Abstract
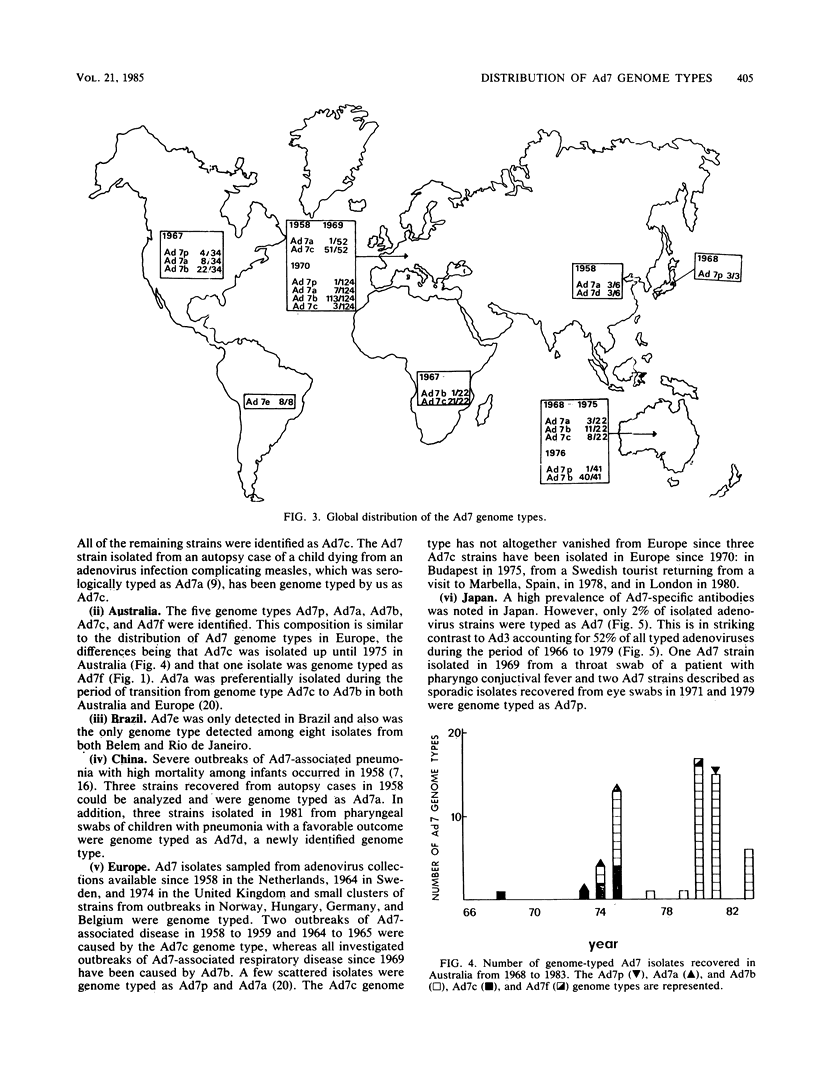
Adenovirus 7 (Ad7) is the adenovirus species that most frequently has been associated with severe illness. Seven distinct genome types of adenovirus 7, Ad7p, Ad7a, Ad7b, Ad7c, Ad7d, Ad7e, and Ad7f, can be identified by using restriction endonucleases BamHI and SmaI. We analyzed the distribution of the different Ad7 genome types among 314 isolates from patients and healthy shedders. The Ad7b and Ad7c genome types accounted for 90% of the isolates from patients and appeared to be mutually exclusive. A shift from Ad7c to Ad7b genome types occurred in 1969 in Europe and in 1975 in Australia. During the last decade, Ad7b genome types predominated in Australia, Europe, and North America. Ad7c was detected in South Africa, Ad7d was detected in China, Ad7e was detected in Brazil, and Ad7f was detected in Australia. The Ad7p and Ad7a genome types dominated among isolates obtained from healthy shedders and appeared scattered through the years and the geographical areas. The prevalence of Ad7 infections is high in Japan as judged by the herd immunity. However, the low percentage (2%) of Ad7 isolates among all adenovirus isolates chiefly from patients, coupled with 30 to 50% antibody prevalence, argues for a high proportion of inapparent infections and, hence, Ad7 strain(s) of low pathogenicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGE T. O., ENGLAND B., MAURIS C., SHUEY H. E., LENNETTE E. H. Etiology of acute respiratory disease among service personnel at Fort Ord, California. Am J Hyg. 1955 Nov;62(3):283–294. doi: 10.1093/oxfordjournals.aje.a119779. [DOI] [PubMed] [Google Scholar]
- Becroft D. M. Histopathology of fatal adenovirus infection of the respiratory tract in young children. J Clin Pathol. 1967 Jul;20(4):561–569. doi: 10.1136/jcp.20.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANY C., LEPINE P., LELONG M., LE T. V., SATGE P., VIRAT J. Severe and fatal pneumonia in infants and young children associated with adenovirus infections. Am J Hyg. 1958 May;67(3):367–378. doi: 10.1093/oxfordjournals.aje.a119941. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Hall C. E., Cooney M. K. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977 Apr;105(4):362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- JEN K. F., TAI Y., LIN Y. C., WANG H. Y. The role of adenovirus in the etiology of infantile pneumonia and pneumonia complicating measles. Chin Med J. 1962 Mar;81:141–148. [PubMed] [Google Scholar]
- JORDAN W. S., Jr, BADGER G. F., DINGLE J. H. A study of illness in a group of Cleveland families. XV. Acquisition of type-specific adenovirus antibodies in the first five years of life; implications for the use of adenovirus vaccine. N Engl J Med. 1958 May 22;258(21):1041–1044. doi: 10.1056/NEJM195805222582104. [DOI] [PubMed] [Google Scholar]
- Kaschula R. O., Druker J., Kipps A. Late morphologic consequences of measles: a lethal and debilitating lung disease among the poor. Rev Infect Dis. 1983 May-Jun;5(3):395–404. doi: 10.1093/clinids/5.3.395. [DOI] [PubMed] [Google Scholar]
- Loker E. F., Jr, Hodges G. R., Kelly D. J. Fatal adenovirus pneumonia in a young adult associated with ADV-7 vaccine administered 15 days earlier. Chest. 1974 Aug;66(2):197–199. doi: 10.1378/chest.66.2.197. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., HUEBNER R. J. Serotype composition of the adenovirus group. Proc Soc Exp Biol Med. 1958 Feb;97(2):465–470. doi: 10.3181/00379727-97-23776. [DOI] [PubMed] [Google Scholar]
- Schmitz H., Wigand R., Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983 Apr;117(4):455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- Spigelblatt L., Rosenfeld R. Hyperlucent lung: long-term complication of adenovirus type 7 pneumonia. Can Med Assoc J. 1983 Jan 1;128(1):47–49. [PMC free article] [PubMed] [Google Scholar]
- Straube R. C., Thompson M. A., Van Dyke R. B., Wadell G., Connor J. D., Wingard D., Spector S. A. Adenovirus type 7b in a children's hospital. J Infect Dis. 1983 May;147(5):814–819. doi: 10.1093/infdis/147.5.814. [DOI] [PubMed] [Google Scholar]
- TAI F. H., GRAYSTON J. T. Adenovirus neutralizing antibodies in persons on Taiwan. Proc Soc Exp Biol Med. 1962 Apr;109:881–884. doi: 10.3181/00379727-109-27366. [DOI] [PubMed] [Google Scholar]
- TENG C. H. Adenovirus pneumonia epidemic among Peking infants and preschool children in 1958. Chin Med J. 1960 Apr;80:331–339. [PubMed] [Google Scholar]
- Top F. H., Jr, Dudding B. A., Russell P. K., Buescher E. L. Control of respiratory disease in recruits with types 4 and 7 adenovirus vaccines. Am J Epidemiol. 1971 Aug;94(2):142–146. doi: 10.1093/oxfordjournals.aje.a121306. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. Two new serotypes of enteric adenovirus causing infantile diarrhoea. Dev Biol Stand. 1983;53:311–318. [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Winberg G., Varsanyi T. M., Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
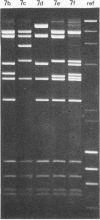
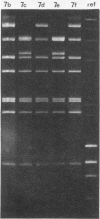
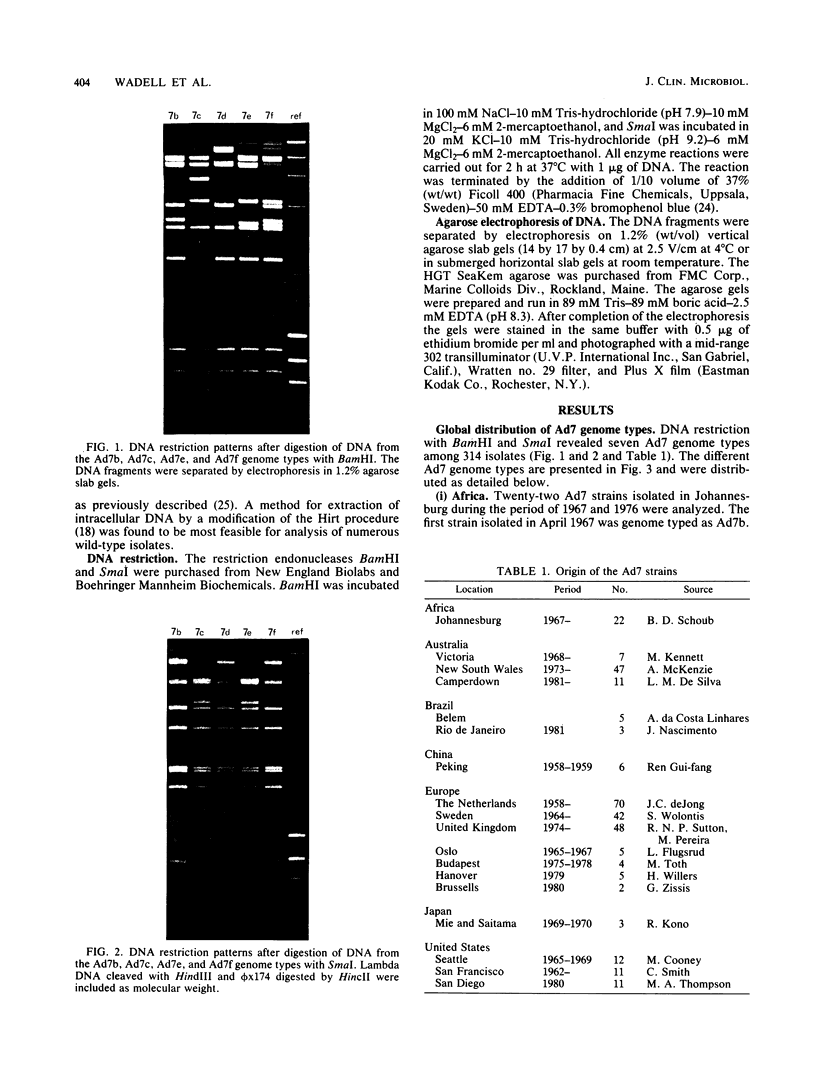
- Wadell G., Varsanyi T. M. Demonstration of three different subtypes of adenovirus type 7 by DNA restriction site mapping. Infect Immun. 1978 Jul;21(1):238–246. doi: 10.1128/iai.21.1.238-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Varsányi T. M., Lord A., Sutton R. N. Epidemic outbreaks of adenovirus 7 with special reference to the pathogenicity of adenovirus genome type 7b. Am J Epidemiol. 1980 Nov;112(5):619–628. doi: 10.1093/oxfordjournals.aje.a113034. [DOI] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect Immun. 1980 Feb;27(2):292–296. doi: 10.1128/iai.27.2.292-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C., Wolontis S. Molecular epidemiology of adenoviruses: alternating appearance of two different genome types of adenovirus 7 during epidemic outbreaks in Europe from 1958 to 1980. Infect Immun. 1981 Nov;34(2):368–372. doi: 10.1128/iai.34.2.368-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Wadell G. Structural polypeptides of adenovirus type 16 incomplete particles. J Virol. 1977 May;22(2):389–401. doi: 10.1128/jvi.22.2.389-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]