Abstract
Cells undergoing apoptosis lose lipid asymmetry that is often manifested by the exposure of phosphatidylserine (PS) to the outer surface of the cell membrane. Macrophages and other cell types recognize externalized PS to signal phagocytosis, thereby eliciting a non-inflammatory response. PS exposure is obligatory in the recognition and clearance of apoptotic cells. Here, we find that externally applied moderate electric field induces PS externalization in a mouse B cell (FOX-NY) membrane without procaspase-3 activation, a major characteristic of apoptotic cells. The field induced PS inversion is caused as a result of electroporation and/or a process involving membrane reorganizations and recovery that ensues following field exposure. Using a mouse macrophage cell line (J7444A.1) from the same strain, we show phagocytic clearance of PS expressing B cells and demonstrate that this is in part due to the apoptosis mimicry of the field exposed cells.
Keywords: Electroporation, Phagocytosis, Phosphatidylserine, Apoptosis, Electropermeabilization, Electric-field
INTRODUCTION
In normal cells, the membrane phospholipids are asymmetrically distributed between the inner and outer leaflets with the amine containing lipids phosphatidylethanolamine (PE) and phosphatidylserine (PS) primarily confined to the cytofacial monolayer. Loss of PS asymmetry has been associated with a number of important cellular, physiological, and pathological events including platelet activation in blood coagulation and adhesion [1], diabetes [2] and in the clearance of apoptotic cells. The latter event has been extensively studied in the past few decades [3]. The apoptotic pathway is a regulated cell death mechanism emanating from a complex set of biochemical events and is characterized by major changes in cell morphology, nuclear DNA fragmentation, chromatin condensation, and the loss of lipid asymmetry via externalization of PS. Exposure of phosphatidylserine is one of the early events that occur in cells undergoing apoptosis [4]. Through receptor and adaptor molecules, macrophages as well as many other cells recognize the surface modifications and externalized PS to signal phagocytosis in dying cells, thereby eliciting a non-inflammatory response.
While it is generally agreed that PS exposure in apoptotic cells is obligatory for recognition by macrophages, one recent report [5] revealed that PS exposure alone in non-apoptotic cells is sufficient for signaling cellular clearance. In this study, HT-1080 fibrosarcoma cells were shown to recognize and engulf viable, non-apoptotic Jurkat cells that have been enriched with brain-derived PS by liposome transfer. Conversely, a cell line (PLB 985) that is known for its failure to lose PS lipid asymmetry during apoptosis was not recognized by phagocytes after UV induced apoptosis, but was readily cleared when the outer plasma membrane was enriched with exogenously added PS liposomes. This data suggests that other means by which PS can be exposed on any cell surface might be a useful method to target cells of interest for phagocytic clearance. One such process is the application of external electric fields.
External electric fields affect cellular systems in a number of ways ranging from low field mediated cell signaling and wound healing, to relatively large pulsed fields which could induce transient membrane pore formation [6–9]. The latter effect is known as electroporation or electropermeabilization. An early electroporation study had shown that transbilayer lipid movement could occur upon electroporation of erythrocyte membranes [10]. In effect, the field induced hydrophilic membrane pores form a contiguous monolayer between the upper and lower leaflets of the membrane, thus allowing previously sequestered lipids to redistribute by lateral diffusion. A number of recent molecular dynamics (MD) simulations have further shown negatively charged PS can be driven by an externally imposed large electric potential across the membrane [11, 12]. However, these same authors have also found the extremely high fields used can induce apoptosis in cells [13, 14], inline with earlier findings by Schoenbach [15].
In this study we show PS externalization is selectively effected in healthy B cells by moderate electric field (~200 μs 2.1 kV/cm) without inducing apoptosis. The process occurs via relatively long-lived transient membrane pore formation and/or through a process involving membrane reorganization and recovery that ensues after electric field exposure. In the latter case, we find the field exposure results in extensive and long-lived membrane blebs whose constrained structure on the membrane surface may offer favorable conditions for spontaneous flipping of phoshatidylserine. Results from cell culture study shows significant recognition and clearance of the electric field permeabilized B-cells by the macrophages. This engulfment-digestion process was further facilitated by the presence of exogenously added Annexin-I, while it was suppressed when field induced externalized PS was blocked by the addition of Annexin-V; consistent with the notion that the uptake process was in part due to the apoptosis mimicry induced by electric field treated cells.
MATERIALS AND METHODS
Cell lines and culture
Mouse B cell lymphoblast, FOX-NY (ATCC, CRL-1732), and mouse Macrophage, J774A.1 (ATCC, TIB-67), were obtained from American Type Culture Collection, ATCC (Rockville, MD). The mouse B-cells were grown in RPMI 1640 media containing 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. The macrophages were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4 mM L-glutamine, 4.5 g/L glucose, 1.5×103 mg/L sodium bicarbonate, 10% fetal bovine serum, 50 Units/ml penicillin, and 50 μg/ml streptomycin. All cell culture media, serum, and antibiotic supplements were from Invitrogen (Gaithersburg, MD). Macrophages were detached by trypsin-EDTA (Invitrogen) treatment and sub-cultured for experiments on a two-well chambered cover glassed slides (Lab Tek, Winooski, VT) to ~70–80% confluency. Cell lines were maintained for growth in a 37 ° 5% CO2 incubator.
Fluorescent markers, reagents and microscopy
Ethidium Homodimer (EthD-1), Alexa Fluor-488 and Alexa Fluor-594 conjugated Annexin-V, Cell tracker green BODIPY, Ca+2 ionophore A-23187, and Di-8-ANEPPES were purchased from Molecular Probes - Invitrogen (Eugene, OR, USA). Bovine lung derived Annexin-I was from BIODESIGN International (Saco, Maine, USA). NaCl, HEPES, Na/K-Phosphates, Triton X-100, sucrose and CaCl2 were from Fisher (St Louis, USA). Rabbit monoclonal antibody directed against caspase-3 and anti-rabbit polyclonal Ab were from Cell Signaling Technology (Danvers, MA). Immunoreactive species were visualized using the Odyssey infrared imaging system (Li-Cor, Lincoln, NE). Fluorescence images were taken on a Zeiss LSM 5 Pascal laser scanning confocal microscope (Carl Zeiss, Jena, Germany).
Electric pulse generator and electrodes
The electroporation equipment (Velonix Model 360, Pollock Pines, CA) is as previously described [16]. Output from the pulse generator was directly connected to a chamber holding 1mm gap electroporation cuvettes (BioRad) in parallel with a 200 resistive load. The pulse across the sample cell suspension (~ 1×106 cells/ml) in an iso-osmotic electroporation buffer (250 mM sucrose, 2 mM phosphate, pH 7.4) was monitored with a 1000:1 probe (Tektronix 6501, Beaverton, OR) on a 500 MHz oscilloscope (Hewlett Packard Model 5661B, Palo Alto, CA). Field strength was calculated from the measured pulse amplitude and electrode separation.
Assay for surface exposed PS and phagocytosis
Surface exposed phosphatidylserine was probed with Alexa Fluor-488 conjugated Annexin-V (488-Annexin-V) following the binding protocol provided by the manufacturer (Molecular Probes - Invitrogen, Eugene, OR). The Annexin-V binding buffer consisted of 10mM HEPES, 140mM NaCl, 2.5 mM CaCl2, pH 7.4. EthD-1 was used as a counter stain to mark plasma membrane integrity for identifying dead/dying cells. Cells were harvested after treatment and re-suspended in 488-Annexin-V binding buffer at a cell density of ~ 1×106 cells/ml. A 250 μL of this cell suspension was incubated with 50 μL of 488-Annexin-V pre-packaged solution for 15 min, after which cells were washed (x3) with serum-free growth media and re-suspended in the same media. 488-Annexin-V positive and EthD-1 negative cells were assessed on the confocal microscope as a qualitative measure of positive PS exposure. For control experiment designed to block field exposed PS, 594-Annexin-V was used. To assess phagocytosis of B-cells, the adherent macrophages were first stained with Di-8-ANEPPES (red fluorescence) for 3 minutes and the unincorporated dye washed out (x3) with PBS. Di-8-ANEPPES is a fast-responding electrochromic dye typically used for measurement of trans-membrane potentials, but is used here for cell tracking purposes due to its membrane retention properties. The B-cells were separately loaded with Cell-tracker green (green fluorescence) for 15 mins, and the excess dye was removed in 3 successive spin/wash cycles. The cells were then co-incubated with the adherent macrophages at an approximate B-cell:macrophage ratio of 15:1 for two hours, after which the B-cells were gently aspirated and the chamber slide washed twice with fresh media. The macrophages incorporating the green fluorescence and appearing yellow (red/green) in the merged images were scored as positive for uptake after bleed-through corrections for the green and red emission channels. The percentage of positive cells for uptake (# of macrophages taking up B cells per total macrophages) was calculated from several randomly selected reading of cells on the microscope field of view.
Western blot analysis of procaspase-3 activation
Apoptosis was induced in B-cells by incubating cells in the presence of 5μM Ca+2 ionophore (A-23187) for 12 hours. Procaspase-3 activation was monitored by Western blot analysis using monoclonal anti-caspase-3 antibody for control, electric field exposed, and calcium ionophore treated cells. After treatment, cells were collected by centrifugation and the pellets were solubilized in SDS-polyacrylamid gel electrophoresis (SDS PAGE) loading buffer. Protein concentration was determined by Lowry method using RC DC protein assay kit (Bio-Rad laboratories, Hercules, CA) according to the manufacturer recommendation. Equal amount of protein (25 μg) was subjected to electrophoresis using SDS PAGE. Separated proteins were thereafter transferred onto nitrocellulose membrane and probed over night with a Rabbit monoclonal antibody directed against caspase-3 (dilution ratio, 1:1000) in blocking buffer (LI-COR, Biosciences), containing 0.1% (vol/vol) Tween-20. After washing with PBST buffer three times, the membrane was then incubated in the dark for 1hr with goat anti-rabbit polyclonal Ab labeled with IR800CW 926-32211 (Rockland, Gilbertsville, PA) as a secondary Ab (dilution ratio, 1:20000). Immunoreactive species were visualized using the Odyssey infrared imaging system (Li-Cor, Lincoln, NE).
RESULTS AND DISCUSSION
The results for the electric field induced PS exposure in B-cell are shown in Fig. 1. On all the panels shown Fig.1A-C, the top frames in each panel (left to right) show the marker for loss of membrane integrity (EthD-1 positive, red channel), and the probe for the presence of surface exposed PS (488-Annexin-V positive, green channel) fluorescence images, respectively. The bottom frames (left to right) show the phase, and triple merged images of the same cells, respectively. As shown on the green channel image of Fig. 1A, very faint 488-Annexin-V binding is found in these control cells implying PS remains sequestered in the intracellular side of the cell membrane. In the few 488-Annexin-V positive cells observed, they were also positive for EthD-1 indicating loss of membranes integrity. In Fig. 1B, cells (~ 1×106 cells/ml) were first suspended in the electroporation buffer and pulsed with a ~200 μSec, ~2.1 kV/cm field. Following the field exposure, cells were transferred to a culture dish and incubated for ~30 minutes in growth media to allow recovery of the membrane pores. PS externalization and membrane integrity were then probed as described in Methods. Fig. 1B shows a substantial increase in 488-Annexin-V fluorescence compared to that observed for control cells. These results revealed that PS was exposed to the extracellular side, while essentially no significant increase in EthD-1 fluorescence indicating a minimal loss of membrane integrity. As a positive control, a 0.2% (final concentration) Triton X-100 surfactant solution was added to the sample shown in Fig. 1B. Fig. 1C shows the cytosolic and nuclear EthD-1 staining after surfactant treatment of cells shown in the same field of view as that of Fig. 1B.
Fig 1.
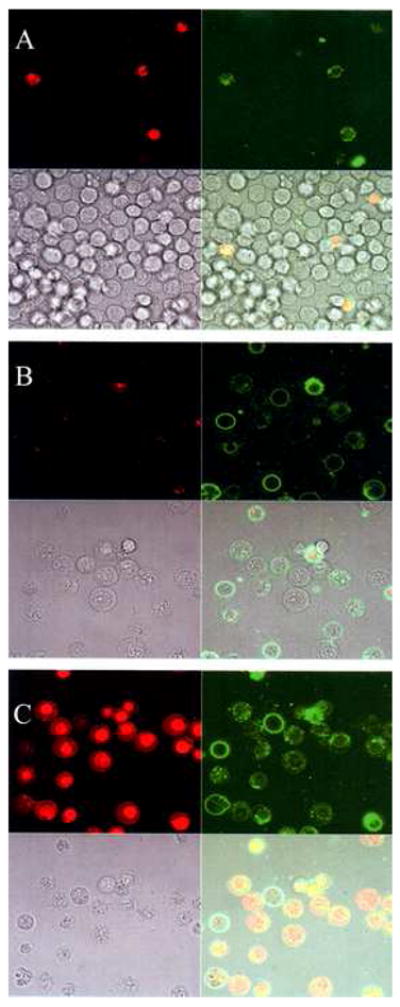
Phase and fluorescence (Annexin (+) and EthD-1 (−) ) microscope images of electric field induced PS externalization in B-cells. (A) Control – No Electric field. (B) Electroporated B-cells. E ~ 2.1 kV/cm, ~ 200 μSec pulse. (C) Addition of 0.2% Triton X-100 to the sample shown in (B) to permeabilize the membrane.
One of the significant morphological changes observed following electroporation of the B-cells is the immediate formation of extensive blebbing as shown in Fig. 2. In the absence of applied electric field, the control cells (Fig. 2A) showed a limited number of membrane blebs. It should be pointed out that short-lived membrane blebs were also observed when cells underwent spin/wash cycles and media changes. By contrast, Fig 2B reveals that electroporated cells show extensive bleb formation. Some of these blebs remain as long as ~30 minutes after the electric pulse before their retraction. Similar field-induced bleb formation and retraction has been reported in other B-cell strains [17, 18]. The image in Fig. 2B was taken ~10 minutes after a ~200 μSec, ~2.1 kV/cm field exposure. In some cells, a membrane envelope was observed around recovering cells possibly due to expansion and coalescence of the blebs into larger entities that could eventually detach from the cortical cytoskeleton. These extensive field induced bleb formation, expansion, and eventual retraction could provide a pathway for enhancing PS inversion and prolong their presence on the outer membrane, thus, providing an efficient mechanism for macrophage mediated clearance.
Fig 2.
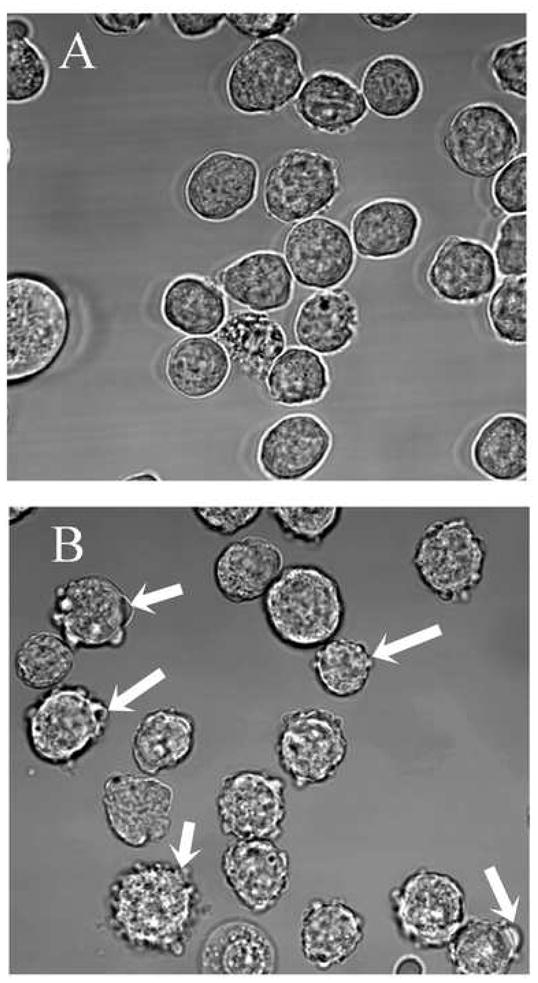
Blebbing and morphological changes in B cells exposed to ~200 μSec, ~2.1 kV/cm electroporating pulse. (A) Control – No Electric field applied. (B) Cells exposed to external field showing bleb formation. White arrows point to blebs in some of the cells. Image was taken ~10 min after field application.
Fig. 3A-C, show the effect of electric field-induced PS externalization on B cell clearance by macrophages. For the phagocytosis experiments, the adherent macrophages were stained with Di-8-ANEPPES and the B-cells were electroporated with ~2.1 kV/cm field strength for ~ 200 μsec and immediately incubated in media containing 25 μM of Cell-tracker green for ~ 30 minutes to allow for Cell tracker loading and membrane recovery. The excess dye was washed out and these electric field treated and Cell-tracker green labeled cells were incubated further at B-cell:macrophage ratio of ~15 for ~2 hrs. The B-cells were then aspirated off and fluorescence images acquired. As shown in Fig. 3A, very little of the control B-cells are recognized for uptake, typically amounting to less than ~ 4% of the macrophages being positive for uptake. On the other hand, the electroporated B-cells were easily recognized and ingested by macrophage as shown in Fig. 3B.
Fig 3.
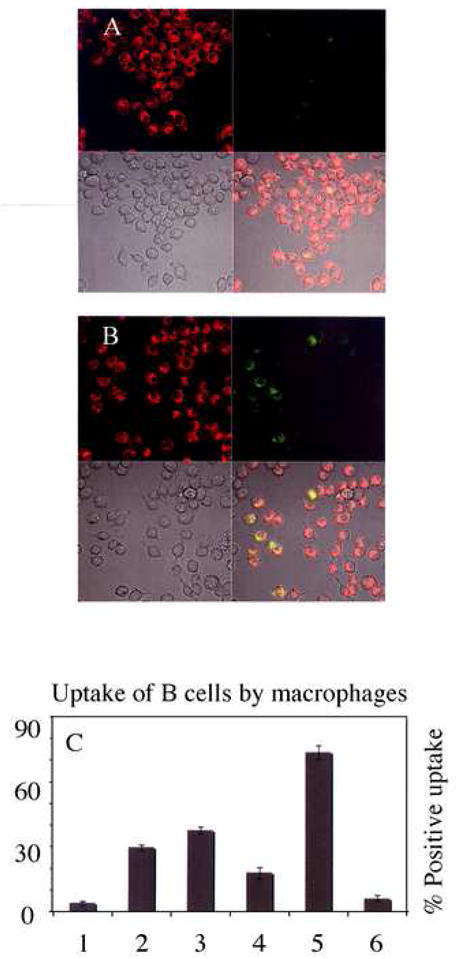
Macrophage uptake of PS expressing cells. (A) Control. No Electric field (B) Field treated B-cells. E ~ 2.1 kV/cm, ~ 200 μSec. See Results section for description of frames in each panel. (C) Percentage of macrophages positive for uptake under conditions shown: Lanes 1- Control; 2-Field exposed; 3-Field exposed + Annexin I; 4-Field exposed + Annexin V blocked; 5-Ca+2 ionophore treated for 12 hrs; 6-Field exposed + 12 hrs incubation. Error bars represent the standard error.
Following the assay used in Fig. 3A–B, the percentage of macrophages positive for uptake under various conditions was scored and the results are shown in Fig. 3C. Electric field exposed B-cells were recognized in ~ 30% (lane 2), relative to ~4% (lane 1) for the control cells of the macrophages when co-incubated for ~2 hrs after electric field treatment. Consistent with the notion that the observed phagocytic uptake is linked to extracellular exposed PS, Fig 3C (lane 3) shows that the uptake was enhanced when cells were co-incubated in the presence of 2 μM Annexin-I, a protein known to facilitate the interaction between the phosphatidylserine receptor and PS exposed in cells. Enhanced caspases activation in apoptotic cells has been shown to correlate with elevated expression of Annexin I, and its translocation to the membrane is believed to serve as a bridging molecule in the PSR-PS complex [19]. Conversely, blocking the field induced externalized PS with Alexa Fluor-594 tagged Annexin-V (594-Annexin-V) prior to incubation with the macrophages suppressed the uptake of cells (Fig. 3C, lane 4), indicating that the recognition and clearance of B cells is in part due to the apoptosis mimicry of the electric field exposed cells. As a positive control to PS mediated macrophage uptake, apoptosis was also induced in B-cells by incubating cells with 5 μM of the calcium ionophore, A-23781, for 12 hours. Co-incubation of these cells with the macrophages resulted in > 70% recognition as shown in Fig. 3C (lane 5). Lastly, to assess the transient nature of field induced PS inversion, electroporated cells were incubated for 12 hrs to allow their recovery from the field effects. These recovered cells were only recognized at levels similar to the non-field treated control cells (Fig. 3C, lane 6). To rule out field-induced apoptosis, caspase-3 activation, a hallmark of apoptosis, was monitored in the control, field exposed, and ionophore treated cells using Western blot analysis. The results (Fig. 3D, see supplementary material figure) show that significant amount of active caspase-3 was only observed in the ionophore treated B-cells (Fig. 3D, lane 3).
Together, these results can be rationalized by a mechanistic scheme shown in Fig. 4. Pathway-A shows lipid inversion can occur via either field driven unidirectional transport of PS (shown with red headgroups) or through diffusion along the walls of the metastable-resealing membrane pores. This process is likely inefficient when short (nanosecond) pulses are used due to the limited size and lifetime of the pores [11, 12]. By contrast, as shown in our earlier study [7] as well as the present data, electric field pulses with moderate field parameters can generate membrane pores that could remain for tens of minutes, thus allowing reasonable timescale for lipid diffusion along pores. Fig. 4, pathway-B shows an additional and probably a more significant pathway for PS inversion in electroporated B-cells. This pathway takes into account the electric field induced bleb formations observed (Fig. 2B) in our experiments. Blebbing is a vesicular bulging of the plasma membrane lipid components from the supporting cortical-cytoskeletal network on the cell surface. Blebs are filled with cytoplasmic fluid and devoid of cortex and associated proteins. Although it is not fully known how the blebs develop as a result of the field exposure, it is reasonable to assume that weakening of the lipid-cytoskelton adhesion at sites where pores are created could facilitate the detachment of lipids to form the nucleus of expanding blebs. This process can be further enhanced if cell shrinkage accompanies fluid exchange through the membrane electro-pores. Whatever the mechanism, however, the semi-spherical structures that are formed lead to constrained lipid packing due to the acute curvatures at the base and apex of the bleb (see Fig. 4 Scheme-B), thus favoring the scrambling of the inner and outer lipid leaflets to enable PS exposure. The constrained lipid packing and subsequent PS inversion has recently been implicated to account for PS-enriched apoptosome-vesicles in dying B-cells [20].
Fig 4.
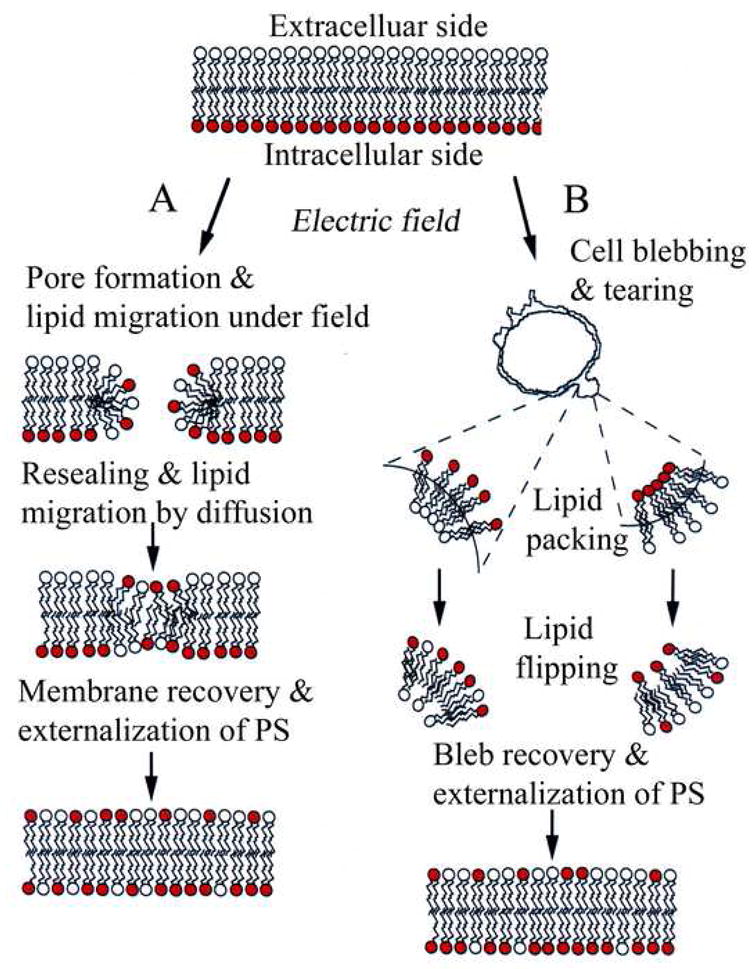
Schematic for electric field induced PS (red head group) externalization. Pathway-A: Lipid inversion could occur either through field driven transport of the negatively charged PS and/or via PS diffusion along the walls of hydrophilic membrane pores. Pathway-B: Electroporation leads to bleb formation similar to those shown in Fig. 2B. The bleb geometry constrains lipid packing due to curvature, thus favoring the scrambling of the inner and out lipid leaflets resulting in PS exposure. Exposed PS diffuses along the outer membrane periphery and remains exposed for extended time till bleb retraction and eventual sequestration to the cytoplasmic side.
The extended lifetime of the field-induced blebs observed here is of particular interest compared to other cellular blebs associated with motion generated from the contractile cortex [21, 22]. In these reports, retraction of blebs occurs in time scales on the order of ~ 2 mins and involves the recruitment and assembly of a number of proteins including actin, actin bundling proteins and myosin. It is reasonable to assume the retraction in field treated cells could be significantly delayed due to the disruption of the cytoskeleton during pore formation. This could also imply that enzymes normally responsible for regulating PS distribution may not access their substrates when the plasma membrane is detached from the cellular cortex and may explain the extended presence of PS found in our experiments.
In summary, our experiments demonstrate externally applied moderate electric fields can induce PS inversion to the outer leaflet of normal B-cell membranes via several possible pathways and that this process suffices to elicit recognition and removal of PS expressing cells by macrophages. Use of electric fields to induce PS exposure on cells is of interest since the field parameters can be tailored for selective electroporation [8, 23] for use in cellular research as well as in clinical settings for selective removal of pathogenic cells through non-inflammatory phagocytosis of the ‘apoptosis-mimetic’ cells.
Supplementary Material
Acknowledgments
The Intramural Research Program of the NIH, NHLBI, supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bevers EM, Comfurius P, Zwaal RF. Changes in membrane phospholipid distribution during platelet activation. Biochim Biophys Acta. 1983;736:57–66. doi: 10.1016/0005-2736(83)90169-4. [DOI] [PubMed] [Google Scholar]
- 2.Wali RK, Jaffe S, Kumar D, Kalra VK. Alternations in organization of phospholipids in erythrocytes as a factor in adherence to endothelial cells in diabetes mellitus. Diabetes. 1988;37:104–111. doi: 10.2337/diab.37.1.104. [DOI] [PubMed] [Google Scholar]
- 3.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 5.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 6.Neumann E, Sowers AE, Jordan CA, editors. Electroporation and Electrofusion in Cell Biology. Plenum Press; New York: 1989. [Google Scholar]
- 7.Tekle E, Astumian RD, Chock PB. Electroporation by using bipolar oscillating electric field: An improved method for DNA transfection of NIH3T3 cells. Proc Natl Acad Sci USA. 1991;88:4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenbach KH, Katsuki S, Stark RH, Buescher ES, Beebe SJ. Bioelectrics – New applications for pulsed power technology. IEEE Trans Plasma Sci. 2002;30:293–300. [Google Scholar]
- 9.Teissie J, Golzio M, Rols MP. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of ?) knowledge. Biochim Biophys Acta. 2005;1724:270–280. doi: 10.1016/j.bbagen.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Dressler V, Schwister K, Haest CW, Deuticke B. Dielectric breakdown of erythrocyte membrane enhances transbilayer mobility of phospholipids. Biochim Biophys Acta. 1983;732:304–307. doi: 10.1016/0005-2736(83)90216-x. [DOI] [PubMed] [Google Scholar]
- 11.Hu Q, Joshi RP, Schoenbach KH. Simulations of nanopore formation and phosphatidylserine externalization in lipid membranes subjected to a high-intensity ultrashort electric pulse. Phys Rev E. 2005;72:031903. doi: 10.1103/PhysRevE.72.031902. [DOI] [PubMed] [Google Scholar]
- 12.Vernier PT, Ziegler MJ, Sun Y, Chang WV, Gundersen MA, Tieleman DP. Nanopore formation and phosphatidylserine externalization in a phospholipids bilayer at high transmembrane potential. J Am Chem Soc. 2006;128:6288–6289. doi: 10.1021/ja0588306. [DOI] [PubMed] [Google Scholar]
- 13.Vernier TP, Li A, Marcu L, Craft CM, Gundersen MA. Ultrashort pulsed electric fields induce membrane phospholipids translocation and caspase activation: Differential sensitivities of Jurkat T lymphoblasts and Rat Glioma C6 cells. IEEE Trans Dielectrics and Electrical Insulation. 2003;10(5):795–809. [Google Scholar]
- 14.Beebe SJ, Fox PM, Rec LJ, Willis LK, Schoenbach KH. Nanosecond high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003;30:286–292. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 15.Schoenbach KH, Beebe SJ, Buescher ES. Intracellular effect of ultrashort electrical pulses. J Bioelectromagnetics. 2001;22:440–448. doi: 10.1002/bem.71. [DOI] [PubMed] [Google Scholar]
- 16.Tekle E, Astumian RD, Friauf WA, Chock PB. Asymmetric pore distribution and loss of membrane lipid in electroporated DOPC vesicles. Biophys J. 2001;81:960–968. doi: 10.1016/S0006-3495(01)75754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gass GV, Chernomordik LV. Reversible large scale deformations in the membranes of electrically-treated cells: electroinduced bleb formation. Biochim Biophys Acta. 1990;1023:1–11. doi: 10.1016/0005-2736(90)90002-6. [DOI] [PubMed] [Google Scholar]
- 18.Neumann E, Toensing K, Kakorin S, Budde P, Frey J. Mechanism of electroporative dye uptake by mouse B cells. Biophys J. 1998;74:98–108. doi: 10.1016/S0006-3495(98)77771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, William M, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 20.Elliott JI, Alessandro S, Cooper JC, Alexander DR, Devanture S, Chimini G, Higgins CF. Phosphatidyl exposure in B lymphocytes: a role for lipid packing. Blood. 2006;108:1611–1617. doi: 10.1182/blood-2005-11-012328. [DOI] [PubMed] [Google Scholar]
- 21.Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477–490. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charras GT, Coughlin M, Mitchison TJ, Mahadevan L. Life and times of a cellular bleb. Biophys J. 2008;94:1836–1853. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tekle E, Oubrahim H, Dzekunov SM, Kolb JF, Schoenbach KH, Chock PB. Selective field effects on intracellular vacuoles and vesicle membranes with nanosecond electric pulses. Biophys J. 2005;89:274–284. doi: 10.1529/biophysj.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


