Abstract
With the emergence of Src inhibitors in clinical trials, improved knowledge of the molecular responses of cancer cells to these agents is warranted. This will facilitate the development of tests to identify patients who may benefit from these agents, allow drug activity to be monitored and rationalize the combination of these agents with other treatment modalities. This study evaluated the functional and molecular effects of Src inhibitor AZD0530 in human lung cancer cells. Src was activated in four of five cell lines tested and the level corresponded with the invasive potential and the histological subtype. Clinically relevant, sub-micromolar concentrations of AZD0530 blocked Src and focal adhesion kinase (FAK), resulting in significant inhibition of cell migration and Matrigel invasion. Reactivation of STAT3 and upregulation of JAK indicated a potential mechanism of resistance. AZD0530 gave a potent and sustained blockage of AKT and enhanced the sensitivity to irradiation. This indicated that AZD0530, aside from being a potent inhibitor of tumor cell invasion which could translate to inhibition of disease progression in the clinic, may also lower resistance of lung cancer cells to pro-apoptotic signals.
Keywords: Lung cancer, invasion, metastasis, Src, signal transducer and activator of transcription, protein kinase B
Introduction
Lung cancer is the leading cause of cancer-related death among men and women in the United States (1). Approximately 80% of all lung cancer patients present with metastases and are considered incurable. Systemic treatment for these patients includes chemotherapy, angiogenesis inhibitors and epidermal growth factor receptor (EGFR) inhibitors. Although these drugs can induce tumor responses, their activity is only transient and additional treatment options are needed. Due to the high invasive potential of lung cancer, inhibition of invasion and metastasis is an appealing concept in this tumor type. A large number of molecular factors associated with lung cancer metastasis have been identified and are currently being evaluated as drug targets (2, 3).
Src is part of a nine-member protein family of non-receptor tyrosine kinases (including Src, Yes, Yrk, Fyn, Fgr, Lyn, Hck, Lck and Blk). Src is closely associated with growth factor and cytokine receptors and plays a key role in the regulation of multiple cellular mechanisms, which are important in both normal and cancer cells, including cell migration, adhesion, invasion, survival, proliferation, angiogenesis and inflammation (4). Src regulates important intracellular signaling pathways, including the focal adhesion kinase (FAK), the phosphatidylinositol 3-kinase (PI3K), and the signal transducer and activator of transcription 3 (STAT3) pathways (5-7). Src is frequently overexpressed in lung cancer. In non-small cell lung cancer (NSCLC), accounting for approximately 80% of all lung cancers, Src levels, determined by immunoblotting and immunohistochemistry, were elevated in 50-80% of samples tested, and Src expression correlated with poor differentiation (8). The levels of activated Src, determined by enolase-assays, were higher in lung cancers compared with adjacent normal tissue (9). Another study revealed elevated Src kinase activity in NSCLC compared with small cell lung cancer (SCLC) (10). Activating mutations of Src have been described in colon cancer, but as yet, neither activating mutations nor gene amplifications have been detected in lung cancer (11, 12). In lung cancer cells, EGFR signaling appears to be the dominant mechanism of Src activation. This view is supported by studies demonstrating significant induction of apoptosis by Src inhibition in EGFR-dependent cell lines (13, 14).
A growing number of pharmacological Src inhibitors are currently being tested in the clinic, but biomarker identification is still in early development (14, 15). The dual Src/Abl inhibitor dasatinib (BMS-354825) has shown preclinical antitumor activity in solid tumors and clinical activity in leukemia (16). A six gene expression signature was shown to predict the sensitivity of breast and lung cancer cells to dasatinib in vitro (17), as did levels of activated Src in colon cancer xenografts (18). The orally available Src inhibitor AZD0530 is currently being tested in phase I-II trials. In breast cancer cells, AZD0530 reduced migration and, in combination with tamoxifen, blocked proliferation (19). In orthotopic colon cancer and prostate cancer models, AZD0530 blocked metastasis (20, 21). The present study evaluated the effects of AZD0530 in lung cancer cells and aimed to identify molecular factors associated with drug response or resistance.
Materials and Methods
Cell lines
The NSCLC cell lines A549, Calu-1 and Calu-6 were acquired from the American Type Culture Collection (Rockville, MD). The small cell lung cancer (SCLC) cell lines H69 and H526 were provided by Dr. R. Bold (UC Davis Cancer Center). NSCLC cells were cultured in DMEM (GIBCO, Carlsbad, CA) supplemented with heat-inactivated sterile-filtered 10% fetal bovine serum, 2 mM of L-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin. SCLC cells were cultured in RPMI (GIBCO) supplemented with 10% fetal bovine serum, 1.5 mg/ml of sodium bicarbonate, 10 mM of HEPES, and 1 mM of sodium pyruvate. Cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
Reagents
AZD0530 (AstraZeneca, Macclesfield, UK), PP2 (EMD Chemicals, Gibbstown, NJ) and LY294002 (Cell Signaling Technology, Beverly, MA) were solubilized in DMSO to 100 mM stock solutions.
Immunoprecipitation
Cells were harvested with trypsin-EDTA and lysed with IP buffer containing 150 mM NaCl, 25 mM Tris (pH 7.4), 1 mM EDTA, 1 mM EGTA, 2 mM Na3VO4, 10 mM NaF, 1% NP40, 10% glycerol, aprotinin (10 mg/ml) and leupeptin (10 mg/ml) for 30 min on ice. Lysates were centrifuged and supernatants were collected. Equal amounts of protein were incubated with total Src antibody (Upstate, Lake Placid, NY) overnight at 4°C. Protein G was added and samples were incubated at 4°C for 2 hours before pellets were rinsed twice with IP buffer, resuspended in 15 μl Laemmli buffer, and boiled at 95°C for 5 min. The supernatant was separated by SDS-PAGE and Western blotting was performed as described below.
Western blotting
Protein extracts were prepared from cell pellets in modified RIPA lysis buffer and proteins were quantified using BCA assay (Pierce, Rockford, IL). Equal amounts of protein were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked using 5% nonfat dry milk in TBST (Tris-buffered saline with Triton-X) and incubated at 4°C overnight with the following primary antibodies: Anti-Src and anti-FAK (Upstate, Lake Placid, NY); anti-phospho-Src family (Y419), anti-Abl, anti-phospho-Akt (S473), anti-phospho-FAK (Y576/577), anti-STAT3, anti-phospho-STAT3 (Y705), anti-JAK1, anti-JAK2, and anti-JAK3 (Cell Signaling Technology, Beverly, MA); anti-Actin, and anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-Bcl-xL (Transduction Labs, Lexington, KY). Membranes were incubated for 2 hours with appropriate HRP-conjugated secondary antibodies (Promega, Madison, WI) followed by visualization with ECL reagent and X-ray films (Amersham Biosciences, Amersham, UK).
Migration and invasion
Confluent monolayers of cells were scratched with a sterile pipette tip. Cells were washed twice with PBS (phosphate-buffered saline) and incubated with AZD0530 at the concentrations indicated. Control cells were incubated with DMSO. After 12 and 24 hours, migrating cells were photographed and counted by microscopy. For invasion, transwell inserts (BD Biosciences, San Jose, CA) were coated with 50 μl of Matrigel (BD Biosciences, Bedford, MA) and placed into 24-well plates. Cells (5×104) were placed into upper wells in the presence of 1 μM of AZD0530. Control cells were incubated with DMSO. After 48 hours, cells in the upper compartment were removed and cells on the lower side of the filter were fixed with 3.7% formaldehyde, stained with 0.5% methylene blue, photographed and counted by microscopy.
MTT assay
Cells were plated (103 per well) in 96-well plates and incubated with 1 μM of AZD0530, 1 μM of PP2 or 10 μM of LY294002. After 24 hours, plates were irradiated with a single dose of 2, 5 or 10 Gy, using a 6 MV linear accelerator (Varian, Palo Alto, CA). After another 72 hours, plates were analyzed using MTT. Cells were incubated with 5 mg/ml MTT [3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma-Aldrich, St. Louis, MO) for 3.5 hours at 37°C. The supernatant was removed, cells were lysed in 100% DMSO for 15 minutes, and absorbance at 570 nm was measured on a Benchmark plus photometer (Bio-Rad, Camarillo, CA). Control cells were incubated with DMSO. All samples were performed in triplicate.
Quantitative real time RT-PCR
Total RNA was extracted using the Trizol protocol (Invitrogen, Carlsbad, CA) and 1 μg of RNA was reversely transcribed using the M-MuLV reverse transcriptase (Fermentas, Hanover, MD). Equal amounts of cDNA were subjected to quantitative real time PCR using the iQ Sybr Green Supermix and the iCycler detection system (Biorad, Hercules, CA). Primers: JAK1-F 5 ′-GGAAATCTGCTACAATGGCGAG-3′; JAK1-R 5 ′-CCCCTTACAAATCTGAACGGC-3′, 18S-F 5′-CGCCGCTAGAGGTGAAATTCT-3′, 18S-R 5′-CGAACCTCCGACTTTCGTTCT-3′. Standard dilutions, melting curve analysis and agarose gel electrophoresis of PCR products were performed to confirm accuracy of the method. Results were analysed using Q-GENE software (22). JAK1 expression values were normalized for 18S rRNA and compared with DMSO control.
Statistical Analysis
Each experiment was performed at least twice; values represent the mean plus standard error of triplicate or quadruplicate wells. Two-sided t-tests were used and p-values <0.05 were considered significant.
Results
Target validation
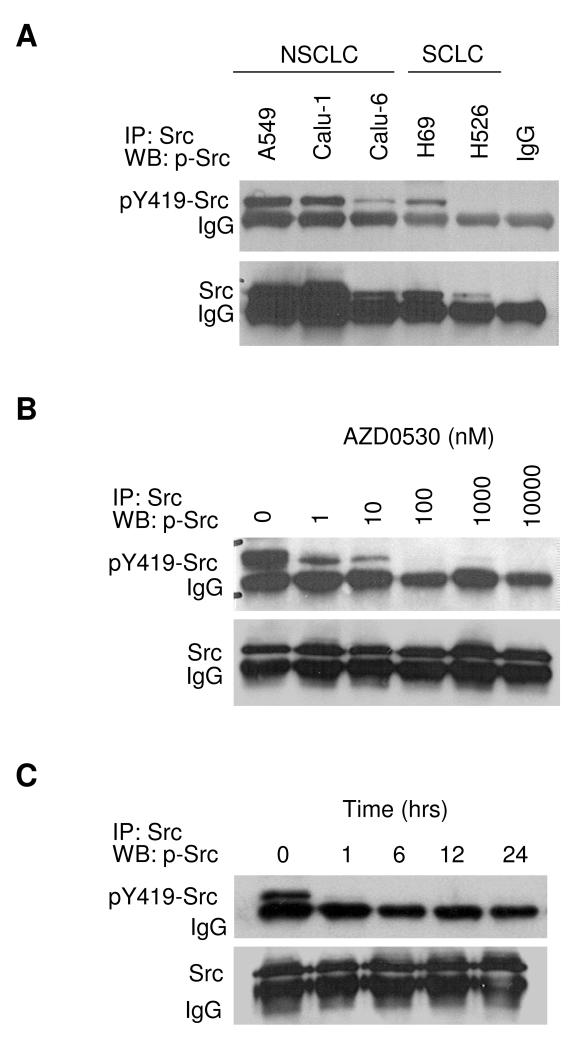
In a panel of 5 human lung cancer cell lines, basal expression levels of activated and total Src were determined by Src-immunoprecipitation and Western blotting for pY419-Src (Figure 1A). Overall, the levels of pSrc corresponded to the levels of Src. The NSCLC cell lines A549 and Calu-1 expressed the highest levels of pSrc and Src, Calu-6 (NSCLC) and H69 (SCLC) cells expressed moderate levels, and the SCLC cell line H526 expressed undetectable levels of pSrc.
Figure 1. Target validation.
A) Basal expression levels of pY419-Src and total Src were determined by immunoprecipitation with anti-Src antibody followed by Western blotting for pY419-Src and total Src. B) A549 cells were incubated with AZD0530 for 24 hours at the doses indicated, followed by immunoprecipitation and Western blotting as in A. C) A549 cells incubated with 1 μM of AZD0530 at the times indicated, followed by immunoprecipitation and Western blotting.
Next, A549 cells were incubated with AZD0530 at concentrations ranging from 1 to 10,000 nM (time ranging from 1 to 24 hours), followed by Src-immunoprecipitation and Western blotting for pY419-Src (Figure 1B and 1C). AZD0530 decreased pSrc levels in a time and dose dependent way and had a potent effect at low nanomolar concentrations. Total Src levels were not affected. Because AZD0530 has moderate activity against Abl (IC50 30 nM) in addition to its activity against Src, total levels of Abl were determined in the 5 lung cancer cell lines (data not shown). Only H69 cells expressed significant levels of Abl, which confirmed that Abl was not highly expressed in most lung cancer cells.
Migration, invasion and FAK signaling
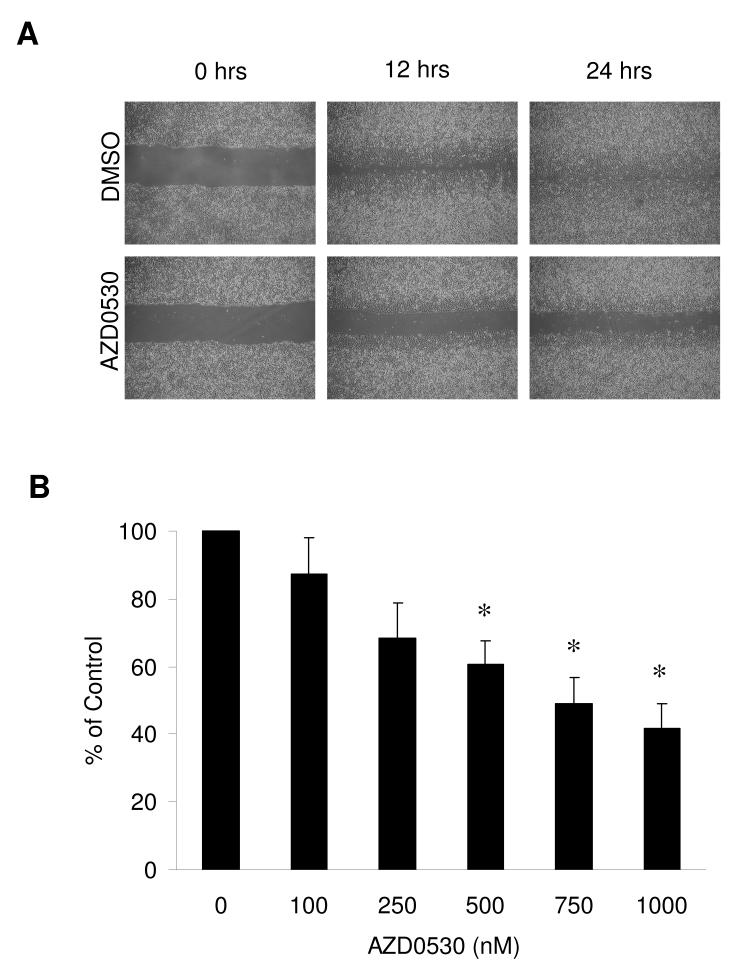
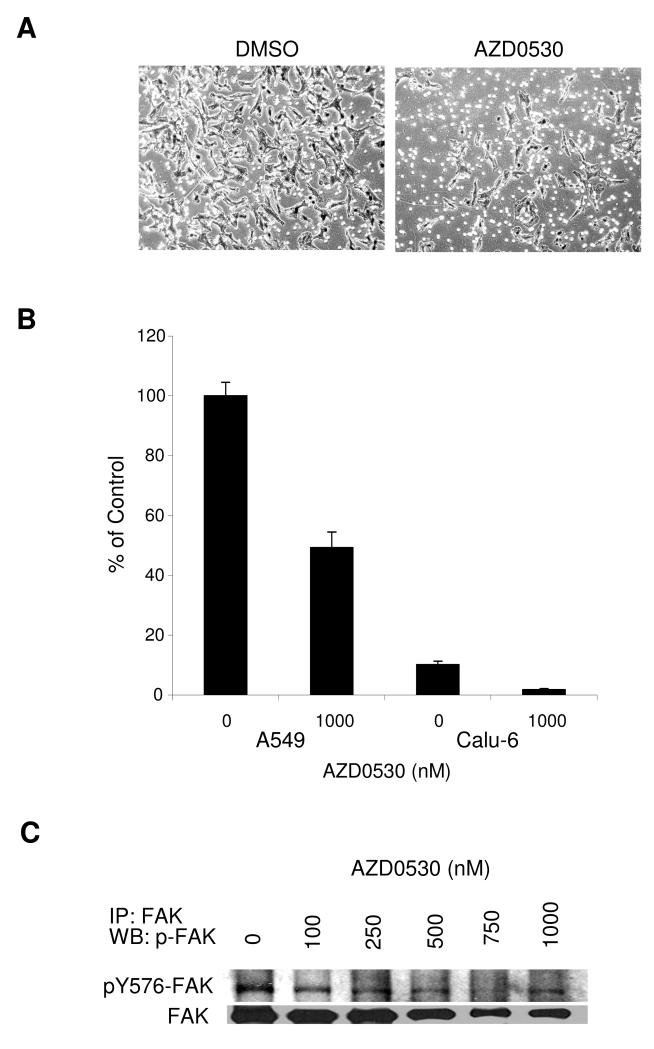
A549 cells were grown to confluent monolayers, which were scratched with a pipette tip and incubated with AZD0530 at concentrations ranging from 100 to 1000 nM (Figure 2A). DMSO-treated control cells continuously migrated into the scratch and nearly closed the scratch within 24 hours. Cell migration was significantly inhibited by AZD0530 in a dose-dependent way (Figure 2B). At the highest dose tested (1 μM), AZD0530 reduced A549 cell migration by more than 60%. Cell invasion was tested using a modified Matrigel assay with A549 (high Src levels) and Calu-6 (low Src levels) cells (Figure 3A and B). Cells were incubated with DMSO or 1 μM of AZD0530 for 48 hours. The invasive potential of A549 cells was almost 10-fold higher compared with Calu-6 cells, reflecting the basal level of Src in these cells. AZD0530 significantly reduced Matrigel invasion in A549 and Calu-6 cells by 51% and 82%, respectively.
Figure 2. Effect on cell migration.
A) Confluent monolayers of A549 cells were scratched and incubated with DMSO or AZD0530 at 500 nM. Migrating cells were photographed at 12 and 24 hours. B) Cells migrating into the scratch were counted and represented as a percentage of the DMSO control at 12 hours of incubation with indicated doses of AZD0530. Results are expressed as the mean ±SEM of three independent experiments. Asterisks represent two-sided t-tests with p-values <0.05.
Figure 3. Effect on Matrigel invasion.
A) A549 cells were plated in the upper chamber of a transwell coated with Matrigel with DMSO or AZD0530 at 1 μM. After 48 hours, cells invading through the Matrigel were photographed. B) A549 and Calu-6 cells invading through the Matrigel were fixed, stained with 0.5% methylene blue and counted. Results presented as a percentage of the A549 DMSO control (±SEM of quadruplicate wells). C) A549 cells were incubated with AZD0530 at indicated doses for 24 hours, followed by FAK-immunoprecipitation and Western blotting for pY576-FAK and total FAK.
To study the effect of AZD0530 on FAK, A549 cells were incubated with AZD0530 at concentrations in the range of 100 to 1000 nM, followed by FAK-immunoprecipitation and Western blotting for pY576-FAK (Figure 3C). AZD0530 at concentrations of 100 nM and 250 nM decreased the level of pFAK, whereas total FAK levels remained unchanged. AZD0530 concentrations above 500 nM decreased both pFAK and total FAK levels.
Reactivation of the JAK-STAT3 pathway
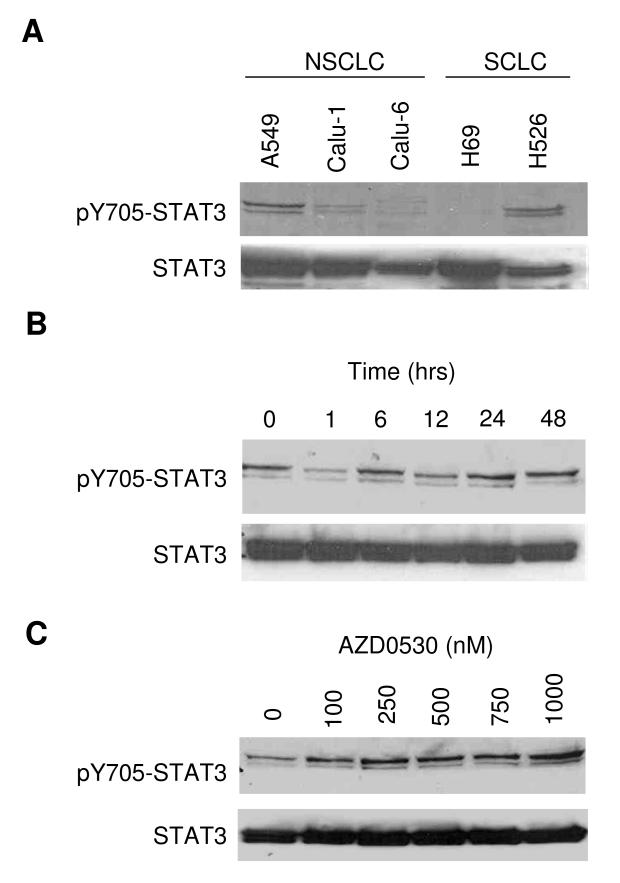
Basal expression levels of pY705-STAT3 and total STAT3 in lung cancer cells were determined by Western blotting (Figure 4A). All cell lines tested expressed STAT3. pSTAT3 levels were highest in A549 and low in Calu-1, consistent with a previous report (23). A549 cells were then incubated with 500 nM of AZD0530 (Figure 4B). Western blotting showed a short decrease of the pSTAT3 levels at 1 hour, followed by a rise at later time points. At the 24 hour time point, incubation with AZD0530 led to a dose-dependent increase of the pSTAT3 level (Figure 4C). Total STAT3 levels were not affected.
Figure 4. Reactivation of STAT3.
A) Basal expression levels of pY705-STAT3 and total STAT3 were determined by Western blotting in A549, Calu-1, Calu-6, H69 and H526 cell lines. B) A549 cells were incubated with 500 nM of AZD0530 for the times indicated, followed by Western blotting for pY705-STAT and STAT3. C) A549 cells were incubated with indicated doses of AZD0530 for 24 hours, followed by Western blotting for pY705-STAT3 and STAT3.
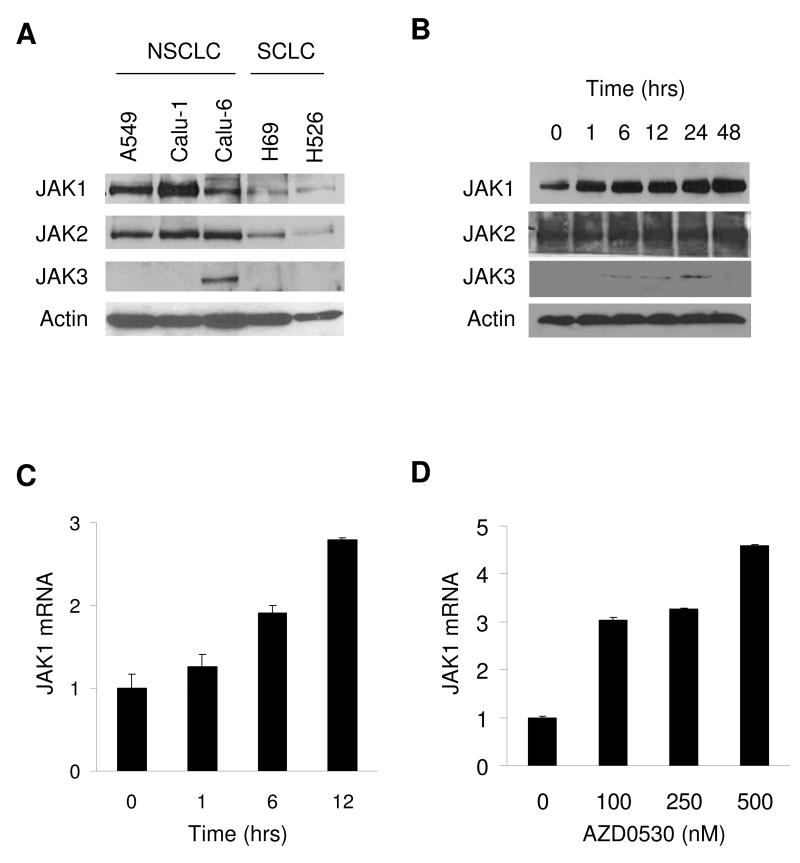
Because JAK can activate STAT3, the basal expression of JAK1-3 in lung cancer cells was determined (Figure 5A). All cell lines expressed JAK1 and JAK2, while JAK3 was only observed in Calu-6. A549 cells were incubated with AZD0530 and JAK1-3 protein levels were determined by Western blotting (Figure 5B). JAK1 mRNA levels were measured by qRT-PCR. AZD0530 increased JAK1 mRNA and JAK1-3 protein levels in a dose and time dependent way (Figures 5B-C).
Figure 5. Upregulation of JAK.
A) Basal expression levels of JAK1, JAK2, and JAK3 were determined by Western blotting. B) Incubation of A549 cells with 500 nM of AZD0530 for the times indicated, followed by Western blotting for JAK1-3. C) Incubation of A549 cells with 500 nM of AZD0530 cells for 24 hours, followed by JAK1 and 18S qRT-PCR. JAK1 expression was normalized for 18S expression. D) Incubation of A549 cells with AZD0530 for 24 hours followed by JAK1 and 18S qRT-PCR.
Inhibition of AKT and enhancement of radiosensitivity
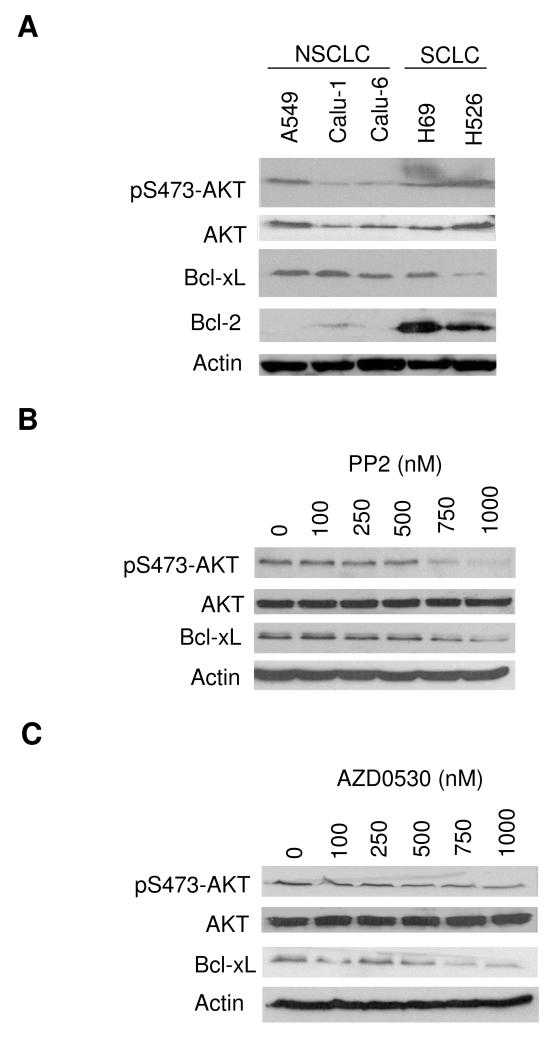
Basal expression of pS473-AKT, AKT, Bcl-XL and Bcl-2 in lung cancer cells was determined by Western blotting (Figure 6A). All cell lines tested expressed AKT. pAKT levels corresponded with AKT, and Bcl-XL levels (Figure 6A) corresponded with Src levels (Figure 1A). Bcl-2 levels were higher in SCLC compared with NSCLC cells. Next, A549 cells were incubated with AZD0530 or PP2 (another selective Src inhibitor (24)), at concentrations ranging from 100 to 1000 nM (Figure 6B and C). AZD0530 and PP2 decreased pAKT and Bcl-XL levels in a dose dependent way, whereas the levels of total AKT remained unchanged.
Figure 6. Inhibition of AKT and Bcl-xL.
A) Basal expression of pS473-AKT, AKT, Bcl-XL and Bcl-2 by Western blotting in a panel of lung cancer cell lines. B) A549 cells were incubated with increasing concentrations of PP2 for 24 hours, followed by Western blotting for pS473-AKT, AKT, Bcl-XL and Actin. C) A549 cells were incubated with AZD0530 for 24 hours at the doses indicated, followed by Western blotting for pS473-AKT, AKT, Bcl-XL and Actin.
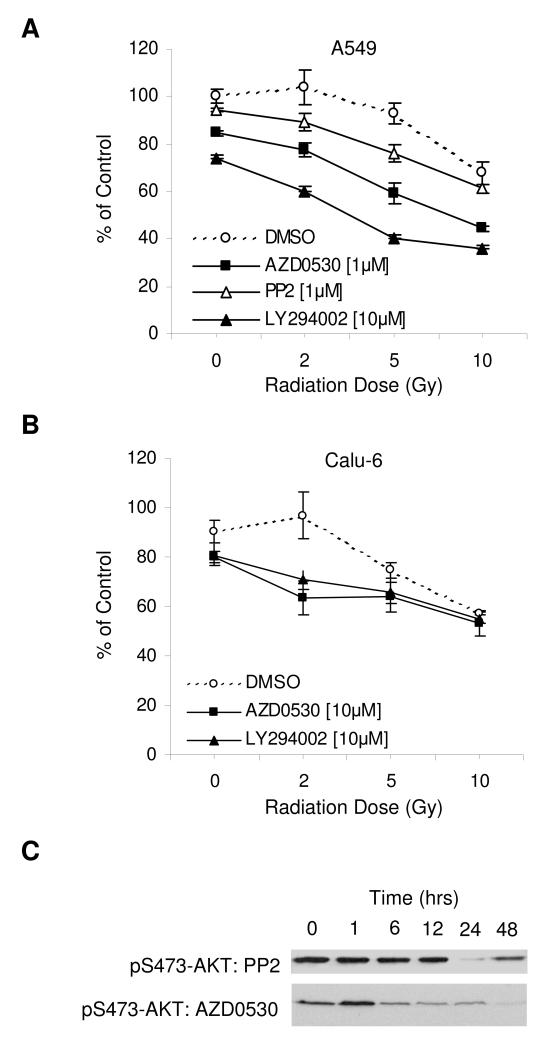
LY294002 is a selective inhibitor of PI3K-AKT and has been shown to sensitize cancer cells to pro-apoptotic signals (25, 26). To examine whether AZD0530 and PP2 have a similar effect, we performed irradiation experiments with A549 and Calu-6 cells. Cells were incubated with DMSO control, 1 and 10 μM of AZD0530, 1 μM of PP2 or 10 μM of LY294002 for 24 hours, followed by no irradiation, or irradiation with a single dose of 2, 5 or 10 Gy (Figure 7A and B). Irradiation alone had a dose dependent effect on cell viability in the MTT assay, which was enhanced by AZD0530 and LY294002, but not by PP2. To find an explanation for the failure of PP2, A549 cells were incubated with AZD0530 or PP2 over different periods of time (ranging from 1 hour to 48 hours), followed by Western blotting for pS473-AKT (Figure 7C). AZD0530 induced rapid and persistent suppression of pAKT, whereas the effect of PP2 on pAKT was delayed and transient.
Figure 7. Enhancement of sensitivity to irradiation.
A and B) A549 and Calu-6 cells were incubated with AZD0530, PP2 or LY294002 at the concentrations indicated for 24 hours and then not irradiated (0 Gy), or irradiated with doses of 2, 5 or 10 Gy. After 72 hours, MTT was used to assess cell viability. C) A549 cells were incubated with PP2 (10 μM) or AZD0530 (500 nM) for the times indicated, followed by Western blotting for pS473-AKT.
Discussion
The Src inhibitor AZD0530 is currently in phase I-II trials in patients with cancer (15). The availability of molecular markers to select patients and monitor drug effects may greatly enhance the development of this agent, as well as other Src inhibitors, in the clinic.
A previous report demonstrated that AZD0530 potently reduced migration and invasion in human breast cancer cell lines (19). The present study confirmed this effect in human lung cancer cell lines. AZD0530 abrogated the levels of activated Src, reduced the levels of activated FAK and significantly blocked cell migration and Matrigel invasion (Figures 1-3). Functional effects were achieved by a single administration of AZD0530 in a range between 100 and 1000 nM. Whether such concentrations can be reached in patients, remains to be shown by clinical trials. It is also possible that off-target activity of the compound may have contributed to these effects to some degree. We observed a concordance between the baseline level of activated Src, the histologic subtype and the differential invasive potential of the cell lines. However, AZD0530 blocked invasion irrespective of the level of activated Src in the cells, suggesting that other molecular markers might be more informative, including FAK, paxillin, ERK and other members of the integrin signaling pathway. In this context, we recently demonstrated that AZD0530 targets the bone morphogenetic protein (BMP) signaling pathway and found that the inhibitor of differentiation 1 (Id1) protein is a potential biomarker for Src inhibitor treatment (27). In the present study, we studied the effect of AZD0530 on two other important pathways regulated by Src.
In lung cancer cells, Src regulates the JAK-STAT3 pathway, which in turn promotes cell proliferation and survival (23, 28). Consequently, the Src inhibitor SU6656 decreased the levels of activated STAT3 and induced apoptosis in a significant proportion of lung cancer cells in a previous study (29). However, the Src inhibitor dasatinib induced reactivation of STAT3 in lung cancer cells, similar to the effect of doxorubicin in breast cancer cells (16, 30). The present study revealed that AZD0530 transiently lowered the levels of activated STAT3, followed by a rapid dose and time dependent reactivation of STAT3 (Figure 4). This was consistent with the observation that clinically relevant concentrations of AZD0530 had a negligible effect on cell viability and did not significantly alter the expression of the STAT3-target genes VEGF and IL-8 (data not shown). Also, the present study for the first time to our knowledge indicated that in lung cancer cells, inhibition of Src induced upregulation of JAK, suggesting that JAK is involved in the compensatory reactivation of STAT3 (Figure 5). Further studies are planned to confirm these data.
Src also interacts with the PI3K-AKT pathway, which regulates cell survival in a way that is partially overlapping with the JAK-STAT pathway (25). Previous studies revealed that cytotoxic drugs activate AKT and that inhibition of AKT enhances the sensitivity to pro-apoptotic signals in cancer cells (31, 32). Consistent with these data, the present study demonstrated that AZD0530 potently and sustainedly blocked AKT and enhanced the sensitivity of lung cancer cells to irradiation (Figure 7). The effect of AZD0530 was comparable with the effect of the PI3K-AKT inhibitor LY294002. PP2 failed in combination with irradiation, likely because PP2 is 5-10 times less potent than AZD0530. This may explain the diverging results in previous studies including PP2 in combination with irradiation and cytotoxic drugs in breast and colon cancer cells, respectively (33, 34).
In conclusion, our results demonstrate that AZD0530 is a potent inhibitor of lung cancer cell migration and invasion. Whilst this may be sufficient to provide clinical benefit through inhibition of disease progression and tumour spread, the profound inhibition of AKT and preliminary data from the combination experiments suggest that AZD0530 warrants further study in combination with irradiation and chemotherapy.
Acknowledgments
We thank AstraZeneca (Alderley Park, UK) for providing AZD0530, and our colleagues Dr. J. Yang, Dr. R. Bold, W.S. Holland and H.L. Devlin at the University of California Davis Cancer Center for their valuable support. OG was supported by the Swiss Foundation for Medical-Biological Grants/Swiss National Science Foundation (1184/PASMA-108925) and the Swiss Cancer League/Oncosuisse (BIL OCS 01949-08-2006).
References
- 1.Gautschi O, Gumerlock PH. Recent advances in the biology of lung cancer. In: Hansen HH, editor. Lung Cancer Therapy. Taylor and Francis Ltd.; Philadelphia. Pa: 2006. [Google Scholar]
- 2.Xi L, Coello MC, Litle VR, et al. A combination of molecular markers accurately detects lymph node metastasis in non-small cell lung cancer patients. Clin Cancer Res. 2006;12:2484–2491. doi: 10.1158/1078-0432.CCR-05-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Blackhall F, Seiden-Long I, et al. Modeling of lung cancer by an orthotopically growing H460SM variant cell line reveals novel candidate genes for systemic metastasis. Oncogene. 2004;23:6316–6324. doi: 10.1038/sj.onc.1207795. [DOI] [PubMed] [Google Scholar]
- 4.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 5.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Yu Q, Liu JH, et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278:40057–40066. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Tay A, Guy GR, et al. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazurenko NN, Kogan EA, Zborovskaya IB, et al. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Eur J Cancer. 1992;28:372–377. doi: 10.1016/s0959-8049(05)80056-5. [DOI] [PubMed] [Google Scholar]
- 9.Masaki T, Igarashi K, Tokuda M, et al. pp60c-src activation in lung adenocarcinoma. Eur J Cancer. 2003;39:1447–1455. doi: 10.1016/s0959-8049(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 10.Budde RJ, Ke S, Levin VA. Activity of pp60c-src in 60 different cell lines derived from human tumors. Cancer Biochem Biophys. 1994;14:171–175. [PubMed] [Google Scholar]
- 11.Irby RB, Mao W, Coppola D, et al. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 12.Kiefer PE, Bepler G, Kubasch M, et al. Amplification and expression of protooncogenes in human small cell lung cancer cell lines. Cancer Res. 1987;47:6236–6242. [PubMed] [Google Scholar]
- 13.Song L, Morris M, Bagui T, et al. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–5548. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Kalyankrishna S, Wislez M, et al. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D, Gautschi O. Clinical development of SRC tyrosine kinase inhibitors in lung cancer. Clin Lung Cancer. 2006;7:381–384. doi: 10.3816/clc.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 16.Johnson FM, Saigal B, Talpaz M, et al. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 17.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 18.Serrels A, Macpherson IR, Evans TR, et al. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 19.Hiscox S, Morgan L, Green TP, et al. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–274. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 20.Phillips K, Parikh N, Kuwai T, et al. Inhibition of colon tumor metastasis in an orthotopic nude mouse model with the dual selective Src/Abl kinase inhibitor, AZD0530. J Clin Oncol (Meeting Abstracts) 2007;25:14032. [Google Scholar]
- 21.Chang YM, Bai L, Liu S, et al. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008 doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19:1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 23.Blaskovich MA, Sun J, Cantor A, et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 24.Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 25.Brognard J, Clark AS, Ni Y, et al. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 26.Vlahos CJ, Matter WF, Hui KY, et al. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 27.Gautschi O, Tepper CG, Purnell PR, et al. Regulation of Id1 expression by SRC: implications for targeting of the bone morphogenetic protein pathway in cancer. Cancer Res. 2008;68:2250–2258. doi: 10.1158/0008-5472.CAN-07-6403. [DOI] [PubMed] [Google Scholar]
- 28.Haura EB. SRC and STAT pathways. J Thorac Oncol. 2006;1:403–405. [PubMed] [Google Scholar]
- 29.Song L, Turkson J, Karras JG, et al. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 30.Gariboldi MB, Ravizza R, Molteni R, et al. Inhibition of Stat3 increases doxorubicin sensitivity in a human metastatic breast cancer cell line. Cancer Lett. 2007;258:181–188. doi: 10.1016/j.canlet.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, et al. Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int J Cancer. 2005;117:755–763. doi: 10.1002/ijc.21242. [DOI] [PubMed] [Google Scholar]
- 32.Shah AN, Gallick GE. Src, chemoresistance and epithelial to mesenchymal transition: are they related? Anticancer Drugs. 2007;18:371–375. doi: 10.1097/CAD.0b013e32801265d7. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths GJ, Koh MY, Brunton VG, et al. Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem. 2004;279:46113–46121. doi: 10.1074/jbc.M408550200. [DOI] [PubMed] [Google Scholar]
- 34.Contessa JN, Abell A, Mikkelsen RB, et al. Compensatory ErbB3/c-Src signaling enhances carcinoma cell survival to ionizing radiation. Breast Cancer Res Treat. 2006;95:17–27. doi: 10.1007/s10549-005-9023-9. [DOI] [PubMed] [Google Scholar]