Robbins DJ, Serra R. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–24. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Ibanez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7:831–7. doi: 10.1038/sj.embor.7400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosz ME, Bellows CG, Aubin JE. Effects of transforming growth factor beta and epidermal growth factor on cell proliferation and the formation of bone nodules in isolated fetal rat calvaria cells. J Cell Physiol. 1989;140:386–95. doi: 10.1002/jcp.1041400225. [DOI] [PubMed] [Google Scholar]
- Aouadi M, Laurent K, Prot M, Le Marchand-Brustel Y, Binétruy B, Bost F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes. 2006;55:281–9. doi: 10.2337/diabetes.55.02.06.db05-0963. [DOI] [PubMed] [Google Scholar]
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–42. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Heersche JN. The frequency of common progenitors for adipocytes and osteoblasts and of committed and restricted adipocyte and osteoblast progenitors in fetal rat calvaria cell populations. J Bone Miner Res. 2001;16:1983–93. doi: 10.1359/jbmr.2001.16.11.1983. [DOI] [PubMed] [Google Scholar]
- Borton AJ, Frederick JP, Datto MB, Wang XF, Weinstein RS. The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J Bone Miner Res. 2001;16:1754–64. doi: 10.1359/jbmr.2001.16.10.1754. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nino DL, Long F, Linsenmayer TF. Regulation of endochondral cartilage growth in the developing avian limb: cooperative involvement of perichondrium and periosteum. Dev Biol. 2001;240:433–42. doi: 10.1006/dbio.2001.0471. [DOI] [PubMed] [Google Scholar]
- Dunker N, Krieglstein K. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem. 2000;267:6982–8. doi: 10.1046/j.1432-1327.2000.01825.x. [DOI] [PubMed] [Google Scholar]
- Dünker N, Krieglstein K. Tgfbeta2 -/- Tgfbeta3 -/- double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol. 2002;206:73–83. doi: 10.1007/s00429-002-0273-6. [DOI] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274:37413–20. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Gazit D, Ebner R, Kahn AJ, Derynck R. Modulation of expression and cell surface binding of members of the transforming growth factor-beta superfamily during retinoic acid-induced osteoblastic differentiation of multipotential mesenchymal cells. Mol Endocrinol. 1993;7:189–98. doi: 10.1210/mend.7.2.8385738. [DOI] [PubMed] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–8. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- Haaijman A, Karperien M, Lanske B, Hendriks J, Löwik CW, Bronckers AL, Burger EH. Inhibition of terminal chondrocyte differentiation by bone morphogenetic protein 7 (OP-1) in vitro depends on the periarticular region but is independent of parathyroid hormone-related peptide. Bone. 1999;25:397–404. doi: 10.1016/s8756-3282(99)00189-1. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hipskind RA, Bilbe G. MAP kinase signaling cascades and gene expression in osteoblasts. Front Biosci. 1998;3:d804–16. doi: 10.2741/a323. [DOI] [PubMed] [Google Scholar]
- Horner A, Kemp P, Summers C, Bord S, Bishop NJ, Kelsall AW, Coleman N, Compston JE. Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. Bone. 1998;23:95–102. doi: 10.1016/s8756-3282(98)00080-5. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Iseki S, Osumi-Yamashita N, Miyazono K, Franzén P, Ichijo H, Ohtani H, Hayashi Y, Eto K. Localization of transforming growth factor-beta type I and type II receptors in mouse development. Exp Cell Res. 1995;219:339–47. doi: 10.1006/excr.1995.1237. [DOI] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Blank U, Helgadottir H, Bjornsson JM, Ehinger M, Goumans MJ, Fan X, Leveen P, Karlsson S. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–35. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. Embo J. 2001;20:1663–73. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TF, Chen D, Wu Q, Chen M, Sheu TJ, Schwarz EM, Drissi H, Zuscik M, O’Keefe RJ. Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J Biol Chem. 2006;281:21296–304. doi: 10.1074/jbc.M600514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunobu T, Tanaka K, Nakamura T, Nakatani F, Sakimura R, Hanada M, Li X, Okada T, Oda Y, Tsuneyoshi M, Iwamoto Y. The possible role of EWS-Fli1 in evasion of senescence in Ewing family tumors. Cancer Res. 2006;66:803–11. doi: 10.1158/0008-5472.CAN-05-1972. [DOI] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111:131–43. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–66. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–70. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–6. [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115:1091–105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–42. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–90. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res. 1999;14:1145–52. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–70. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–16. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro F, Holtrop ME, Glimcher MJ. Organization and cellular biology of the perichondrial ossification groove of ranvier: a morphological study in rabbits. J Bone Joint Surg Am. 1977;59:703–23. [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spagnoli A, O’rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL. TGF-{beta} signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–17. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Nakayama K, Matsumoto T. Differentiation and cell surface expression of transforming growth factor-beta receptors are regulated by interaction with matrix collagen in murine osteoblastic cells. J Biol Chem. 1996;271:3938–44. doi: 10.1074/jbc.271.7.3938. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J. 2008;55:11–21. doi: 10.1507/endocrj.kr-110. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002a;17:101–10. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002b;277:36181–7. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- Yanagi Y, Suzawa M, Kawabata M, Miyazono K, Yanagisawa J, Kato S. Positive and negative modulation of vitamin D receptor function by transforming growth factor-beta signaling through smad proteins. J Biol Chem. 1999;274:12971–4. doi: 10.1074/jbc.274.19.12971. [DOI] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–91. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–74. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–14. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.