Abstract
Repeated training on a perceptual task can result in performance deterioration. In the case of vision, this practice-dependent decrease, or perceptual deterioration is restored by changing the target orientation, spatial location, or by taking a daytime nap. Behavioral studies suggest the locus of these performance changes to be primary visual cortex. The present study utilizes functional magnetic resonance imaging (fMRI) to directly probe whether perceptual deterioration and nap-dependent maintenance of performance can be detected at the level of primary visual cortex. We also ask whether these changes are due to a bottom-up, stimulus-driven response or a top-down plasticity of attentional mechanisms. Subjects were scanned while performing the texture discrimination task. Half the subjects took a nap between sessions. We measured the relationship between changes in performance and changes in BOLD signal modulation between the two groups. Non-nappers showed performance deterioration that was significantly correlated with decreased BOLD signal modulation, exclusively in area V1 and limited to the bottom-up condition. In contrast, no change was detected in performance and BOLD response in the two conditions for nappers. These results indicate that napping prevented performance deterioration, which was reflected in the fMRI response of neurons in V1. Without a nap, perceptual deterioration was related to decreases in the stimulus-driven, bottom-up representation, rather than decreases in attentional modulation to the stimulus.
Introduction
Experience-dependent plasticity of cortical neurons has been shown in electrophysiological recordings in animals and with fMRI in humans. Plasticity is associated with adaptive changes to the organization of the brain due to experience with new information that leads to learning (Kandel, Kupfermann et al. 2000). These adaptive changes have been found in populations of cells (Zohary, Celebrini et al. 1994), individual neuronal tuning functions(Schoups, Vogels et al. 2001; Yang 2004, Raiguel et al 2006), and even at the once-thought immutable level of the receptive field (Meliza 2006). Some of these changes have been associated with sleep-dependent behavioral improvements on perceptual tasks (Karni, 1994, Walker, 2003). Neural changes associated with sleep-dependent perceptual learning corresponds to the functional nature of the particular neurons tested (e.g., improved orientation discrimination after a night of sleep associated with increased BOLD signal in primary visual cortex (Schwartz 2002; Furmanski, Schluppeck et al. 2004)).
More recently, work has investigated the contrary phenomenon of performance deterioration. Deterioration occurs, for example, on a visual texture discrimination task due to repeated within-day training (Gais, Plihal et al. 2000; Mednick, Nakayama et al. 2002; Mednick, Nakayama et al. 2003; Mednick, Arman et al. 2005, Censor, Karni et al. 2006). By testing whether deterioration in performance transfers to untested primitive stimulus features, these studies have made inferences regarding the exact neural group responsible for these performance changes. Perceptual deterioration does not transfer to new target locations(Mednick, Nakayama et al. 2002) or new target orientations(Mednick, Arman et al. 2005). However, deterioration is unaffected by changes to distractor orientations, or switching to the untrained eye. Interestingly, subjects are not aware of the target orientation change despite the recovery in performance. In contrast,, the obvious change to the distractor orientation is universally noted, but does not lead to recovered performance(Mednick, Arman et al. 2005). These findings show that perceptual deterioration is not due to global fatigue and may not be related to conscious attention. Rather, these studies suggest the changes may be due to the fatigue of neurons early in the visual processing stream.
One intervention outside of the task parameters that can reverse perceptual deterioration is a midday nap. Previous studies have shown that inter- test session napping prevented perceptual deterioration (Mednick, Nakayama et al. 2002). Sleep appeared necessary for reversal of the deterioration to occur, as substituting the nap with 60 minutes of eyes closed, or increasing subjects’ motivation with monetary reward did not alter the course of the deterioration. The lack of effect of motivation supports the hypothesis that overt attentional mechanisms do not control deterioration or lack thereof.
Collectively, these results are consistent with the hypothesis that perceptual deterioration is a phenomenon occurring at the level of primary visual neurons and is not modulated with conscious attention. Indeed, previous research on perceptual learning have demonstrated that sleep-dependent improvement on this task is likely to occur at the level of primary visual cortex (Karni and Sagi 1991; Walker, Stickgold et al. 2005). Behavioral studies examining transfer of learning or deterioration, however, may not necessarily implicate a specific underlying neuronal population. In his review, Ghose delineates the limitations of such studies with the following point: 1) orientation selectivity can be found from V1 up through inferotemporal cortex, 2) replication of some specificity findings such as monocularity have been unsuccessful, and 3) perceptual learning models propose that the source of improvement may just as likely be due to changes in selectivity at higher rather than lower visual areas (Ghose 2004). Similarly, one potential explanation for performance deterioration is that repeated testing reduces top-down attentional mechanisms that help modulate the primary sensory system’s representation or processing of the stimulus.
In order to disentangle these competing hypotheses, the source of deterioration needs to be examined at multiple levels of the visual system with careful control of attentional modulation. Functional neuroimaging is a good method for examining the influence of top-down attention versus bottom-up stimulus-driven processing (Gandhi, Heeger et al. 1999).
Using fMRI, we studied subjects before and after extensive exposure to a texture discrimination task. Half the subjects were given a daytime nap between the second and third test session. The study had two main aims. 1) To examine which areas of visual cortex (i.e. V1, V2, V3, V4v and V3A) correlated with performance decreases due to repeated testing compared to performance maintenance after a nap. We hypothesized that these performance differences would be associated with changes to the BOLD signal in orientation-selective, retinotopically-specific areas early in visual processing (i.e. V1). 2) To test whether perceptual deterioration was associated with decreased bottom-up stimulus-driven response, or decreased ability of top-down attentional mechanisms to modulate that response. We hypothesized that perceptual deterioration would be associated with fatigue of bottom-up mechanisms. . .
Materials and Methods
Experimental Protocol
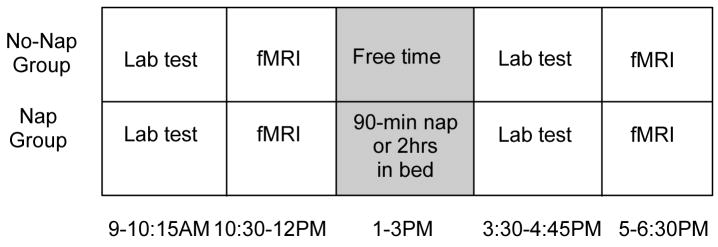
Each subject was tested on a version of the texture discrimination task four times in one day: twice in the laboratory (9AM and 3:30PM) and twice inside the scanner (10:30AM and 5PM) (Figure 1 for study timeline). Each session normally lasted 60–75 min.
Figure 1.
Testing schedule for nap and no-nap groups
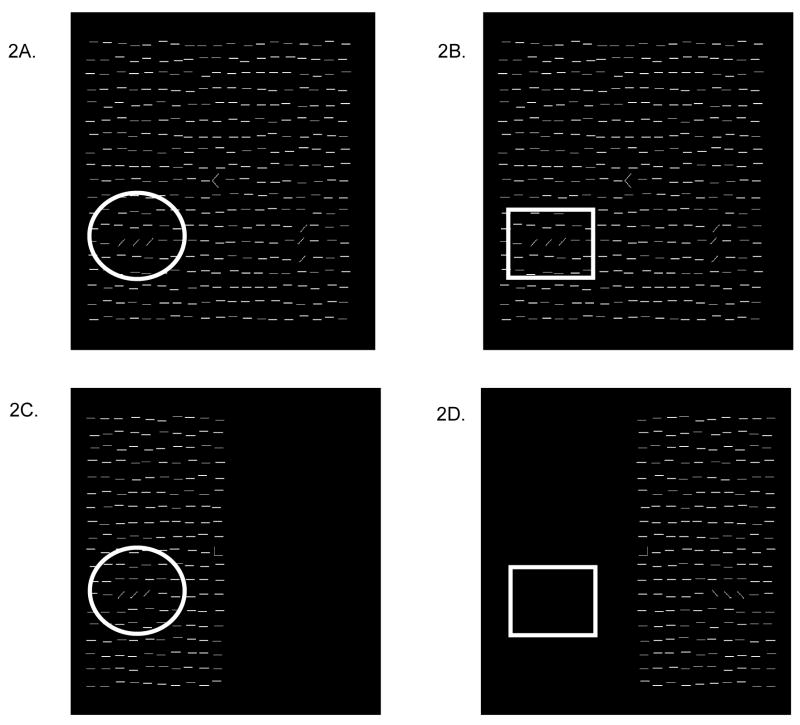
We utilized essentially the same fMRI task design as in the study of spatial attention by Gandhi, Heeger, and Boynton(Gandhi, Heeger et al. 1999). In the first condition, we measured the contrast between the fMRI response to the texture stimulus plus the attention to the task stimuli compared to no stimulus (labeled Stimulus+Attention). The task was the same as that used for training, including the full-field mask, and fixation letter discrimination task, with the exception that the texture stimulus on the hemifield opposite to the target hemifield was removed (see Figure 2C and D). An arrow appeared before each trial to indicate the hemifield to which subjects were to attend. Since the mask was presented in both hemifields throughout the scan, we expected the fMRI activation in each brain hemisphere to be modulated both by attention and the presence of the texture stimulus vs. the absence of both attention and stimulus.
Figure 2.
fMRI conditions: Scans were structured in a blocked design in which half of the trials directed the subject to perform the task with the target on the trained side and half on the untrained side. The Attention Only condition (2A & 2B) measured top-down modulation to a region of interest where there was an attended stimulus (indicated by circle) versus an unattended region with a stimulus (indicated by square). The Stimulus + Attention condition (2C. & 2D) measured bottom-up modulation to a region of interest where there was an attended stimulus (indicated by circle) versus an unattended region with no stimulus (indicated by square).
In the second condition, we measured the effect of attention alone (labeled Attention Only). A block design was used in which the texture stimulus, including the peripheral target array, appeared in both hemifields simultaneously, while an arrow directed the subject to alternate attention to one hemifield at a time (see Figure 2A and B). Thus fMRI modulation in a given hemisphere was only due to the attentional modulation to the hemifield, since the physical properties of the stimulus remain constant throughout the scan.
In order to test our a priori hypothesis that perceptual deterioration was produced by a stimulus-driven (bottom-up) fatigue of V1 neurons, we devised four difference scores. For each of the two conditions, one difference score was devised to test the change in BOLD signal modulation to the trained target from fMRI session one to session two. The other score was the same subtraction but to the untrained target and served as a retinotopic control condition for the first difference score. These difference scores were calculated for the selected visual areas: V1, V2, V3, V4v, V3A.
Subjects and Procedures
A total of 34 subjects gave informed consent to participate in the study, which was approved by the institutional review boards of both the University of California San Diego and the Salk Institute for Biological Studies. All subjects, ages 18–30, had normal or corrected-to-normal vision and no history of neurological, mental or physical illness. Participants were restricted from caffeine the day of the study, alcohol starting the evening before test day, and were asked to get at least seven hours of sleep the night before the study.
Nap Procedures
Half of the subjects (n=17) were randomly assigned to a napping group. At 12:30pm, nappers were taken to the UCSD Gillin Laboratory for Sleep and Chronobiology where they were fitted with standard monitors for polysomography and were in bed by 1pm. Sleep stages and nap duration was visually monitored and scored in real time. Subjects got out of bed after 90-minutes of sleep or two-hours in bed, whichever came first.
Data Elimination
We tested 17 nappers and 16 non-nappers. Functional data from 2 nappers and 2 non-nappers contained an uncorrectable artifact (apparently produced by the head coil) confined to the occipital pole. We excluded these subjects in the analysis of the behavioral and fMRI data.
Texture Discrimination Task
In-Laboratory Testing
Laboratory testing used a simulator that matched the optical conditions used in the fMRI environment. The simulator included the same LCD projector (NEC, Rancho Cordova, CA), back-projection screen, stimulus-generating computer (Macintosh Powerbook G3 laptop) and viewing distances as that in fMRI environment. Laboratory testing took place in a dimly lit room using a chin rest, at a distance of 57.5 cm from the back-projection screen. The task was programmed in Matlab and PsychToolbox(Pelli 1997).
Participants performed a texture discrimination task similar to that developed by Karni and Sagi(Karni and Sagi 1991). Participants were asked to discriminate two targets per trial: a central letter (‘T’ or ‘L’), and a peripheral line array (vertical or horizontal orientation) in one of the lower quadrants at 2.5–5.9 deg eccentricity from the center of the screen. The peripheral array consisted of three diagonal bars that were either positioned in a horizontal array or a vertical array against a background of horizontally oriented bars, which created a texture difference between the target and background (see Figure 2a).
An experimental trial consisted of the following sequence: central fixation cross, target screen for 32 ms, blank screen for a duration between 0 and 600 ms (the inter-stimulus-interval, or ISI), mask for 16 ms followed by the response time interval before the next trial. Subjects reported both the letter at central fixation (T or L) and the orientation of the peripheral, three-element array (horizontal or vertical) by making two key presses. The central task controlled for eye movements.
Each block consisted of 50 trials, each with the same ISI, and lasting approximately 2 minutes. A threshold was determined from the performance across 20 blocks, with a progressively shorter ISI, starting with 600 msec and ending with 0 msec. The specific sequence of ISIs across an entire session was [600, 500, 400, 350, 300, 250, 200, 175, 150, 125, 100, 80, 60, 40, 20, 0]. A psychometric function of percent correct for each block was fit with a Weibull function to determine the ISI at which performance yielded 80% accuracy.
Participants controlled the onset of each block and were instructed to take as many breaks as they needed between blocks. Once a block began, a new trial initiated every 2 seconds, regardless of whether or not the subject made a response. Training, which occurred at the beginning of the 9AM test session, consisted of 15 trials of an easy version of the task (ISI of 1000 – 1500 msec), and 50 trials of the easiest block of the actual task (ISI of 600 msec). This training ensured that participants understood the task and were discriminating the peripheral target between 90% and 100% correct on the easiest version of the task.
Performance Difference Scores
Difference scores were calculated to measure the change in performance by subtracting the second in-laboratory test session threshold from the first in-laboratory test session threshold. Negative difference scores indicate perceptual deterioration.
In-Scanner Testing
The task design described in the experimental procedures section was adapted for administration in the scanner to measure the fMRI responses to the texture stimulus. FMRI requires incorporating a contrast within each scan session, which eliminated the possibility of using the same behavioral task inside and outside the scanner. This requirement, however, allowed us to design a contrast that compared BOLD signal modulation in both the trained and untrained (i.e. control) hemifield. Each scanning session lasted 60–75min. Scans alternated between two conditions of the task. Each condition was administered four times, for a total of eight functional scans. Both conditions of the test utilized block designs in which the texture stimulus alternated between the trained and untrained visual hemifield every 20 seconds (i.e., every 10 trials). A reference scan ended each scanning session.
The task parameters were also altered to conform to the scanner. Each fMRI session consisted of eight 4-minute scans. Within each scan there were six 40-second cycles. Each cycle consisted of a block of trials in the trained hemifield and a block of trials in the untrained hemifield. Whether the first block of a scan was in the trained and untrained hemifield was counterbalanced across scans and subjects. Each block contained ten 2-second trials. Each trial consisted of a 100 msec fixation interval with a cue, a texture stimulus presented for 17msec, a blank inter-stimulus-interval (ISI) (duration of ISI was determined with an online three-up/one-down staircase procedure), a 17 msec mask, and a 1.5-second response interval during which subjects made a response to the fixation (T vs. L) and texture (vertical vs horizontal) targets. Again, the fixation target controlled for eye movements.
The staircase procedure was implemented inside the scanner in order to maximize the number of trials (and thus BOLD signal modulation) at threshold (80% correct). These thresholds, however, were not used in the statistical analysis comparing changes in performance and changes in BOLD for three reasons. First, the staircase procedure produced extremely noisy data. Second, the thresholds obtained in the laboratory were used instead in order to have an independent measure of performance change. Three, perceptual deterioration has been shown to increase linearly with the amount of training. In the present study, performance deterioration in the second in-laboratory session (third session overall) showed the same linear increase in ISI that we have previously reported (i.e. Mednick (2005) reported a difference score of 43 ms after second training session, while the present study found a difference score of 63 ms after third session). Since behavioral data from inside the scanned was not used, we were also unable to analyze performance in the untrained hemifield.
Statistical Analysis
To evaluate the relationship between changes in behavioral performance with changes in BOLD signal modulation, multiple regression tests were run with one dependent variable: the behavioral difference score: TDT difference and two independent variables: Group (napper vs. non-napper) and the difference score for BOLD signal modulation associated with each condition. The results are presented by visual area.
MRI Data Collection
Functional Images
FMRI experiments were conducted at the Center for Functional Magnetic Resonance Imaging at the University of California, San Diego. Echo-planar imaging was performed on a whole-body 3-Telsa GE MRI scanner using a low-bandwidth echo-planar pulse sequence. An eight-channel array surface coil was used to maximize signal-to-noise in the occipital areas. 150 volumes of 32 axial slices were acquired per run with TR = 2sec, TE = 30ms, FOV = 25cm, in-plane resolution = 64×64.
Retinotopic mapping
Prior to the experimental scans, all subjects had their visual areas mapped using standard retinotopic mapping techniques to segregate the subject’s retinotopic visual areas (V1, V2, V3, V3A and V4v). The polar angle and eccentricity components of the retinotopic maps were measured by recording the fMRI response to slowly rotating wedge and expanding ring stimuli, respectively. These retinotopy measurements were visualized on a computationally flattened region of the gray matter in the occipital lobe from a high-resolution MRI of each subject’s brain (Engel, Glover et al. 1997). FMRI data from subsequent sessions were then aligned to a common three-dimensional coordinate grid, which allowed us to project regions of interest across sessions.
Reference Scan
Each experimental session ended with a reference scan that determined the region within each retinotopic area associated with the textured target. This also served to eliminate inactive voxels suffering from partial volume effects. The reference scan consisted of a contrast reversing 8.3-Hz, 1-cycle/degree checkerboard restricted to the same peripheral apertures as texture target in the main experiments. The stimulus alternated (20 sec on, 20 sec off) with a uniform gray field. Active voxels that correlate with a 40-second sinusoid (r>0.23) were included for analysis in the subsequent scans.
Results
Task Performance
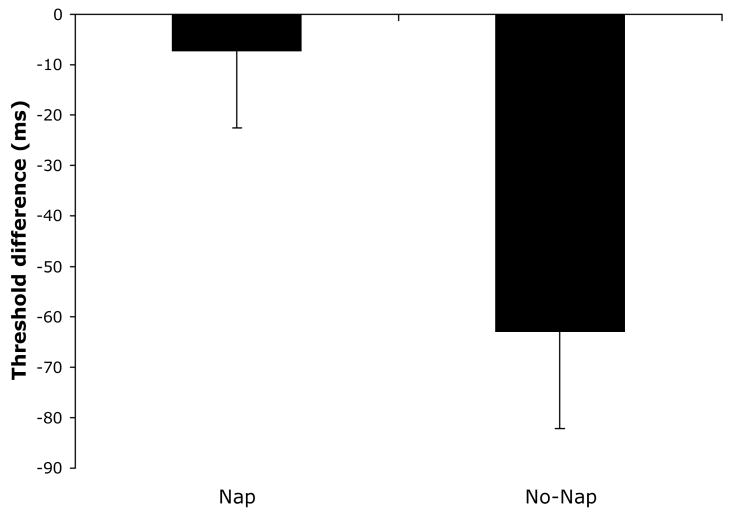
Replicating earlier findings that napping prevents perceptual deterioration, we found significant differences between nappers and non-nappers on the difference score, (t=2.56, p=.01, −7.3 ms. and −63.0 ms, nappers and non-nappers respectively, Figure 3).
Figure 3.
Texture discrimination difference threshold in nappers and non-nappers.
BOLD fMRI Data
V1
We tested the difference between nappers and non-nappers on the two conditions in the scanner, using behavioral performance as the dependent measure, modeling the effects of Group, Attention Only condition, Stimulus+Attention condition, and the interactions between Group X Attention Only, and Group X Stimulus+Attention. A significant relationship was found between the change in behavioral performance (TDT difference) and BOLD signal modulation across sessions (omnibus F=3.39, p=.01, r2=.41). Amongst the terms, there was a significant effect of Group (F=6.88, p=.01), and significance in the interaction Group X Stimulus+Attention (F=−2.05, p=.05). There were no further significant terms: Attention Only (F=.95, p=.34); Stimulus+Attention (F=2.9, p=.10) or the interaction Group X Attention Only (F=.67, p=.42).
Stimulus+Attention/Trained-side
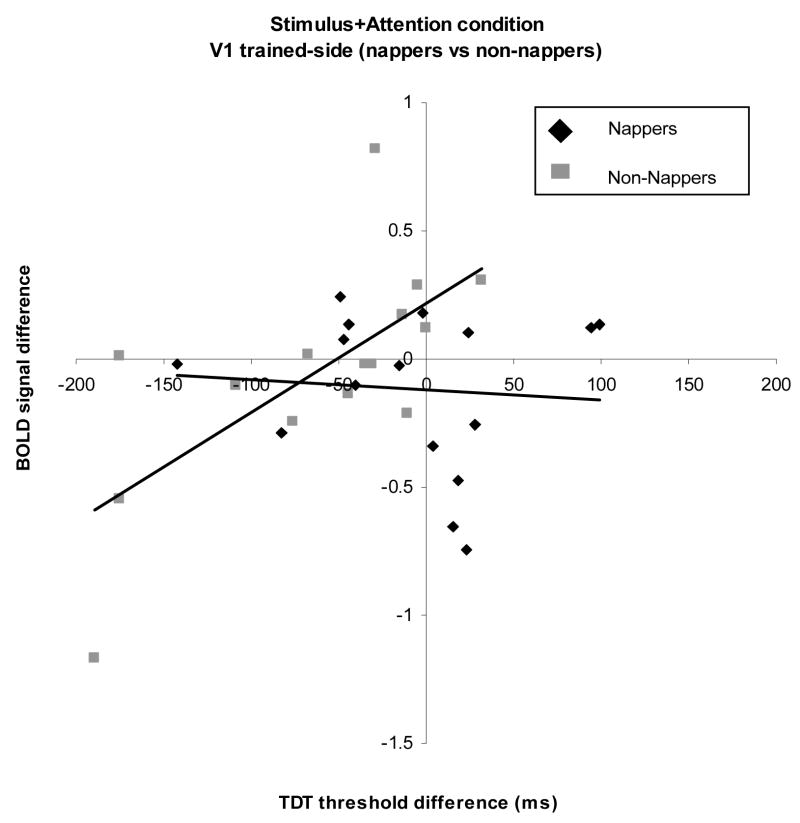
The Attention + Stimulus condition measures the response associated with attention and the target stimulus compared to the response to no stimulus. Since the above-mentioned omnibus F statistic was significant, we repeated the regression separately for nappers and non-nappers. In the nappers, we found no relationship between TDT difference and changes in BOLD signal modulation (F=.08, p=.77) (Figure 4).
Figure 4.
Plot of difference scores for TDT and BOLD signal of the Stimulus+Attention condition on the trained side of V1 in nappers and non-nappers. In non-nappers, a linear relationship was found between decreases in task performance and decreases in BOLD signal.
Non-nappers, however, showed a significant relationship (F=10.95, p=.005, r2=.47), such that the decrement in perceptual deterioration in the second session was positively correlated with the decrement in BOLD signal in the second session (Figure 5). We examined the behavioral and BOLD signal data to ensure that this significant correlation was not due to outliers (values more than three standard deviations from the mean). No data fit this outlier definition indicating that the correlation between BOLD signal modulation and performance was not skewed by individual data points.
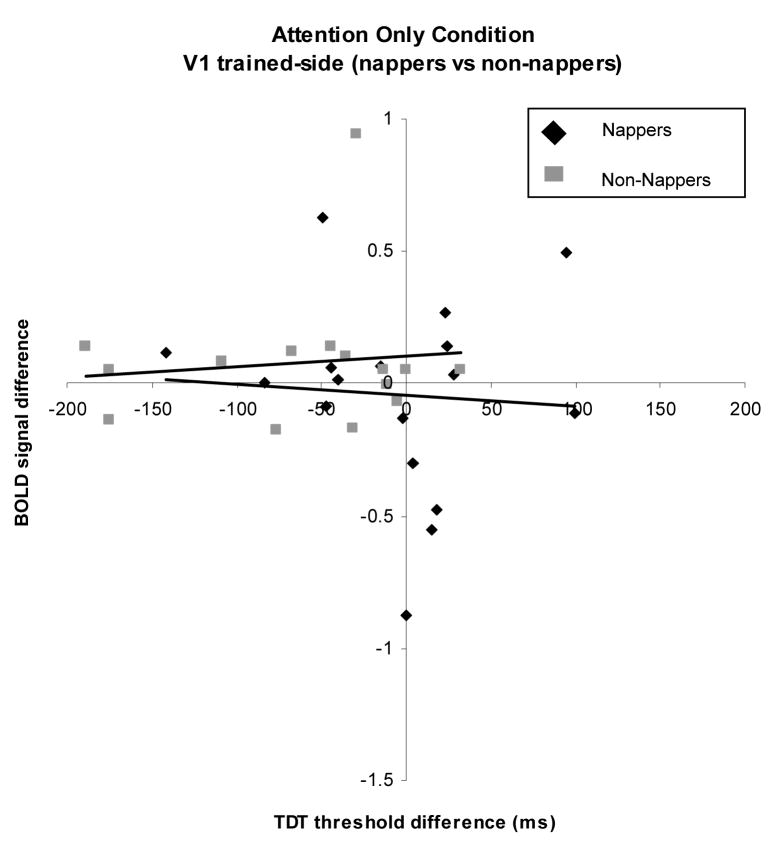
Figure 5.
Plot of difference scores for TDT and BOLD signal of the Attention Only condition on the trained side of V1 in nappers and non-nappers. No relationship was found between changes in task performance and BOLD signal modulation.
Stimulus+Attention/Untrained-side
In the untrained side of V1, the omnibus test was not significant (F=2.11, p=.11, r2=.19). Amongst the modeling effects, Group was significant (F=5.13, p.04) but neither the condition, p=.83; nor interaction, p.30 were significant, indicating a lack of a relationship between performance and BOLD signal modulation on the untrained side in nappers and non-nappers. By considering the untrained side as a control, we found that the relationship between changes in performance and in BOLD signal was present in the trained side of V1 only.
Attention Only/Trained-side
The Attention Only condition measured the brain response to an attended versus an unattended target. The omnibus regression of group and session on TDT difference was not significant (F=1.71, p=.18). The term Group was significant (F=4.96 p=.03). But no other terms reached significance: Attention Only (F=.02, p=.86), interaction: (F=.2, p=.65)). When each group was analyzed separately, there was no relationship between the performance change and the change in BOLD signal to the attention condition for either group (nappers: (F=.05, p=.8 (Figure 6)), non-nappers: (F=.13 p=.72 (Figure 7)). Thus, the top-down condition was not correlated with the decreases in performance in the non-nappers.
Attention Only/Untrained-side
In the untrained side of V1, there was no significant relationship between TDT difference and changes in BOLD signal modulation between session one and two (omnibus F=1.98, p=.14, r2=.18). The term Group was significant (F=5.12, p=.02), but none of the other terms reached significance Attention Only (p=.35), interaction (p=.37), indicating a lack of a relationship between performance and BOLD signal modulation on the untrained side in nappers and non-nappers.
V2–V4
No significant differences were found for any of the analyses conducted within the other of the visual areas examined (i.e. V2, V3 and V4) (all p> .2).
Discussion
Neural correlates of perceptual deterioration
We found that performance decreases due to repeated, within day testing was reflected in fMRI response in the trained hemisphere of primary visual cortex. This relationship was not seen in higher visual areas of V2, V3, V4v, V3A or in the untrained side of V1. Decreased BOLD signal in the non-nappers was found in the bottom-up condition which compared fMRI signal modulation to the stimulus in the attended side with no stimulus in the unattended side. No such relationship was found between behavioral performance and BOLD signal in the top-down condition, which compared fMRI signal modulation to the stimulus in the attended side with the stimulus in the unattended side. These data suggest perceptual deterioration is a stimulus-driven effect that is related to the fatigue of early visual neurons. We did not find evidence that deterioration is due to an inability to allocate attention to the visual stimulus.
Prior behavioral studies suggested that perceptual deterioration is due to fatigue of target-selective, orientation-specific, binocular neurons. The present study adds to the characterization of this phenomenon by demonstrating that deterioration involves bottom-up fatigue of V1 neurons and is not modulated by top-down attentional processes. This finding shows further evidence of a discrepancy between the activity of V1 neurons and awareness.
The relationship between BOLD signal modulation and performance decreases was not found in areas outside of V1. Other findings also support a dissociation between V1 and conscious awareness. Sasaki and Watanabe recently reported that attentional modulation on a color filling-in task enhanced BOLD signal modulation only in the primary visual cortex (Sasaki and Watanabe 2004). The activity of later visual areas is not necessary for the generation of the motor signal needed for the behavioral response, as the signals that drive the motor system do not originate in the visual cortex regardless of the stimuli or induced perception. The stimulus-driven signals generated in the visual system are sent to the frontal-eye-fields (FEFs), an area implicated in decision making (Glimcher 2002). Studies have shown that the final stimulus based response is determined by the output of the FEFs (Hanes and Schall 1996; Schall and Thompson 1999). The observed activation pattern of visual cortical areas beyond V1 is consistent with the notion that subject’s are deliberately attending to a target which was texturally different from a uniform background (Zipser et al 1996). The fact that BOLD signal in higher visual areas V2, V3, V4v did not correlate with performance indicates that, given the stimuli used, V1 is sufficient for process target features, but that the attentional resources of this area were not able to overcome the build up of training induced fatigue.
Perceptual and neural maintenance
Napping prevented deterioration from occurring both at the level of behavior and the fMRI response. Nappers maintained baseline performance and BOLD signal for each visual area in both task conditions. These results replicate previous findings that an hour nap restored performance to baseline on the texture discrimination task(Mednick, Nakayama et al. 2002), and go further to demonstrate the neural consequence of napping versus not napping. A recent surge in examination of the benefits of napping has produced a wide range of results in learning and memory research. Along with the benefits to perceptual learning (Mednick, Nakayama et al. 2003), napping has been shown to produce the same amount of memory increases as a full night sleep on tasks of declarative memory(Takashima, Petersson et al. 2006; Tucker, Hirota et al. 2006), motor memory(Walker, Brakefield et al. 2003) and spatial memory(Peigneux, Laureys et al. 2004). The present results suggest that napping can also prevent early sensory cortical areas from succumbing to fatigue that would otherwise have happened without a nap.
One difference between perceptual deterioration and perceptual learning may be the cortical level of processing that drives both phenomena. We have shown that performance decreases correlated with the BOLD signal in the bottom-up stimulus-driven condition in V1, but no evidence for influence of top-down processes or higher visual areas. Contrasting these results with models of perceptual learning, it appears that these two phenomena (i.e. learning and deterioration) may be opposing forces acting in parallel. Current theories of perceptual learning have shifted from strictly bottom-up models of plasticity of early visual neurons (Fiorentini and Berardi 1980; Fahle and Edelman 1993) to the proposal that learning is governed by top-down mechanisms (Ahissar and Hochstein 2004; Polley, Steinberg et al. 2006).
Generally speaking, top-down models propose that performance improvement is shaped by the task and environmental demands, as well as the state of expectation and attention of the subject, which refine access to sensory input via top-down mechanisms. Li et al. showed that for monkeys trained in a shape discrimination task, V1 neurons took on novel functional properties that were specifically related to the task demands and not tied to the primitive stimulus features of the task(Li, Piech et al. 2004). Walker and co-workers utilized fMRI with the same task used here to show that nocturnal sleep-dependent improvement was related not only to enhanced BOLD signal in primary visual cortex, but also in regions associated with higher levels of processing such as the occipital temporal junction, the medial temporal lobe and the inferior parietal lobe (Walker, Stickgold et al. 2005). While only focusing on the visual system, our data are consistent with Walker, showing that a nap produced parallel results for both behavior and BOLD signal. In our case, however, this was perceptual and neural maintenance, not improvement.
An interesting extension of these findings would be an investigation into the dynamics of these two processes. For example, it is not clear to what extent increasing levels of perceptual deterioration may affect perceptual learning. A parallel example of a deterioration in processing with repeated training in the motor system may be motor dystonia, a movement disorder characterized by sustained involuntary muscle contractions that often requires extensive repetition of a stereotypic movement to emerge (e.g. writer’s cramp)(Hallett 1998). People with this disorder show higher thresholds in a task involving discrimination of two electric stimuli closely related temporally, an abnormality that correlates with the degree of severity of dystonia(Bara-Jimenez, Catalan et al. 1998). Further, deficient activation of premotor cortex and decreased correlation between premotor cortical regions and putamen suggest a dysfunction of the premotor cortical network in patients with writer’s cramp(Ibanez, Sadato et al. 1999). Perhaps submitting a local region of primary visual cortex to an orientation discrimination task over an extended period of time may slow the neural response and decrease perception. Further research in this area may have implications for occupational related injuries due to repetitive visual processing, and in planning optimized training strategies integrating napping to learning schedules.
In summary, we have conducted the first study utilizing functional neuroimaging to demonstrate that napping can prevent training-induced fatigue of early visual neurons. Some limitations of the present study include 1) performance inside the scanner was not used in the regression with BOLD signal, 2) although the overall task inside and outside the scanned were similar; the exact stimuli were not the same across conditions, 3) low power of the study. Future studies will address these issues. Nevertheless, these perceptual changes may be fundamental to sensation and perception in everyday life. The specificity of deterioration to spatial location and configuration of the stimulus indicates that this phenomenon is not simply the result of general fatigue or changes in arousal. The results presented here suggest that perceptual deterioration is manifested as decreased activity in V1, the earliest stages of visual processing. This is caused by degradation in the feed-forward stimulus-driven, or bottom-up representation of the stimulus, rather than by a decrease in the ability of attentional mechanisms to modulate the representation of the attended stimulus. This implies that once perceptual deterioration has occurred, performance cannot be recovered simply through increasing attention or effort, because information is lost at the sensory level.
Acknowledgments
This work was supported by NIH grant EY12925 and Ruth L. Kirschstein National Research Service Award (NIH F32 EY015564) to S.C. Mednick
References
- Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn Sci. 2004;8(10):457–64. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Catalan MJ, et al. Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol. 1998;44(5):828–31. doi: 10.1002/ana.410440520. [DOI] [PubMed] [Google Scholar]
- Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Research. 2006;(46):4071–74. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375(6527):121–3. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. A framework for consciousness. Nat Neurosci. 2003;6(2):119–26. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, et al. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7(2):181–92. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S. Long-term learning in vernier acuity: Effects of stimulus orientation, range and of feedback. Vision Research. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287(5777):43–4. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Furmanski CS, Schluppeck D, et al. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol. 2004;14(7):573–8. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Gais S, Plihal W, et al. Early sleep triggers memory for early visual discrimination skills. Nature Neuroscience. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, et al. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci USA. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose GM. Learning in mammalian sensory cortex. Curr Opin Neurobiol. 2004;14(4):513–8. doi: 10.1016/j.conb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Glimcher PW. Making choices: the neurophysiology of visual-saccadic decision making. Trends Neurosci. 2001 Nov;24(11):654–9. doi: 10.1016/s0166-2236(00)01932-9. [DOI] [PubMed] [Google Scholar]
- Hallett M. Physiology of dystonia. Adv Neurol. 1998;78:11–8. [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996 Oct 18;274(5286):427–30. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, et al. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology. 1999;53(1):96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Kupfermann I, et al. Learning and memory. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. New York: McGraw-Hill; 2000. pp. 1227–1246. [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Science of the United States of America. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D. Dependence on REM Sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Li W, Piech V, et al. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7(6):651–7. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, et al. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6(7):697–8. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Arman AC, et al. The time course and specificity of perceptual deterioration. Proc Natl Acad Sci U S A. 2005;102(10):3881–5. doi: 10.1073/pnas.0407866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, et al. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5(7):677–81. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49(2):183–9. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–42. [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, et al. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26(18):4970–82. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky A, Blake R, et al. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3(11):1153–9. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci. 2006 Jun 14;26(24):6589–602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Watanabe T. The primary visual cortex fills in color. Proc Natl Acad Sci U S A. 2004;101(52):18251–6. doi: 10.1073/pnas.0406293102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, et al. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412(6846):549–53. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci U S A Dec 24. 2002;99(26):17137–42. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103(3):756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–59. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Tse PU, Martinez-Conde S, et al. Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proc Natl Acad Sci U S A. 2005;102(47):17178–83. doi: 10.1073/pnas.0508010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006 doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, et al. Sleep and the time course of motor skill learning. Learn Mem. 2003;10(4):275–84. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, et al. The functional anatomy of sleep-dependent visual skill learning. Cereb Cortex. 2005;15(11):1666–75. doi: 10.1093/cercor/bhi043. [DOI] [PubMed] [Google Scholar]
- Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neuroscience. 2004;24(7):1617–26. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser Y, Lamme VA, Schiller PH. Contextual modulation in primary visual cortex. J Neurosci. 1996 Nov 15;16(22):7376–89. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Celebrini S, et al. Neuronal plasticity that underlies improvement in perceptual performance. Science. 1994;263(5151):1289–92. doi: 10.1126/science.8122114. [DOI] [PubMed] [Google Scholar]