Abstract
BACKGROUND:
Although various studies have examined the short-term effects of a ketogenic diet in reducing weight in obese patients, its long-term effects on various physical and biochemical parameters are not known.
OBJECTIVE:
To determine the effects of a 24-week ketogenic diet (consisting of 30 g carbohydrate, 1 g/kg body weight protein, 20% saturated fat, and 80% polyunsaturated and monounsaturated fat) in obese patients.
PATIENTS AND METHODS:
In the present study, 83 obese patients (39 men and 44 women) with a body mass index greater than 35 kg/m2, and high glucose and cholesterol levels were selected. The body weight, body mass index, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, fasting blood sugar, urea and creatinine levels were determined before and after the administration of the ketogenic diet. Changes in these parameters were monitored after eight, 16 and 24 weeks of treatment.
RESULTS:
The weight and body mass index of the patients decreased significantly (P<0.0001). The level of total cholesterol decreased from week 1 to week 24. HDL cholesterol levels significantly increased, whereas LDL cholesterol levels significantly decreased after treatment. The level of triglycerides decreased significantly following 24 weeks of treatment. The level of blood glucose significantly decreased. The changes in the level of urea and creatinine were not statistically significant.
CONCLUSIONS:
The present study shows the beneficial effects of a long-term ketogenic diet. It significantly reduced the body weight and body mass index of the patients. Furthermore, it decreased the level of triglycerides, LDL cholesterol and blood glucose, and increased the level of HDL cholesterol. Administering a ketogenic diet for a relatively longer period of time did not produce any significant side effects in the patients. Therefore, the present study confirms that it is safe to use a ketogenic diet for a longer period of time than previously demonstrated.
Keywords: Diet, Ketosis, Obesity
Obesity has become a serious chronic disease in both developing and developed countries. Furthermore, it is associated with a variety of chronic diseases (1–4). It is estimated that in the United States alone approximately 300,000 people die each year from obesity-related diseases (5,6). Different methods for reducing weight using reduced calorie and fat intake combined with exercise have failed to show sustained long-term effects (7–9). Recent studies from various laboratories (10,11), including our own (12), have shown that a high fat diet rich in polyunsaturated fatty acids (ketogenic diet) is quite effective in reducing body weight and the risk factors for various chronic diseases. The ketogenic diet was originally introduced in 1920 (13). In this diet, the fat to carbohydrate ratio is 5:1. While there was a significant decrease in the weight of obese patients who were on a ketogenic diet (12), the reverse occurred when the diet changed to one high in carbohydrates (14).
It should be noted that the concept that fat can be eaten ad libitum and still induce weight loss in obese subjects is not a recent one (13–33). Ketosis occurs as a result of the change in the body’s fuel from carbohydrate to fat. Incomplete oxidation of fatty acids by the liver results in the accumulation of ketone bodies in the body. A ketogenic diet maintains the body in a state of ketosis, which is characterized by an elevation of D-b-hydroxybutyrate and acetoacetate.
Mild ketosis is a natural phenomenon that occurs in humans during fasting and lactation (19,20). Postexercise ketosis is a well-known phenomenon in mammals. Although most of the changes in the physiological parameters induced following exercise revert back to their normal values rapidly, the level of circulating ketone bodies increases for a few hours after muscular activity ceases (21). It has been found that in trained individuals, a low blood ketone level protects against the development of hypoglycemia during prolonged intermittent exercise (22). In addition, ketosis has a significant influence on suppressing hunger. Thus, a ketogenic diet is a good regulator of the body’s calorie intake and mimics the effect of starvation in the body.
It is generally believed that high fat diets may lead to the development of obesity and several other diseases such as coronary artery disease, diabetes and cancer. This view, however, is based on studies carried out in animals that were given a high fat diet rich in polyunsaturated fatty acids. In contrast, our laboratory has recently shown that a ketogenic diet modified the risk factors for heart disease in obese patients (12).
Although various short-term studies examining the effect of a ketogenic diet in reducing the weight of obese patients have been carried out (10), its long-term effects in obese subjects are not known (15). Therefore, the purpose of the present study was to investigate the long-term effects of a ketogenic diet on obesity and obesity-associated risk factors in a large population of obese patients.
PATIENTS AND METHODS
Patients and biochemical analysis
The prospective study was carried out at the Academic Department of Surgery, Consultation and Training Centre, Faculty of Medicine, Kuwait University (Jabriya, Kuwait) in 83 obese subjects (39 men and 44 women). The body mass index (BMI) of men and women was 35.9±1.2 kg/m2 and 39.4±1.0 kg/m2, respectively. The mean age was 42.6±1.7 years and 40.6±1.6 years for men and women, respectively. The mean age, initial height, weight and BMI for all patients are given in Table 1. Fasting blood tests were carried out for all of the subjects. Initially, all patients were subjected to liver and renal function tests, and glucose and lipid profiles, using fasting blood samples, and a complete blood count. Thereafter, fasting blood samples were tested for total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, triglycerides, blood sugar, urea and creatinine levels at the eighth, 16th and 24th week. In addition, weight and height measurements, and blood pressure were monitored at each visit.
TABLE 1.
Patient data at baseline before treatment with the ketogenic diet
| n | Age (years) | Height (m) | Weight (kg) | Body mass index (kg/m2) | |
|---|---|---|---|---|---|
| Men | 39 | 42.6±1.7 | 1.7±0.01 | 102.4±3.7 | 35.9±1.2 |
| Women | 44 | 40.6±1.6 | 1.6±0.01 | 99.8±2.9 | 39.4±1.0 |
All data are mean ± SEM
Protocol for ketogenic diet-induced body weight reduction
All 83 subjects received the ketogenic diet consisting of 20 g to 30 g of carbohydrate in the form of green vegetables and salad, and 80 g to 100 g of protein in the form of meat, fish, fowl, eggs, shellfish and cheese. Polyunsaturated and monounsaturated fats were also included in the diet. Twelve weeks later, an additional 20 g of carbohydrate were added to the meal of the patients to total 40 g to 50 g of carbohydrate. Micronutrients (vitamins and minerals) were given to each subject in the form of one capsule per day (Table 2).
TABLE 2.
Composition of the capsule*
| Para-aminobenzoic acid (PH) | 30 mg |
| Vitamin B1 (thiamin mononitrate) (BP) | 15 mg |
| Vitamin B2 (riboflavin) (BP) | 3 mg |
| Vitamin B5 (nicotinamide) (BP) | 25 mg |
| Vitamin B3 (calcium pantothenate) (PH) | 3 mg |
| Vitamin B6 (pyridoxine HCI) (BP) | 5 mg |
| Vitamin B12 (cyanocobalamin) (BP) | 10 μg |
| Biotin (PH) | 5 μg |
| Folic acid (BP) | 100 μg |
| Vitamin C (ascorbic acid) BP | 60 mg |
| Vitamin A (retinol) (USP; 2000 IU) | 0.6 mg |
| Vitamin D (calciferol) (INN; 200 IU) | 5 μg |
| Vitamin E (tocopherol acetate) (USNF) | 10 mg |
| Lecithin (PH) | 40 mg |
| Wheat germ oil | 100 mg |
| Lysine (FP) | 40 mg |
| Methionine (DAB) | 60 mg |
| Rutin (DAB) (rutoside) (INN) | 10 mg |
| Iron (as fumarate; BP) | 12 mg |
| Calcium (as dicalcium phosphate) (BP) | 52 mg |
| Phosphorus (as dicalcium phosphate) (BP) | 40 mg |
| Potassium (as KCl) (BP) | 2 mg |
| Zinc (as ZnSO4) (BP) | 8 mg |
| Copper (as CuSO4) (BP) | 1 mg |
| Manganese (as MnSO4) (BP) | 2 mg |
| Iodine (as potassium iodide) (BP) | trace |
| Ginseng (Siberian) (5:1 concentrated extract) | 4 mg |
Net weight 45 g. BP British Pharmacopoeia; DAB German Pharmacopoeia; FP French Pharmacopoeia; INN International nonpropietary names; IU International units; PH Swiss Pharmacopoeia; USNF United States National Formulary; USP United States Pharmacopoeia
Statistical analysis
Statistical differences between body weight, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, level of fasting blood sugar, and urea and creatinine levels before and after the administration of the ketogenic diet were analyzed using a paired Student’s t test using the Stat-view version 4.02 (Abacus Concepts Inc, USA). Weight, BMI and all biochemical parameters are expressed as mean ± SEM.
RESULTS
The mean initial weight of the subjects was 101.03±2.33 kg. The weight decreased significantly during all stages of the treatment period. The body weights at the eighth, 16th and 24th week were 91.10±2.76 kg, 89.39±3.4 kg and 86.67±3.70 kg, respectively (Figure 1). Similar to the loss in body weight, a significant decrease was observed in the BMI of the patients following the administration of the ketogenic diet. The initial BMI, and the BMI after the eighth, 16th and 24th week were 37.77±0.79 kg/m2, 33.90±0.83 kg/m2, 33.24±1.00 kg/m2 and 32.06±1.13 kg/m2, respectively (Figure 2).
Figure 1).
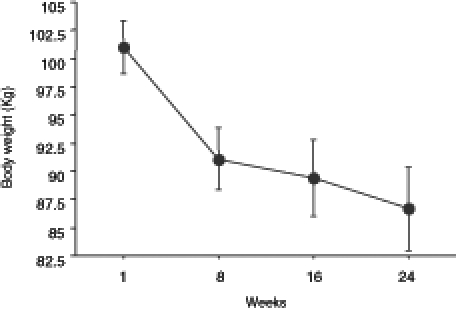
Reduction in body weight at eight, 16 and 24 weeks following the administration of the ketogenic diet in obese patients. The weights are expressed as mean ± SEM
Figure 2).
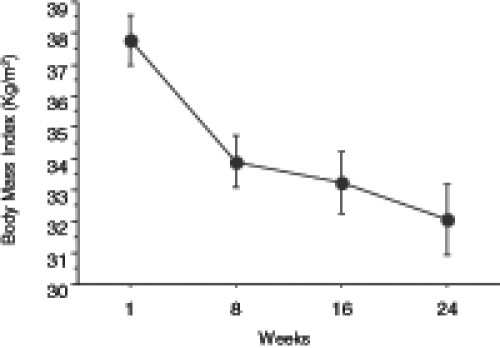
Decrease in body mass index at eight, 16 and 24 weeks during the administration of a ketogenic diet in obese patients. The values are expressed as mean ± SEM
The level of total cholesterol showed a significant decrease from week 1 to week 24 (Figure 3). The level of HDL cholesterol significantly increased (Figure 4), whereas LDL cholesterol levels significantly decreased with treatment (Figure 5). The level of triglycerides decreased significantly after 24 weeks of treatment. The initial level of triglycerides was 2.75±0.23 mmol/L, whereas at week 24, the level decreased to 1.09±0.08 mmol/L (Figure 6). The level of blood glucose significantly decreased at week 24. The initial blood glucose level and its level at the eighth, 16th and 24th week were 7.26±0.38 mmol/L, 5.86±0.27 mmol/L, 5.56±0.19 mmol/L and 5.62±0.18 mmol/L, respectively (Figure 7). The changes in the levels of urea (Figure 8) and creatinine (Figure 9) were not statistically significant.
Figure 3).
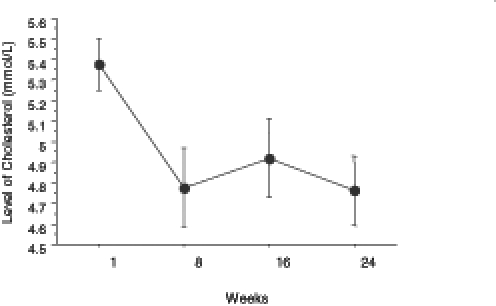
Decreased levels of total cholesterol (expressed as mean ± SEM) in obese patients at eight, 16 and 24 weeks during the administration of a ketogenic diet
Figure 4).
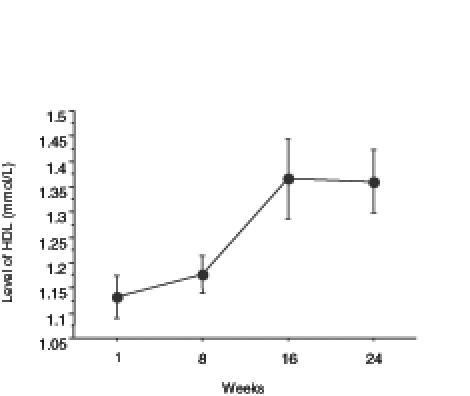
Changes in the level of high density lipoprotein (HDL) cholesterol in obese patients during treatment with a ketogenic diet for a period of 24 weeks. Data are expressed as mean ± SEM
Figure 5).
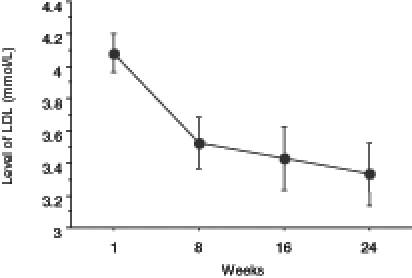
Changes in the level of low density lipoprotein (LDL) cholesterol during treatment with a ketogenic diet in obese patients at eight, 16 and 24 weeks. The values are expressed as mean ± SEM
Figure 6).
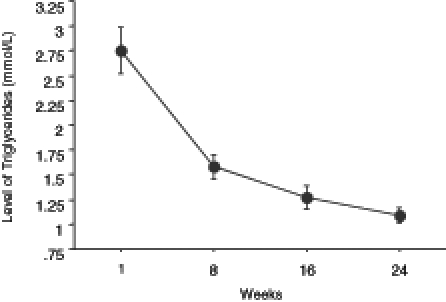
Changes in the level of triglycerides in obese patients during treatment with a ketogenic diet over a period of 24 weeks. The values are expressed as mean ± SEM
Figure 7).
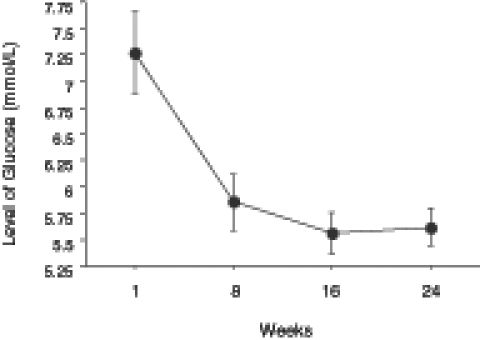
Decreased levels of blood glucose (expressed as mean ± SEM) in obese patients at eight, 16 and 24 weeks during the administration of a ketogenic diet
Figure 8).
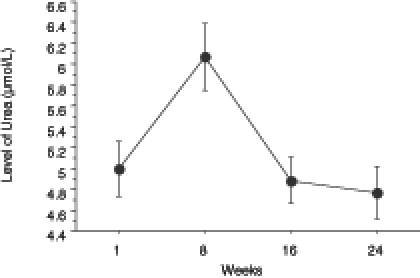
Changes in the level of urea in obese patients during a 24-week ketogenic diet. The level of urea is expressed as mean ± SEM
Figure 9).
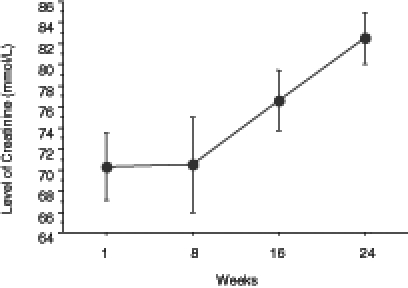
Changes in the level of creatinine in obese patients during a 24-week ketogenic diet. Values are expressed as mean ± SEM
DISCUSSION
Until recently, ketosis was viewed with apprehension in the medical world; however, current advances in nutritional research have discounted this apprehension and increased public awareness about its favourable effects. In humans, ketone bodies are the only additional source of brain energy after glucose (23,24). Thus, the use of ketone bodies by the brain could be a significant evolutionary development that occurred in parallel with brain development in humans. Hepatic generation of ketone bodies during fasting is essential to provide an alternate fuel to glucose. This is necessary to spare the destruction of muscle from glucose synthesis.
A ketogenic diet is clinically and experimentally effective in antiepileptic and antiobesity treatments; however, the molecular mechanisms of its action remain to be elucidated. In some cases, a ketogenic diet is far better than modern anticonvulsants (25). Recently, it has been shown that a ketogenic diet is a safe potential alternative to other existing therapies for infantile spasms (27). It was further shown that a ketogenic diet could act as a mood stabilizer in bipolar illness (28). Beneficial changes in the brain energy profile have been observed in subjects who are on a ketogenic diet (28). This is a significant observation because cerebral hypometabolism is a characteristic feature of those who suffer from depression or mania (28). It has also been found that a ketogenic diet affects signal transduction in neurons by inducing changes in the basal status of protein phosphorylation (29). In another study (30), it was shown that a ketogenic diet induced gene expression in the brain. These studies provide evidence to explain the actions of a ketogenic diet in the brain.
One of the mechanisms of a ketogenic diet in epilepsy may be related to increased availability of beta-hydroxybutyrate, a ketone body readily transported through the blood-brain barrier. In support of this hypothesis, it was found that a ketogenic diet was the treatment of choice for glucose transporter protein syndrome and pyruvate dehydrogenase deficiency, which are both associated with cerebral energy failure and seizures (26).
One argument against the consumption of a high fat diet is that it causes obesity. The major concern in this regard is whether a high percentage of dietary fat promotes weight gain more than a low percentage of fat intake. Because fat has a higher caloric density than carbohydrate, it is thought that the consumption of a high fat diet will be accompanied by a higher energy intake (31). On the contrary, recent studies from our laboratory (12) and many other laboratories (24,32–34) have observed that a ketogenic diet can be used as a therapy for weight reduction in obese patients.
It has been found that a sugary diet is the root cause of various chronic diseases of the body. A recent study (35) showed that sugar can accelerate aging. Several recent studies (36,37) have pointed to the fact that a diet with a high glycemic load is independently associated with the development of cardiovascular diseases, type II diabetes and certain forms of cancer. Glycemic load refers to a diet of different foods that have a high glycemic index. Glycemic index is a measure of the elevation of glucose levels following the ingestion of a carbohydrate. The classification of a carbohydrate based on its glycemic index provided a better predictor of risk for coronary artery diseases than the traditional method of classification of carbohydrate into simple or complex forms (38). In other studies (38–46), it was shown that the risk of dietary glycemic load from refined carbohydrates was independent of other known risk factors for coronary diseases.
It is now evident that high carbohydrate diets increase fasting plasma triglyceride concentrations (47–51) and decrease HDL cholesterol concentrations (52–55). These changes are associated with enhanced atherogenesis (55). However, it has been shown that short-term ketogenic diets improve the lipid disorders that are characteristic of atherogenic dyslipidemia (56). It has also been found that sugary drinks decreased blood levels of vitamin E, thus reducing the amount of antioxidants in the body. It has been proven, beyond a doubt, that disrupting the oxidant-antioxidant status of the cell will lead to various diseases of the body (57).
The relation between a high fat diet and cancer is not conclusive. Recent epidemiological studies (17,58–60) could not explain a specific causal relationship between dietary fat and cancer. It has been found that altered energy metabolism and substrate requirements of tumour cells provide a target for selective antineoplastic therapy. The supply of substrates for tumour energy metabolism can be reduced by dietary manipulation (eg, ketogenic diet) or by pharmacological means at the cellular level (eg, inhibitors of glycolysis or oxidative phosphorylation). Both of these techniques are nontoxic methods for controlling tumour growth in vivo (61). Sugar consumption is positively associated with cancer in humans and test animals (58–61). This observation is quite logical because tumours are known to be enormous sugar absorbers. It has also been found that the risk of breast cancer decreases with increases in total fat intake (16). Further studies on the role of a ketogenic diet in antineoplastic therapy are in progress in our laboratory.
A link between low fat diets and osteoporosis has been suggested. Very low fat diets are considered to be low in calcium content. Women on low fat diets excrete most of the calcium they consume; therefore, they are more prone to osteoporosis. However, a high fat diet can rectify this situation (62).
In the present study, a control population on a low fat diet was not included due to the difficulties in recruiting subjects for a control group. However, several studies (63,64) with appropriate control groups that compared the effect of a low fat diet with a low carbohydrate ketogenic diet have recently been published. In this regard, these two recent studies are comparable with the present study. Brehm et al (23) showed that obese women on a low carbohydrate ketogenic diet lost 8.5 kg over six months compared with 4.2 kg lost by those in the low fat diet group (P<0.001). Twenty-two subjects from the low carbohydrate ketogenic diet and 20 subjects from the low fat diet completed the study, with both groups reducing their energy intake by approximately 450 kcal from the baseline level. In another study performed in 132 severely obese subjects for six months (24), there was greater weight loss in the low carbohydrate ketogenic diet group than in the low fat diet group (5.8 kg versus 1.9 kg, P=0.002). Both of these studies support the findings presented in the present paper.
CONCLUSIONS
The data presented in the present study showed that a ketogenic diet acted as a natural therapy for weight reduction in obese patients. This is a unique study monitoring the effect of a ketogenic diet for 24 weeks. There was a significant decrease in the level of triglycerides, total cholesterol, LDL cholesterol and glucose, and a significant increase in the level of HDL cholesterol in the patients. The side effects of drugs commonly used for the reduction of body weight in such patients were not observed in patients who were on the ketogenic diet. Therefore, these results indicate that the administration of a ketogenic diet for a relatively long period of time is safe. Further studies elucidating the molecular mechanisms of a ketogenic diet are in progress in our laboratory. These studies will open new avenues into the potential therapeutic uses of a ketogenic diet and ketone bodies.
REFERENCES
- 1.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Barnett JP. Metabolic and health complications of obesity. Dis Mon. 1990;36:641–731. [PubMed] [Google Scholar]
- 3.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–60. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 4.Simopoulos AP, Van Itallie TB. Body weight, health, and longevity. Ann Intern Med. 1984;100:285–95. doi: 10.7326/0003-4819-100-2-285. [DOI] [PubMed] [Google Scholar]
- 5.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–12. [PubMed] [Google Scholar]
- 6.Thomas PR, editor. Washington: National Academy Press; 1995. Weighing the Options: Criteria for Evaluating Weight-Management Programs. [PubMed] [Google Scholar]
- 7.Andersen T, Stokholm KH, Backer OG, Quaade F. Long-term (5-year) results after either horizontal gastroplasty or very-low-calorie diet for morbid obesity. Int J Obes. 1988;12:277–84. [PubMed] [Google Scholar]
- 8.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: Patterns of regain among men and women. Int J Obes. 1989;13:123–36. [PubMed] [Google Scholar]
- 9.Peni MG. Improving maintenance of weight loss following treatment by diet and lifestyle modification. In: Wadden TA, Van Itallie TB, editors. Treatment of the Seriously Obese Patient. New York: Guilford; 1992. pp. 456–77. [Google Scholar]
- 10.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factors in overweight adolescents. J Pediatr. 2003;142:253–8. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 11.Yancy WS, Jr, Guyton JR, Bakst RP, Westman EC. A randomized, controlled trial of a low-carbohydrate ketogenic diet versus a low-fat diet for obesity and hyperlipidemia. Am J Clin Nutr. 2002;72:343S. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dashti HM, Bo-Abbas YY, Asfar SK, et al. Ketogenic diet modifies the risk factors of heart disease in obese patients. Nutrition. 2003;19:901–2. doi: 10.1016/s0899-9007(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 13.Wilder RM. The effect of ketonemia on the course of epilepsy. Mayo Clin Proc. 1921;2:307–8. [Google Scholar]
- 14.Pilkington TR, Rosenoer VM, Gainsborough H, Carey M. Diet and weight-reduction in the obese. Lancet. 1960;i:856–8. doi: 10.1016/s0140-6736(60)90736-4. [DOI] [PubMed] [Google Scholar]
- 15.Howard BV, Wylie-Rosett J. Sugar and cardiovascular disease: A statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2002;106:523–7. doi: 10.1161/01.cir.0000019552.77778.04. Erratum in 2003;107:2166. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi S, Favero A, Decarli A, et al. Intake of macronutrients and risk of breast cancer. Lancet. 1996;347:1351–6. doi: 10.1016/s0140-6736(96)91008-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Manson JE, Stantpfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin. 2001;73:560–6. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 18.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein and risk of myocardial infarction. Circulation. 1997;96:2520–5. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 19.Kreitzman SN. Factors influencing body composition during very-low-caloric diets. Am J Clin Nutr. 1992;56(l Suppl):217S–23S. doi: 10.1093/ajcn/56.1.217S. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, et al. Medical aspects of ketone body metabolism. Clin Invest Med. 1995;18:193–216. [PubMed] [Google Scholar]
- 21.Koeslag JH. Post-exercise ketosis and the hormone response to exercise: A review. Med Sci Sports Exerc. 1982;14:327–34. [PubMed] [Google Scholar]
- 22.Winder WW, Baldwin KM, Holloszy JO. Exercise-induced increase in the capacity of rat skeletal muscle to oxidize ketones. Can J Physiol Pharmacol. 1975;53:86–91. doi: 10.1139/y75-011. [DOI] [PubMed] [Google Scholar]
- 23.Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions. J Neurosci Res. 1999;56:565–70. doi: 10.1002/(SICI)1097-4547(19990615)56:6<565::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 24.Amiel SA. Organ fuel selection: Brain. Proc Nutr Soc. 1995;54:151–5. doi: 10.1079/pns19950044. [DOI] [PubMed] [Google Scholar]
- 25.Singhi PD. Newer antiepileptic drugs and non surgical approaches in epilepsy. Indian J Pediatr. 2000;67:S92–8. [PubMed] [Google Scholar]
- 26.Janigro D. Blood-brain barrier, ion homeostatis and epilepsy: Possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Res. 1999;37:223–32. doi: 10.1016/s0920-1211(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 27.Kossoff EH, Pyzik PL, McGrogan JR, Vining EP, Freeman JM. Efficacy of the ketogenic diet for infantile spasms. Pediatrics. 2002;109:780–3. doi: 10.1542/peds.109.5.780. [DOI] [PubMed] [Google Scholar]
- 28.El-Mallakh RS, Paskitti ME. The ketogenic diet may have mood-stabilizing properties. Med Hypotheses. 2001;57:724–6. doi: 10.1054/mehy.2001.1446. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler DR, Araujo E, Rotta LN, Perry ML, Goncalves CA. A ketogenic diet increases protein phosphorylation in brain slices of rats. J Nutr. 2002;132:483–7. doi: 10.1093/jn/132.3.483. [DOI] [PubMed] [Google Scholar]
- 30.Cullingford TE, Eagles DA, Sato H. The ketogenic diet upregulates expression of the gene encoding the key ketogenic enzyme mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in rat brain. Epilepsy Res. 2002;49:99–107. doi: 10.1016/s0920-1211(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 31.Prentice AM. Manipulation of dietary fat and energy density and subsequent effects on substrate flux and food intake. Am J Clin Nutr. 1998;67(3 Suppl):535S–41S. doi: 10.1093/ajcn/67.3.535S. [DOI] [PubMed] [Google Scholar]
- 32.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 33.He K, Merchant A, Rimm EB, et al. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ. 2003;327:777–82. doi: 10.1136/bmj.327.7418.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westman EC, Mavropoulos J, Yancy WS, Volek JS. A review of low-carbohydrate ketogenic diets. Curr Atheroscler Rep. 2003;5:476–83. doi: 10.1007/s11883-003-0038-6. [DOI] [PubMed] [Google Scholar]
- 35.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 37.Leeds AR. Glycemic index and heart disease. Am J Clin Nutr. 2002;76:286S–9S. doi: 10.1093/ajcn/76/1.286S. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycaemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–61. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 39.Sims EA, Danford E, Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–96. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- 40.Golay A, DeFronzo RA, Ferrannini E, et al. Oxidative and non-oxidative glucose metabolism in non-obese type 2 (non-insulin dependent) diabetic patients. Diabetologia. 1988;31:585–91. doi: 10.1007/BF00264764. [DOI] [PubMed] [Google Scholar]
- 41.Defronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: A common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23:313–9. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- 42.Defronzo RA, Diebert D, Hendler R, Felig P. Insulin sensitivity and insulin binding in maturity onset diabetes. J Clin Invest. 1979;63:939–46. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Hollenbeck B, Y-Di Chen, Reaven GM. A comparison of the relative effects of obesity and non-insulin dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes. 1984;33:622–6. doi: 10.2337/diab.33.7.622. [DOI] [PubMed] [Google Scholar]
- 44.Kolterman OG, Gray RS, Griffin J, et al. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981;68:957–69. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: A follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–65. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 46.Hansen BC, Bodkin NL. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–14. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- 47.Coulston AM, Liu GC, Reaven GM. Plasma glucose, insulin and lipid responses to high-carbohydrate low-fat diets in normal humans. Metabolism. 1983;32:52–6. doi: 10.1016/0026-0495(83)90155-5. [DOI] [PubMed] [Google Scholar]
- 48.Chen YDI, Swami S, Skowronski R, Coulston AM, Reaven GM. Effects of variations in dietary fat and carbohydrate intake on postprandial lipemia in patients with non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;76:347–51. doi: 10.1210/jcem.76.2.8432777. [DOI] [PubMed] [Google Scholar]
- 49.Chen YD, Hollenbeck CB, Reaven GM, Coulston AM, Zhou MY. Why do low-fat high-carbohydrate diets accentuate postprandial lipemia in patients with NIDDM? Diabetes Care. 1995;18:10–6. doi: 10.2337/diacare.18.1.10. [DOI] [PubMed] [Google Scholar]
- 50.Gardner CD, Kraemer HC. Monosaturated versus polyunsaturated dietary fat and serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1917–25. doi: 10.1161/01.atv.15.11.1917. [DOI] [PubMed] [Google Scholar]
- 51.Jeppesen J, Schaaf P, Jones C, Zhoue MY, Chen YD, Reaven GM. Effects of low-fat, high-carbohydrate diets on risk factors for ischemic heart disease in post-menopausal women. Am J Clin Nutr. 1997;65:1027–33. doi: 10.1093/ajcn/65.4.1027. [DOI] [PubMed] [Google Scholar]
- 52.Mensink RP, Katan MN. Effect of dietary fatty acids on serum lipids and lipoproteins. Arterioscler Thromb. 1992;12:911–9. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 53.Groot PH, Van Stiphout WA, Krauss XH, et al. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11:653–62. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- 54.Patsch JR, Miesenbock G, Hopferweiser T, et al. Relation of triglyceride metabolism and coronary artery disease studies in the postprandial state. Arterioscler Thromb. 1992;12:1336–45. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- 55.Abbasi F, McLaughlin T, Lamendola C, et al. High carbohydrate diets, triglyceride-rich lipoproteins and coronary heart disease risk. Am J Cardiol. 2000;85:45–8. doi: 10.1016/s0002-9149(99)00604-9. [DOI] [PubMed] [Google Scholar]
- 56.Sharman MJ, Kraemer WJ, Love DM, et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002;132:1879–85. doi: 10.1093/jn/132.7.1879. [DOI] [PubMed] [Google Scholar]
- 57.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–3. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 58.Kaaks R. Nutrition and colorectal cancer risk: The role of insulin and insulin-like growth factor-1. European Conference on Nutrition and Cancer. International Agency for Research on Cancer and Europe Against Cancer Programme of the European Commission; Lyon, France. June 21 to 21; 2001. A0.14. (Abst) [Google Scholar]
- 59.Berrino F, Bellati C, Oldani S, et al. DIANA trial on diet and endogenous hormones. European Conference on Nutrition and Cancer. International Agency for Research on Cancer and Europe Against Cancer Programme of the European Commission; Lyon, France. June 21 to 24; 2001. A0.27. (Abst) [Google Scholar]
- 60.Willett WC. Cancer prevention: Diet and risk reduction: Fat. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 5th edn. New York: Lippincott-Raven; 1997. pp. 559–66. [Google Scholar]
- 61.Fearon KC. Nutritional pharmacology in the treatment of neoplastic disease. Baillieres Clin Gastroenterol. 1988;2:941–9. doi: 10.1016/0950-3528(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 62.Wolf RL, Cauley JA, Baker CE, et al. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr. 2000;72:466–71. doi: 10.1093/ajcn/72.2.466. [DOI] [PubMed] [Google Scholar]
- 63.Brehm BJ, Seeley RI, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 64.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]


