Abstract
Peroxisome proliferator-activated receptors (PPARs) are transcription factors belonging to the nuclear receptor superfamily that heterodimerize with the retinoid X receptor and bind to specific response elements in target gene promoters. PPARs have three isoforms: α, β (or δ) and γ. The prostaglandin D2 metabolite, 15-deoxy-12,14-prostaglandin J2, is an endogenous ligand for PPARγ. The antidiabetic thiazolidinediones are synthetic ligands for PPARγ. PPARγ is expressed predominantly in adipose tissue and promotes adipocyte differentiation and glucose homeostasis. PPARγ is also present in various cell types including cardiac myocytes. PPARγ regulates various neurohumoral factors involved in the progression of heart failure; its ligands inhibit cardiac hypertrophy and ischemia-reperfusion injury via, in part, a PPAR-independent pathway. Although experimental studies suggest that PPARγ ligands might have a favourable influence on heart failure, their use in patients with heart failure is limited because of an increase in plasma volume. Further studies are needed to determine whether PPARγ ligands prevent the development of heart disease in clinical settings.
Keywords: Heart failure, Hypertrophy, Ischemia-reperfusion injury, PPARγ, Thiazolidinedione
Peroxisome proliferator-activated receptors (PPARs) are transcription factors belonging to the nuclear receptor superfamily that heterodimerize with the retinoid X receptor and bind to specific response elements termed PPAR responsive elements in target gene promoters. The PPAR responsive elements are formed by a direct repeat of the hexameric consensus sequence AGGTCA, separated by one spacer nucleotide. These nuclear receptors are ligand-dependent transcription factors, and activation of target gene transcription depends on the binding of the ligand to its receptor. PPARs have three isoforms: α, β/δ and γ. Until relatively recently, PPARα was thought to be limited to the regulation of lipid catabolism and peroxisome proliferation in the liver (1), whereas PPARγ was thought to be involved in adipocyte differentiation and glucose homeostasis (2,3). Although PPARβ/δ is almost ubiquitously expressed (4–6), its roles are poorly understood. Earlier observations indicated that PPARα was present in tissues with a high oxidative capacity, such as liver, kidney and heart, while PPARγ was expressed predominantly in adipose tissue (2,3). More recently, it has been demonstrated that PPARγ is also expressed in many other cell types, such as macrophages, vascular smooth muscle cells, endothelial cells and cardiac myocytes of the cardiovascular system (7–11). Thus, interest in PPARγ’s functions in the cardiovascular system has grown and numerous investigations have focused on PPARγ. In the present review, we introduce the current trends of PPARγ research and discuss the function of PPARγ in the heart.
PPARs: THEIR LIGANDS AND INTRACELLULAR SIGNALLING PATHWAYS
The prostaglandin D2 metabolite, 15-deoxy-12,14-prostaglandin J2, was the first endogenous ligand discovered for PPARγ (12,13). Although 15-deoxy-12,14-prostaglandin J2 is the most potent natural ligand of PPARγ, the extent to which its effects are mediated through PPARγ in vivo remains to be determined. Two components of oxidized low-density lipoprotein, 9-hydroxyoctadecadienoic and 13-hydroxyoctadecadienoic acids, are also potent endogenous activators of PPARγ (14,15). Activation of 12/15-lipoxygenase induced by interleukin-4 also induces the endogenous ligands for PPARγ (16). The antidiabetic thiazolidinediones (TZDs), such as troglitazone, pioglitazone HCl, ciglitazone and rosiglitazone maleate, are synthetic ligands of PPARγ (17,18). TZDs bind PPARγ with various affinities and their insulin-sensitizing effects are exerted by activating PPARγ.
The splice variants of the γ isoform, PPARγ1 and PPARγ2, have been cloned; these two forms differ only in their N-terminal 30 amino acids (19). Although PPARγ1 is expressed in various tissues including liver, kidney, spleen, intestine, muscle, brain and lung, PPARγ2 is predominantly expressed in adipose tissue (4,5,20–22). Both PPARγ isoforms are derived from the same gene with alternative promoter usage and splicing. Like other members of nuclear receptors, PPARs have several modular domains (Figure 1) (23). The N-terminal A/B domain, which contains a ligand-independent activating function-1, is the least conserved. The C domain, which is the best conserved and consists of two zinc fingers, is the DNA-binding domain. The D domain allows for bending or conformational alteration of PPAR. The E/F domain is the ligand-binding domain (LBD). Ligand-dependent transcription requires activating function-2, which is located at the C-terminus of the LBD. Ligand binding by PPARγ is regulated by intramolecular interaction between its N-terminal A/B domain and its C-terminal LBD.
Figure 1).
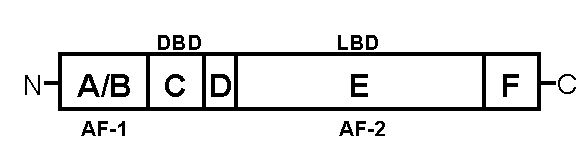
Schematic representation of the peroxisome proliferator-activated receptors (PPAR). The N-terminal A/B domain contains a ligand-independent activating function (AF)-1. The C domain consists of two zinc fingers and is the DNA-binding domain (DBD). The D domain allows for bending or conformational alteration of PPAR. The E/F domain is the ligand-binding domain (LBD) and ligand-dependent transcription requires AF-2, which is located at the C-terminus of the LBD. Ligand binding by PPARγ is regulated by intramolecular interaction between its N-terminal A/B domain and its C-terminal LBD
The activity of PPARs can be modulated by phosphorylation. PPARγ activity is depressed by phosphorylation of a serine residue (Ser112) in the A/B domain, which is mediated by a member of mitogen-activated protein kinase family, the extracellular signal-regulated protein kinase (24,25). C-Jun N-terminal kinase, another member of the mitogen-activated protein kinase family, also phosphorylates PPARγ at Ser82, reducing the transcriptional activity of PPARγ (26). These modifications may control interactions between PPARγ and coactivators or co-repressors that have been shown to interact with many members of the nuclear receptor family (27). Several lines of evidence have implicated the functional significance of the interaction between nuclear receptors and coactivators in transcriptional activation. The cyclic AMP response element binding protein-binding protein (CBP)/p300 is a transcriptional coactivator of PPARα, PPARγ and nuclear factor kappa B (NF-κB) (28–30). Steroid receptor coactivator-1 (SRC-1) is also a coactivator for both PPARγ and NF-κB (31–33). Puigserver et al (34) reported that both CBP/p300 and SRC-1 interact with the PPARγ:retinoid X receptor heterodimer, and that this interaction was mediated by the initial binding of PPARγ coactivator-1. These findings suggest that nuclear competition for limited amounts of CBP/p300 or SRC-1 may occur between PPARs and other transcription factors.
POSSIBLE ROLE OF PPARγ IN HEART FAILURE
Accumulating evidence suggests that PPARγ ligands have multiple antiatherosclerotic effects, including attenuation of the growth and migration of vascular smooth muscle cells (9,35–39) and endothelial cells (40–42), inhibition of the migration of monocytes (43,44), reduction of inflammatory cytokines from monocytes/macrophages (10,45–47), suppression of vascular cell adhesion molecule-1 and intracellular adhesion molecule-1 expression in endothelial cells (45,48), and increase of cholesterol efflux from foam cells (49–51). Diabetes mellitus is one of the leading and growing causes of coronary artery disease and heart failure. The antiatherosclerotic effects of PPARγ ligands may stabilize atherosclerotic lesions in coronary arteries, which might indirectly result in an improvement of cardiac function in diabetic patients with coronary artery disease.
Left ventricular hypertrophy is an important risk factor for ischemic heart disease and cardiac-related mortality. Insulin resistance and hyperinsulinemia are involved in cardiac hypertrophy (52,53). Yamamoto et al (54) demonstrated that the PPARγ activators, troglitazone and 15-deoxy-Delta-(12,14)-prostaglandin J2, inhibited cardiac hypertrophy caused by mechanical strain in neonatal cardiac myocytes, mediated, in part, through the NF-κB pathway (54).
The renin-angiotensin system is activated as cardiac function deteriorates, and inhibition of angiotensin-converting enzyme favourably remodels the myocardium in patients with heart failure (55). Asakawa et al (56) reported that PPARγ ligands, such as troglitazone, pioglitazone HCl and rosiglitazone maleate, inhibited angiotensin II-induced cardiac hypertrophy in neonatal rat cardiac myocytes and pressure overload-induced cardiac hypertrophy in mice, suggesting the potential clinical efficacy of TZDs for the prevention of cardiac hypertrophy.
Evidence suggests that the production of tumour necrosis factor-alpha (TNF-α) by cardiac myocytes promotes the development and progression of heart failure (57). Takano et al (11) reported that both PPARα and PPARγ activators inhibited the cardiac expression of TNF-α, in part, through attenuating NF-κB activation, suggesting that treatment with PPAR activators may prevent the development of congestive heart failure. They used only lipopolysaccharide to induce TNF-α production in cardiac myocytes. Because other cytokines, such as interleukin-1beta, interleukin-2 and interleukin-6, are also involved in the pathogenesis of congestive heart failure, further studies are needed to clarify the effects of PPAR activators on the development of heart failure in vivo.
Circulating endothelin-1 (ET-1) levels are correlated with the severity of hemodynamics and symptoms in patients with congestive heart failure (58,59). Preliminary studies (60–62) suggest that glitazones may reduce ET-1 production, which, in turn, may benefit diabetic patients with heart failure. ET-1 has been shown to induce cardiomyocyte growth in vitro (63,64) and to promote collagen synthesis by cardiac fibrosis (65). Recently, Iglarz et al (66) reported that inhibition of cardiac ET-1 production by both PPARα and PPARγ activators was associated with decreased cardiac fibrosis in deoxycorticosterone acetate-salt rats, a model of ET-1-dependent hypertension.
Shimoyama et al (67) suggested that troglitazone may exert inotropic effects in isolated perfused rat hearts, but the exact mechanism of this response still remains controversial. Ghazzi et al (68) reported that troglitazone enhanced cardiac output and stroke volume in patients with type II diabetes, but that this may have been a result of decreased arterial blood pressure and peripheral resistance.
Recently, TZDs and other ligands of PPARγ were thought to reduce tissue injury caused by regional myocardial ischemia and reperfusion in rodents. Ligands of PPARγ caused a substantial reduction in myocardial infarct size when given before onset of myocardial ischemia in the rat (69–71). Rosiglitazone maleate also improved the functional recovery of rat hearts obtained from diabetic animals subjected to global ischemia and reperfusion (72). Although the mechanisms of the cardioprotective effects of TZDs are not entirely clear, they may be due to inhibition of the activation of NF-κB, reduced expression of inducible nitric oxide synthase, monocyte chemoattractant protein-1 and intracellular adhesion molecule-1, and inhibition of Jun NH2-terminal kinase (70–72). Thus, there is growing evidence that ligands of PPARγ may be useful in the therapy of conditions associated with inflammation and ischemia-reperfusion of the heart and other organs (11,73–82). Shiomi et al (83) reported that pioglitazone HCl administration in mice subjected to infarction significantly reduced left ventricular dysfunction, and that this effect was associated with a decrease in myocyte hypertrophy and interstitial fibrosis, and reduced expression of TNF-α, transforming growth factor-beta and monocyte chemoattractant protein-1. In contrast, Lygate et al (84) did not observe modulation of left ventricular remodelling, and also found increased mortality in rats treated with rosiglitazone maleate when subjected to infarction. However, Frantz et al (85) reported that the administration of pioglitazone HCl had no effect on mortality, left ventricular remodelling, cytokine expression (including TNF-α, interleukin-1β and ET-1), collagen content or endothelial function in mice with chronic myocardial infarction. Thus, further studies are needed to evaluate the effects of TZDs on ischemic myocardium.
The majority of mechanistic and experimental studies suggests that TZDs might favourably influence cardiac hemodynamics in heart failure. However, a large scale clinical trial (86) reported fluid retention and increased plasma volume with glitazone therapy, with an increased incidence of peripheral edema. A large retrospective cohort study (87) suggested that TZD use was predictive of heart failure even after controlling for other variables. This effect may be related to increased endothelial cell permeability induced by glitazone therapy (88,89) and/or, indirectly, the facilitation of insulin’s action in promoting renal sodium retention (90,91). Because an increase in preload may contribute to worsening cardiac function in patients with heart failure, TZDs are contraindicated in patients with heart failure.
CONCLUSIONS
Experimental studies suggest that PPARγ ligands might have a favourable influence on heart failure, as summarized in Figure 2. However, PPARγ ligands increase plasma volume, which contributes to worsening cardiac function. Further studies are needed to clarify the role of PPARγ ligands in heart failure.
Figure 2).
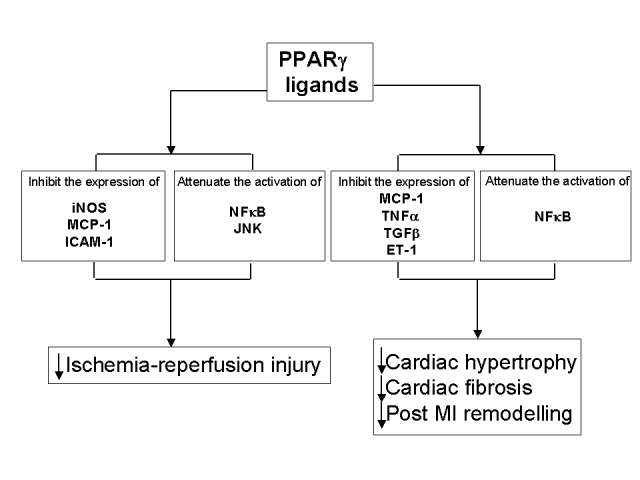
The possible role of peroxisome proliferator-activated receptor gamma (PPARg) on the heart. Experimental evidence suggest that PPARg ligands might have a favourable influence in heart failure. However, PPARg ligands are contraindicated in patients with severe heart failure because these agents increase plasma volume. ET-1 Endothelin-1; ICAM-1 Intracellular adhesion molecule-1; iNOS Inducible nitric oxide synthase; JNK c-Jun N-terminal kinase; MCP-1 Monocyte chemoattractant protein-1; MI Myocardial infarction; NFkB Nuclear factor kappa B; TGFb Transforming growth factor-beta; TNFa Tumour necrosis factor-alpha; ↓ Decreased
REFERENCES
- 1.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–50. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM, Flier JS. Adipogenesis and obesity: Rounding out the big picture. Cell. 1996;87:377–89. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 3.Spiegelman BM. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–9. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–66. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee R, Jow L, Croston GE, Paterniti JR., Jr Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem. 1997;272:8071–6. doi: 10.1074/jbc.272.12.8071. [DOI] [PubMed] [Google Scholar]
- 7.Benson S, Wu J, Padmanabhan S, Kurtz TW, Pershadsingh HA. Peroxisome proliferator-activated receptor (PPAR)-gamma expression in human vascular smooth muscle cells: Inhibition of growth, migration, and c-fos expression by the peroxisome proliferator-activated receptor (PPAR)-gamma activator troglitazone. Am J Hypertens. 2000;13:74–82. doi: 10.1016/s0895-7061(99)00148-x. [DOI] [PubMed] [Google Scholar]
- 8.Iijima K, Yoshizumi M, Ako J, et al. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in rat aortic smooth muscle cells Biochem Biophys Res Commun 1998247353–6.Erratum in: 1999;255:549. [DOI] [PubMed] [Google Scholar]
- 9.Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 11.Takano H, Nagai T, Asakawa M, et al. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Circ Res. 2000;87:596–602. doi: 10.1161/01.res.87.7.596. [DOI] [PubMed] [Google Scholar]
- 12.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 14.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–40. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 15.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 16.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 17.Camp HS, Li O, Wise SC, et al. Differential activation of peroxisome proliferator-activated receptor-gamma by troglitazone and rosiglitazone. Diabetes. 2000;49:539–47. doi: 10.2337/diabetes.49.4.539. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto J, Kimura H, Moriyama S, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–11. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 19.Fajas L, Auboeuf D, Raspe E, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–89. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 20.Auboeuf D, Rieusset J, Fajas L, et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–27. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 21.Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70:1366–75. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- 22.Vidal-Puig AJ, Considine RV, Jimenez-Linan M, et al. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997;99:2416–22. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Berger J, Hu E, et al. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–66. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 25.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–3. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 26.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–7. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 27.Takano H, Komuro I. Roles of peroxisome proliferator-activated receptor gamma in cardiovascular disease. J Diabetes Complications. 2002;16:108–14. doi: 10.1016/s1056-8727(01)00203-3. [DOI] [PubMed] [Google Scholar]
- 28.Dowell P, Ishmael JE, Avram D, Peterson VJ, Nevrivy DJ, Leid M. p300 functions as a coactivator for the peroxisome proliferator-activated receptor alpha. J Biol Chem. 1997;272:33435–43. doi: 10.1074/jbc.272.52.33435. [DOI] [PubMed] [Google Scholar]
- 29.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–32. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizukami J, Taniguchi T. The antidiabetic agent thiazolidinedione stimulates the interaction between PPAR gamma and CBP. Biochem Biophys Res Commun. 1997;240:61–4. doi: 10.1006/bbrc.1997.7602. [DOI] [PubMed] [Google Scholar]
- 31.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395:137–43. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 32.Sheppard KA, Phelps KM, Williams AJ, et al. Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem. 1998;273:29291–4. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Qi C, Calandra C, Rao MS, Reddy JK. Cloning and identification of mouse steroid receptor coactivator-1 (mSRC-1), as a coactivator of peroxisome proliferator-activated receptor gamma. Gene Expr. 1996;6:185–95. [PMC free article] [PubMed] [Google Scholar]
- 34.Puigserver P, Adelmant G, Wu Z, et al. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–71. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 35.Law RE, Meehan WP, Xi XP, et al. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law RE, Goetze S, Xi XP, et al. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–8. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- 37.Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA, Law RE. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1→ S transition in vascular smooth muscle cells. J Biol Chem. 2000;275:22435–41. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- 38.Goetze S, Xi XP, Kawano H, et al. PPAR gamma-ligands inhibit migration mediated by multiple chemoattractants in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1999;33:798–806. doi: 10.1097/00005344-199905000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Abe M, Hasegawa K, Wada H, et al. GATA-6 is involved in PPARgamma-mediated activation of differentiated phenotype in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:404–10. doi: 10.1161/01.ATV.0000059405.51042.A0. [DOI] [PubMed] [Google Scholar]
- 40.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116–21. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 41.Murata T, He S, Hangai M, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:2309–17. [PubMed] [Google Scholar]
- 42.Murata T, Hata Y, Ishibashi T, et al. Response of experimental retinal neovascularization to thiazolidinediones. Arch Ophthalmol. 2001;119:709–17. doi: 10.1001/archopht.119.5.709. [DOI] [PubMed] [Google Scholar]
- 43.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARgamma: Differentiation-dependent peroxisomal proliferator-activated receptor gamma (PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kintscher U, Goetze S, Wakino S, et al. Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. Eur J Pharmacol. 2000;401:259–70. doi: 10.1016/s0014-2999(00)00461-1. [DOI] [PubMed] [Google Scholar]
- 45.Jackson SM, Parhami F, Xi XP, et al. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 46.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 47.Han KH, Chang MK, Boullier A, et al. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J Clin Invest. 2000;106:793–802. doi: 10.1172/JCI10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–8. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 49.Chinetti G, Lestavel S, Bocher V, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–8. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 50.Chawla A, Boisvert WA, Lee CH, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–71. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 51.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe K, Sekiya M, Tsuruoka T, Funada J, Kameoka H. Effect of insulin resistance on left ventricular hypertrophy and dysfunction in essential hypertension. J Hypertens. 1999;17:1153–60. doi: 10.1097/00004872-199917080-00015. [DOI] [PubMed] [Google Scholar]
- 53.Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42:246–53. doi: 10.1016/s0008-6363(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto K, Ohki R, Lee RT, Ikeda U, Shimada K. Peroxisome proliferator-activated receptor gamma activators inhibit cardiac hypertrophy in cardiac myocytes. Circulation. 2001;104:1670–5. doi: 10.1161/hc4001.097186. [DOI] [PubMed] [Google Scholar]
- 55.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 56.Asakawa M, Takano H, Nagai T, et al. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105:1240–6. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- 57.Bryant D, Becker L, Richardson J, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–81. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 58.Pacher R, Stanek B, Hulsmann M, et al. Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J Am Coll Cardiol. 1996;27:633–41. doi: 10.1016/0735-1097(95)00520-x. [DOI] [PubMed] [Google Scholar]
- 59.Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992;85:504–9. doi: 10.1161/01.cir.85.2.504. [DOI] [PubMed] [Google Scholar]
- 60.Fukunaga Y, Itoh H, Doi K, et al. Thiazolidinediones, peroxisome proliferator-activated receptor gamma agonists, regulate endothelial cell growth and secretion of vasoactive peptides. Atherosclerosis. 2001;158:113–9. doi: 10.1016/s0021-9150(01)00430-0. [DOI] [PubMed] [Google Scholar]
- 61.Neve BP, Fruchart JC, Staels B. Role of the peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol. 2000;60:1245–50. doi: 10.1016/s0006-2952(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 62.Delerive P, Martin-Nizard F, Chinetti G, et al. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- 63.Yamazaki T, Komuro I, Kudoh S, et al. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–8. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 64.Ito H, Hirata Y, Adachi S, et al. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J Clin Invest. 1993;92:398–403. doi: 10.1172/JCI116579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27:2130–4. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 66.Iglarz M, Touyz RM, Viel EC, et al. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension. 2003;42:737–43. doi: 10.1161/01.HYP.0000083511.91817.B1. [DOI] [PubMed] [Google Scholar]
- 67.Shimoyama M, Ogino K, Tanaka Y, Ikeda T, Hisatome I. Hemodynamic basis for the acute cardiac effects of troglitazone in isolated perfused rat hearts. Diabetes. 1999;48:609–15. doi: 10.2337/diabetes.48.3.609. [DOI] [PubMed] [Google Scholar]
- 68.Ghazzi MN, Perez JE, Antonucci TK, et al. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. The Troglitazone Study Group. Diabetes. 1997;46:433–9. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- 69.Thiemermann C, Wayman NS. Menarini Academy Cardiovascular Research Awards in Basic Science 2001: Ligands of the orphan receptor peroxisome-proliferator activator-gamma reduce myocardial infarct size. Med Sci Monit. 2001;7:787–9. [PubMed] [Google Scholar]
- 70.Yue TL, Bao W, Jucker BM, et al. Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation. 2003;108:2393–9. doi: 10.1161/01.CIR.0000093187.42015.6C. [DOI] [PubMed] [Google Scholar]
- 71.Wayman NS, Hattori Y, McDonald MC, et al. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16:1027–40. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 72.Khandoudi N, Delerive P, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51:1507–14. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- 73.Nakajima A, Wada K, Miki H, et al. Endogenous PPAR gamma mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120:460–9. doi: 10.1053/gast.2001.21191. [DOI] [PubMed] [Google Scholar]
- 74.Cuzzocrea S, Wayman NS, Mazzon E, et al. The cyclopentenone prostaglandin 15-deoxy-Delta(12,14)-prostaglandin J(2) attenuates the development of acute and chronic inflammation. Mol Pharmacol. 2002;61:997–1007. doi: 10.1124/mol.61.5.997. [DOI] [PubMed] [Google Scholar]
- 75.Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2, ligands of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), reduce ischaemia/reperfusion injury of the gut. Br J Pharmacol. 2003;140:366–76. doi: 10.1038/sj.bjp.0705419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kon K, Ikejima K, Hirose M, et al. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem Biophys Res Commun. 2002;291:55–61. doi: 10.1006/bbrc.2002.6385. [DOI] [PubMed] [Google Scholar]
- 77.Naito Y, Takagi T, Uchiyama K, et al. Suppression of intestinal ischemia-reperfusion injury by a specific peroxisome proliferator-activated receptor-gamma ligand, pioglitazone, in rats. Redox Rep. 2002;7:294–9. doi: 10.1179/135100002125000983. [DOI] [PubMed] [Google Scholar]
- 78.Shiojiri T, Wada K, Nakajima A, et al. PPAR gamma ligands inhibit nitrotyrosine formation and inflammatory mediator expressions in adjuvant-induced rheumatoid arthritis mice. Eur J Pharmacol. 2002;448:231–8. doi: 10.1016/s0014-2999(02)01946-5. [DOI] [PubMed] [Google Scholar]
- 79.Collin M, Thiemermann C. The PPAR-gamma ligand 15-deoxyDelta12,14 prostaglandin J2 reduces the liver injury in endotoxic shock. Eur J Pharmacol. 2003;476:257–8. doi: 10.1016/s0014-2999(03)02179-4. [DOI] [PubMed] [Google Scholar]
- 80.Enomoto N, Takei Y, Hirose M, et al. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther. 2003;306:846–54. doi: 10.1124/jpet.102.047217. [DOI] [PubMed] [Google Scholar]
- 81.Sivarajah A, Chatterjee PK, Patel NS, et al. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol. 2003;23:267–76. doi: 10.1159/000072088. [DOI] [PubMed] [Google Scholar]
- 82.Ito H, Nakano A, Kinoshita M, Matsumori A. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates myocardial ischemia/reperfusion injury in a rat model. Lab Invest. 2003;83:1715–21. doi: 10.1097/01.lab.0000106724.29121.da. [DOI] [PubMed] [Google Scholar]
- 83.Shiomi T, Tsutsui H, Hayashidani S, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106:3126–32. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 84.Lygate CA, Hulbert K, Monfared M, Cole MA, Clarke K, Neubauer S. The PPARgamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc Res. 2003;58:632–7. doi: 10.1016/s0008-6363(03)00289-x. [DOI] [PubMed] [Google Scholar]
- 85.Frantz S, Hu K, Widder J, et al. Peroxisome proliferator activated-receptor agonism and left ventricular remodeling in mice with chronic myocardial infarction. Br J Pharmacol. 2004;141:9–14. doi: 10.1038/sj.bjp.0705585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mudaliar S, Henry RR. New oral therapies for type 2 diabetes mellitus: The glitazones or insulin sensitizers. Annu Rev Med. 2001;52:239–57. doi: 10.1146/annurev.med.52.1.239. [DOI] [PubMed] [Google Scholar]
- 87.Delea TE, Edelsberg JS, Hagiwara M, Oster G, Phillips LS. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: A retrospective cohort study. Diabetes Care. 2003;26:2983–9. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 88.Niemeyer NV, Janney LM. Thiazolidinedione-induced edema. Pharmacotherapy. 2002;22:924–9. doi: 10.1592/phco.22.11.924.33626. [DOI] [PubMed] [Google Scholar]
- 89.Idris I, Gray S, Donnelly R. Rosiglitazone and pulmonary oedema: An acute dose-dependent effect on human endothelial cell permeability. Diabetologia. 2003;46:288–90. doi: 10.1007/s00125-002-1008-1. [DOI] [PubMed] [Google Scholar]
- 90.Stenvinkel P, Bolinder J, Alvestrand A. Effects of insulin on renal haemodynamics and the proximal and distal tubular sodium handling in healthy subjects. Diabetologia. 1992;35:1042–8. doi: 10.1007/BF02221679. [DOI] [PubMed] [Google Scholar]
- 91.Muscelli E, Natali A, Bianchi S, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9:746–52. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]


